Abstract
Laccases are appealing biocatalysts for various industrial utilizations. The fungus Trametes versicolor (L.: Fr.) Pilát causes white rot in wood and has been identified as an important fungal laccase producer. To investigate laccase production and activity in T. versicolor, the native isolate was collected from the host (Quercus castaneifolia) in the forests of Guilan province, northern Iran, and then purified and identified using the molecular marker. Its ability to produce laccase enzyme in the presence of different plant substrates including sawdust and wood chips of oak, poplar, and pine was evaluated. Also, the effect of copper as an enzyme inducer was investigated in vitro. The results showed that adding the wood to the culture medium increased laccase production, and among these, oak sawdust had the greatest effect, a 1.7-fold increase from that in the control (4.8 u/l vs. 2.8 u/l). Also, the enzyme extraction time effect on the optimal recovery yield showed that the 5-h enzyme extraction cycle resulted in the highest yield of the enzyme (18.97 u/l). Moreover, adding different concentrations of copper to the fungal culture medium increased the production of laccase, and the highest amount of enzyme (92.04 u/l) was obtained with 3.5 mM of CuSO4 along with oak sawdust. Based on the results, the addition of host wood sawdust (“oak” in this work) and copper particles together stimulates the fungal growth and the laccase production during submerged cultivation of T. versicolor. Therefore, it would be a safe and cheap strategy for the commercial production of laccase by filamentous fungi.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Genus Trametes belongs to the Polyporaceae family, Polyporales order, Agaricomycotina sub-phylum, and Basidiomycota phylum. It includes many species that decompose the wood of dead trees [1]. In this genus, species T. versicolor (synonym Coriolus versicolor) and T. gibbosa are important ligninolytic fungi that cause white rot in wood and grow on different trees such as oak (Quercus spp.), plum (Prunus spp.), and different coniferous trees such as pine (Pinus spp.) [2]. Trametes versicolor, the rainbow bracket or rainbow fungus, grows on rotten or dead tree trunks [3] and becomes dormant or inactive in dry conditions. The optimal conditions for its growth are 23 to 24 °C and 40 to 50% humidity, and it can also live on wood with a humidity of 80–120% [4]. In addition, the presence of wood preservatives, such as zinc and copper compounds, has been reported to induce laccase production in various white-rot fungi, such as Trametes [5,6,7]. Laccases (EC 1.10.3.2, p-diphenol: dioxygen oxidoreductases) are common biological oxidoreductase enzymes that catalyze the single-electron transfer of diverse substrates to generate four phenoxy radicals with simultaneous reduction of O2 to 2H2O [8]. However, laccases require other small molecules such as 2′-azino-bis-(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS), 1-hydroxybenzotriazole (HBT), syringaldehyde, veratyl alcohol, and guaiacol, which act as mediators and assist the effective oxidation of non-phenolic substrates [9]. The search for new, efficient, and environmentally friendly processes for the textile, pulp, and paper industry has aroused interest in these “green catalysts,” which work with air and produce water as the only by-product. As a result, in recent years, numerous scientific reports have been published focusing on the biochemical properties of these proteins or their use in biological processes, bioremediation, and chemical reactions [10, 11]. Laccase activity has been identified in a wide range of fungi, from Ascomycetes to Basidiomycetes, and from wood rot fungi to symbiotic ectomycorrhizal fungi [12]. White-rot fungus (Basidiomycete) is considered one of the first-rank laccase producers [13]. Commercial application of laccase enzyme has been well investigated in white-rot fungi such as Trametes versicolor, T. pubescens, T. villosa, T. hirsuta, and Pleurotus spp., and the enzyme purification was successful by ordinary approaches [14,15,16,17,18,19,20]. One of the considerable restrictions of large-scale utilization of fungal laccases is the low rate of production by wild-type and recombinant fungal isolates [5]. Therefore, it is necessary to achieve a higher concentration of enzyme by optimizing the fungal culture conditions and changing the substrates in the culture media. Laccase productivity can be enhanced by applying optimization strategies based on the one-factor-at-a-time method or statistical procedures. Changing the growth substrate, cultivation time, moisture, and inducers are some of the factors which were studied [21]. Generally, a small amount of laccase is produced by white-rot fungi in submerged culture or on wood, while adding different aromatic compounds such as 2,5-xylidine and ferulic acid could increase the enzyme concentration. Different response elements in the promoter regions of laccase genes can be induced by certain xenobiotic compounds, heavy metals, or heat shock treatment [22]. Studies showed that copper, as a micronutrient, plays an important role in laccase production by white-rot fungi, but it should be noted that excessive amounts of inducers such as copper impair fungal growth and enzyme production [23,24,25]. Different species of white-rot fungi have different tolerances to heavy metals, which also occurs in strains of the same species. Laccase activity increased by adding copper in the species Phanerochaete chrysosporium ME446 [23], Pycnoporus sanguineus BAFC 2126 [24], Trametes pubescens [5], and Pleurotus ostreatus [26]. Due to heavy metals’ effect on laccase enzyme activity, it is obvious that their presence can affect the biotechnological processes [23]. Jaber et al. [25] evaluated the effect of different amounts of copper sulfate on laccase production by Trichoderma muroiana IS1037 and showed that all concentrations increased laccase production. Backes et al. [27] showed that farnesol is an excellent laccase inducer in T. versicolor and P. sanguineus in solid-state fermentation and enhanced laccase production up to 77.88 ± 5.62 U/g (236% above control) in T. versicolor and 130.95 ± 2.20 U/g (159% increase) in P. sanguineus after 17 days.
Currently, researchers are interested in increasing yield efficiency and reduction of the laccase final price by improving the production and purification process. In the meantime, the identification of effective substrates in increasing the production of industrial laccase enzymes is of undeniable importance. Therefore, this research was carried out to optimize laccase enzyme production in the presence of different plant substrates and also investigate the effect of copper as a stimulant for laccase production in laboratory conditions.
Material and method
Fungal isolation and identification
To conduct experiments, in the November of 2019, the basidiocarps of Trametes versicolor and the associated wood trunk were collected from the western forest of Guilan, northern Iran, and transferred to the laboratory. The dominant plants of the collection area were oriental beech (Fagus orientalis), chestnut-leaved oak (Quercus castaneifolia), European hornbeam (Carpinus betulus), Caucasian alder (Alnus subcordata), velvet maple (Acer velutinum), Cappadocian maple (Acer cappadocicum), Scots elm (Ulmus glabra), common ash (Fraxinus excelsior), and date-plum (Diospyros lotus).
An isolate of T. versicolor was isolated from wild fruit bodies growing on oak logs. A small piece of oak wood colonized by T. versicolor was disinfected with 95% ethanol and 1% sodium hypochlorite and then rinsed with sterile distilled water. After that, it was cultured on malt extract agar (MEA) medium and kept at 25 ± 2 °C. Purification was done by the single hyphal tip method on a 2% water agar medium [28]. Genomic DNA was extracted using Safaie et al.’s [29] method. Identification of the fungal species was carried out by amplification of the ITS1-5.8S-ITS2 region using ITS1 and ITS4 primers [30] and getting it sequenced by Bio Magic Gene Company (http://www.bmgtechno.com). The obtained sequences were subjected to BLAST analysis (BLAST search) in GenBank (National Center for Biotechnology Information) using the “blastn” (Megablast) option. After sequence alignment, the gene tree was drawn using the maximum parsimony method in MEGA X software. Also, Boletopsis leucomelaena was considered as an outgroup [31].
Effect of wood substrate on enzyme production in solid-state fermentation
To investigate the effect of different substrates on laccase production, the isolate was cultivated on different wood substrates, including oak (Quercus castaneifolia), poplar (Populus deltoides), and pine (Pinus teada). Wood substrates were prepared in the form of chips (30 × 15 × 5 mm) and sawdust (80 mesh). The purified T. versicolor isolate was cultured on malt agar medium (pH = 5.5). Then, six agar plugs from 5-day-old fungal culture were added to a liquid medium containing 12.7 g/l of malt extract and 5 g/l of corn steep liquor and shaken for 3 days at 50 rpm. Eventually, 20 ml of this medium was added to 250-ml Erlenmeyer flasks containing 23 g of chips or sawdust previously sterilized at 121 °C for 20 min. All materials were kept at 60% humidity and 29 ± 2 °C under static conditions for 32 days [32]. Three replications were considered, and the sample without fungus was used as a control [32]. Laccase enzyme was extracted at 25 ± 2 °C during two enzyme extraction cycles (5 and 3 h) with 50 mM sodium acetate buffer (pH = 5.5) containing 0.20 g/l Tween 20. In the first step, wood chips were soaked with 50 ml extraction buffer for 5 h. Then, the second extraction cycle was done with 25 ml of extraction buffer for 3 h. Sawdust samples were extracted with 75 ml of buffer for 5 h, and the second run was done with 110 ml of buffer for 3 h. In each step, all collected supernatants were filtered and centrifuged at 5000 rpm for 15 min and applied to enzyme activity measurement. Laccase enzyme activity was measured at 30 °C using 5 mM 2,6-dimethoxyphenol (DMP) in 0.1 M sodium acetate buffer (pH = 3.6). Absorption at 469 nm wavelength (E469 = 27.5 mM−1cm−1) was measured by spectrophotometer (Unico UV/vis double beam). One unit of laccase activity was considered to be the enzyme’s amount needed to oxidize 1 μmol of DMP per minute at 30 °C [32].
Effect of wood substrates on enzyme production in liquid medium
To investigate laccase production in a broth medium containing wood substrate, the methods of Jaber et al. [25] and Stoilova et al. [33] were used. First, the liquid medium containing 10 g/l malt extract, 2 g/l peptone extract, 2 g/l yeast extract, 2 g/l KH2PO4, and 1 g/l MgSO4.7H2O (pH = 4.5) was prepared. Then, sawdust from pine, poplar, and oak trees was used as a substrate. Each culture medium contained 10% (w/v) of the substrate and was incubated in a shaker incubator at 28 ± 2 °C and 120 rpm for 6 days, after inoculation. Laccase enzyme activity was measured every 48 h for 6 days according to the method described in the previous section
The copper’s effect on enzyme production in liquid medium
The effect of copper as an inducer on laccase production was evaluated, by adding different amounts of copper (CuSO4) to the liquid culture medium described in the above section. Oak sawdust was also used as the substrate. Copper concentrations of 1.5, 2, 3, and 3.5 mM were added to the culture medium (pH = 4.5) [25]. After inoculation, the culture media were incubated in a shaker incubator at 28 ± 2 °C and 120 rpm. Laccase enzyme activity was measured every 48 h for 6 days as described before. All the experiments in this research were analyzed in the form of a completely random design using SPSS (version 26) software. Means were compared by Duncan’s method.
Results and discussion
Identification of the fungus
A comparison of the obtained sequence with the deposited ones at NCBI showed that it was more than 95% similar to the sequence Trametes versicolor MW554264, which is from China, and its plant host is not specified. As shown in Fig. 1, isolate B1 was grouped with T. versicolor species in a clade with 100 bootstraps and separated from other Trametes species, which confirms the species. The sequence of T. versicolor isolate B1 is available at NCBI with an accession number of MW811802.
Laccase enzyme assay
Solid-state assay
Investigating the effect of different wood substrates (oak, poplar, and pine) and forms of substrate (sawdust and wood chips) on laccase production in T. versicolor showed that the enzyme extraction cycle (F = 138.98, dft,e = 1, 24, p ≤ 0.0001), host (F = 81.33, dft,e = 2, 24, p ≤ 0.0001), form of substrate (F = 26.42, dft,e = 1, 24, p ≤ 0.0001), and time × host (F = 45.30, dft,e = 2, 24, p ≤ 0.0001) and time × form of substrate (F = 21.13, dft,e =1, 24, p ≤ 0.0001) interactions had significant differences. As seen in Fig. 2a, the highest amount of laccase production (15.621 u/l) was in the presence of oak (sawdust and chips in average) as substrate after 5 h of extraction. Also, laccase production in both enzyme extraction cycles (5 h and 3 h) was higher on sawdust than on wood chips regardless of the wood types used. The results showed that the host sawdust had the greatest effect on increasing laccase production, and a 5-h enzyme extraction cycle resulted in the highest amount of enzyme (10.661 u/l) (Fig. 2b). In a similar study, laccase production was tested in lignocellulosic environments (oak sawdust) to produce adequate amounts of laccase by T. versicolor. Higher enzyme activity was obtained by 0.5% oak sawdust (0.8 u/ml) in 48 h, which demonstrates the oak sawdust’s capability as a desirable substrate for laccase production [34]. As a result, form of the substrate (sawdust or chips) has a remarkable effect on the extracted enzyme activity. Sawdust has a smaller size particle than chips, and the fungal mycelia can attach more to this substrate. The effect of the substrate size on producing more laccase enzymes by providing more surface and thus more lignin available for the basidiomycete, T. versicolor, leads to more growth and production of laccase. In other similar studies, sawdust was the most effective substrate for the production of hydrolytic and oxidative enzymes. For example, the optimization of laccase production by Trichoderma isolates using sawdust substrate was investigated [25]. Among the different tested carbon sources (sawdust, rice straw, sugarcane bagasse, and empty fruit bunches of oil palm), the highest laccase activity (5.84 u/ml) was achieved in Trichoderma muroiana IS1037 in submerged fermentation using sawdust as substrate. Laccase production increased in the presence of 2 mM copper sulfate [25]. Therefore, the results of the present study were consistent with other studies in terms of increased production and activity of laccase enzyme under the influence of sawdust substrate compared to other substrates, as well as the positive effect of oak sawdust substrate in increasing laccase production.
Liquid medium assay
The results showed that the form of plant substrate (F = 43.75, dft,e = 3, 22, p ≤ 0.0001) and enzyme measurement time (F = 9.58, dft,e = 2, 22, p ≤ 0.0001) had a significant difference in laccase production in T. versicolor. As seen in Fig. 3, the addition of substrate had a positive effect on increasing laccase activity compared to the control, and in the meantime, the oak sawdust with an average of 4.8991 (u/l) showed the most positive effect on enzyme production. The results also showed that laccase activity was at the highest level in the first 48 h, and it decreased with time (Fig. 4). Improvement of laccase production by native Trichoderma muroiana IS1037 using rubber sawdust as a substrate showed that sawdust is the most suitable and highly efficient (5.8 u/l) substrate in the enzyme production [25]. Also, another study examined the effect of different forms of substrates on laccase production, and sawdust had the greatest effect on it [35]. Therefore, sawdust is an abundant and low-cost plant substrate as forestry wastes that can be utilized as a carbon source for laccase production. In addition, to solve environmental waste disposal problems, lignocellulosic waste can be considered an interesting alternative substrate.
The effect of copper on laccase enzyme activity
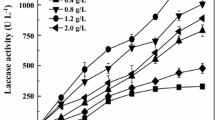
The results showed that the addition of copper (F = 459.135, dft,e = 6, 42, p ≤ 0.0001), time (F = 16.253, dft,e = 2, 42, p ≤ 0.0001), and time × treatment interaction (F = 191.468, dft,e = 12, 42, p ≤ 0.0001) had a significant difference. According to the analysis of variance, the average effect of different concentrations of copper sulfate on laccase activity showed that compared to the control (cultivation of T. versicolor without copper or oak sawdust), the addition of copper, like adding oak sawdust, in all concentrations significantly increased the laccase production and activity in liquid medium (Fig. 5), and the concentration of 3.5 mM copper had the highest effect on laccase induction by 92.04 u/l (Fig. 6). By adding concentrations of 3 and 3.5 mM of copper, it was observed that over time, laccase activity had an increasing trend compared to the control, but in the case of other concentrations, this trend was decreasing.
Average of laccase produced by Trametes versicolor in the presence of different concentrations of copper. B is oak sawdust. The numbers written next to Cu show the millimolar concentration of copper added to the medium. Means labeled with the identical letters show no significant difference (Duncan’s test, p ≤ 0.01)
The amount of fungal laccase production in the culture medium is affected by different environmental (temperature) and culture (pH, medium components, substrate size, inoculation) conditions [36]. This issue allows researchers to optimize laccase enzyme production by adjusting these involved factors. The results of many studies conducted in the last decade to optimize the production of fungal laccase enzyme, such as optimization of culture parameters [37], process optimization [38], optimization of culture medium [39], and overall yield of enzyme production [40], show that among these optimization processes, the most influential factors on laccase production are copper followed by substrate size and inoculum concentration.
Generally, the enzymes’ production in microorganisms is often enhanced by inducer compounds. Laccase production also can be improved by phenolic or aromatic compounds associated with lignin or lignin derivatives, including ferulic acid, guaiacol, and veratyl alcohol [41]. Several studies indicate that copper plays an important role in laccase production by white-rot fungi [23, 24]. For example, a similar enhancement in laccase production was obtained in white-rot basidiomycete Coriolopsis rigida by adding copper to the fungal culture medium. These researchers stated that copper can stimulate and increase the laccase isozymes’ production [42]. In the study by Jaber et al. [25], the effect of different concentrations of copper sulfate (0.5, 1, 1.5, 2, 2.5, and 3 mM) on laccase production in Trichoderma muroiana IS1037 was investigated. All added doses of copper sulfate enhanced laccase production, in comparison to the control (no induction). The highest amount of laccase activity (65 u/l) was reached after 4 days at a concentration of 2 mM copper sulfate, while the lowest amount (5.8 u/l) was obtained in the control (without CuSO4) [25]. Also, the study of laccase production in Pleurotus ostreatus revealed that the effect of copper on enzyme production is dependent on dose or concentration, as laccase activity increases by its concentration [43]. Birhanli and Yesilada [23] showed that the highest laccase activity of Funalia trogii–growing cells in cultures with and without copper was 4.61 ± 0.88 and 12.3 ± 0.79 u/ml, respectively. These values were 2.96 ± 0.34 and 2.34 ± 0.46 u/ml in T. versicolor. However, in repeated cultures of F. trogii and T. versicolor with and without copper, laccase activity increased to 40.29 ± 1.97 and 12.09 ± 0.72 u/ml, respectively. Copper was utilized as a chemical inducer for laccase production, which is required for the enzyme synthesis and activation of existing enzymes. In the present study, as in other studies, it was found that the addition of certain amounts of copper sulfate increases the production and activity of the laccase enzyme.
Conclusion
Based on the results obtained in this research, adding different wood substrates to the culture medium of T. versicolor had a high effect on laccase production and activity. In solid-state fermentation, among the tested forms of the substrate, the best result was obtained when the substrate was made of sawdust. Oak wood substrate increased the enzyme production and activity about 2 times compared to the control. Also, in a parallel experiment in the liquid medium, adding different concentrations of copper sulfate to the fungal culture medium revealed that copper sulfate with a concentration of 3.5 mM increased the laccase production and activity from 13.3 u/l in the control to 92.04 u/l in the treatment, which showed a significant sharp increase. Currently, researchers are trying to increase the yield efficiency and decrease the final price of laccase by improving the production process, so identifying effective substrates for further secretion of this industrial enzyme is of undeniable importance. Hence, this research could be a fundamental step to achieve this important strategy in several laccase industrial applications in Iran.
Data availability
Not applicable.
References
Wong AHH, Wilkes J (1988) Progressive changes in cell wall components of Pinus radiata during decay. Int Biodeterior 24(6):481–487. https://doi.org/10.1016/0265-3036(88)90036-X
Janjušević L, Karaman M, Šibul F et al (2017) The lignicolous fungus Trametes versicolor (L.) Lloyd (1920): a promising natural source of antiradical and AChE inhibitory agents. J Enzyme Inhib Med Chem 32(1):355–362
Schmidt O (2006) Wood and tree fungi. Springer
Taremian A, Faizipour M, Karimi AN, Parsapjoh D (2008) Heterogeneity of the physical properties of Rohtan Noel pine wood (Picea abies) containing compressed wood. Pajouhesh and Sazandegi 20(4):158
Galhaup C, Haltrich D (2001) Enhanced formation of laccase activity by the white-rot fungus Trametes pubescens in the presence of copper. Appl Microbiol Biotechnol 56(1):225–232
Vaithanomsat P, Sangnam A, Boonpratuang T et al (2013) Wood degradation and optimized laccase production by resupinate white-rot fungi in northern Thailand. BioResources 8(4):6342–6360
Chauhan R (2019) Nitrogen sources and trace elements influence Laccase and peroxidase enzymes activity of Grammothele fuligo. Vegetos 32(3):316–323
Sun K, Li S, Si Y, Huang Q (2021) Advances in laccase-triggered anabolism for biotechnology applications. Crit Rev Biotechnol 41(7):969–993
Maciel MJM, Ribeiro HCT (2010) Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: a review. Electron J Biotechnol 13(6):14–15
Riva S (2006) Laccases: blue enzymes for green chemistry. TRENDS Biotechnol 24(5):219–226
Curran LMCLK, Sale KL, Simmons BA (2021) Review of advances in the development of laccases for the valorization of lignin to enable the production of lignocellulosic biofuels and bioproducts. Biotechnol Adv 54:107809
Pourkhanali K, Khayati G, Mizani F, Raouf F (2021) Isolation, identification and optimization of enhanced production of laccase from Galactomyces geotrichum under solid-state fermentation. Prep Biochem Biotechnol 51(7):659–668
Baldrian P (2006) Fungal laccases–occurrence and properties. FEMS Microbiol Rev 30(2):215–242
Dong JL, Zhang YZ (2004) Purification and characterization of two laccase isoenzymes from a ligninolytic fungus Trametes gallica. Prep Biochem Biotechnol 34(2):179–194
Han SH, Lee JH (2005) An overview of peak-to-average power ratio reduction techniques for multicarrier transmission. IEEE Wirel Commun 12(2):56–65
D’Souza C, Taghian M, Lamb P (2006) An empirical study on the influence of environmental labels on consumers. Corp Commun Int J 11(2):162–173
Zouari-Mechichi H, Mechichi T, Dhouib A, Sayadi S, Martinez AT, Martinez MJ (2006) Laccase purification and characterization from Trametes trogii isolated in Tunisia: decolorization of textile dyes by the purified enzyme. Enzyme Microb Technol 39(1):141–148
Haibo Z, Yinglong Z, Feng H, Peiji G, Jiachuan C (2009) Purification and characterization of a thermostable laccase with unique oxidative characteristics from Trametes hirsuta. Biotechnol Lett 31(6):837–843
Mishra VK, Tripathi BD (2009) Accumulation of chromium and zinc from aqueous solutions using water hyacinth (Eichhornia crassipes). J Hazard Mater 164(2-3):1059–1063
Zapata-Castillo P, Villalonga-Santana M, Tamayo-Cortés J, Rivera-Muñoz G, Solís-Pereira S (2012) Purification and characterization of laccase from Trametes hirsuta Bm-2 and its contribution to dye and effluent decolorization. African J Biotechnol 11(15):3603–3611
de Araújo DG, Bezerra DS, de Queiroz DD et al (2019) Toxicological and pharmacologic effects of farnesol (C15H26O): a descriptive systematic review. Food Chem Toxicol 129:169–200
Lassouane F, Aït-Amar H, Amrani S, Rodriguez-Couto S (2019) A promising laccase immobilization approach for Bisphenol A removal from aqueous solutions. Bioresour Technol 271:360–367
Birhanli E, Yesilada O (2006) Increased production of laccase by pellets of Funalia trogii ATCC 200800 and Trametes versicolor ATCC 200801 in repeated-batch mode. Enzyme Microb Technol 39(6):1286–1293
Fonseca MI, Shimizu E, Zapata PD, Villalba LL (2009) WITHDRAWN: Laccase-producing ability and the inducing effect of copper on laccase production of white rot fungi native from Misiones (Argentina). Enzym Microb Technol. https://doi.org/10.1016/j.enzmictec.2009.07.003
Jaber SM, Shah UKM, Asa'ari AZM, Ariff AB (2017) Optimization of laccase production by locally isolated Trichoderma muroiana IS1037 using rubber wood dust as substrate. BioResources 12(2):3834–3849
Větrovský T, Baldrian P, Gabriel J (2013) Extracellular enzymes of the white-rot fungus Fomes fomentarius and purification of 1, 4-β-glucosidase. Appl Biochem Biotechnol 169(1):100–109
Backes E, Kato CG, de Oliveira Junior VA et al (2023) Overproduction of Laccase by Trametes versicolor and Pycnoporus sanguineus in farnesol-pineapple waste solid fermentation. Fermentation 9(2):188
Costa LG, Paes JB, Jesus Junior WC, Brocco VF, Pinho DB (2020) Isolation and identification of fungi with potential for biological stump removal of Eucalyptus spp. J Trop For Sci 32(2):154–160
Safaie N, Alizadeh AA, Saeidi A, Adam G, Rahimian H (2005) Molecular characterization and genetic diversity among iranian populations of Fusarium graminearum, the causal agent of wheat headblight. Iran J Plant Pathol 41(2):171–189
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc Guid Methods Appl 18(1):315–322
Justo A, Hibbett DS (2011) Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five–marker dataset. Taxon 60(6):1567–1583
Giorgio EM, Fonseca MI, Tejerina MR et al (2012) Chips and sawdust substrates application for lignocellulolytic enzymes production by solid state fermentation. Int Res J Biotechnol 3(7):120–127
Stoilova I, Krastanov A, Stanchev V (2010) Properties of crude laccase from Trametes versicolor produced by solid-substrate fermentation. Adv Biosci Biotechnol 1:208–215
Bertrand B, Martínez-Morales F, Tinoco-Valencia R, Rojas S, Acosta-Urdapilleta L, Trejo-Hernández MR (2015) Biochemical and molecular characterization of laccase isoforms produced by the white-rot fungus Trametes versicolor under submerged culture conditions. J Mol Catal B Enzym 122:339–347
Daâssi D, Zouari-Mechichi H, Frikha F, Rodríguez-Couto S, Nasri M, Mechichi T (2016) Sawdust waste as a low-cost support-substrate for laccases production and adsorbent for azo dyes decolorization. J Environ Heal Sci Eng 14(1):1–12
Niku-Paavola ML, Karhunen E, Kantelinen A, Viikari L, Lundell T, Hatakka A (1990) The effect of culture conditions on the production of lignin modifying enzymes by the white-rot fungus Phlebia radiata. J Biotechnol 13(2-3):211–221
Sabarathinam S, Jayaraman V, Balasubramanian M, Swaminathan K (2014) Optimization of culture parameters for hyper laccase production by Trichoderma asperellum by Taguchi design experiment using L-18 orthogonal array. Malaya j Biosci 1(4):214–225
Abd El Aty AA, Wehaidy HR, Mostafa FA (2014) Optimization of inulinase production from low cost substrates using Plackett–Burman and Taguchi methods. Carbohydr Polym 102:261–268
Petlamul W, Prasertsan P (2014) Medium optimization for production of Beauveriabassiana BNBCRC spores from biohydrogen effluent of palm oil mill using Taguchi design. Int J Biosci Biochem Bioinforma 4(2):106
Azin M, Moravej R, Zareh D (2007) Production of xylanase by Trichoderma longibrachiatum on a mixture of wheat bran and wheat straw: optimization of culture condition by Taguchi method. Enzyme Microb Technol 40(4):801–805
Revankar MS, Lele SS (2006) Enhanced production of laccase using a new isolate of white rot fungus WR-1. Process Biochem 41(3):581–588
Saparrat MCN, Guillén F, Arambarri AM, Martínez AT, Martínez MJ (2002) Induction, isolation, and characterization of two laccases from the white rot basidiomycete Coriolopsis rigida. Appl Environ Microbiol 68(4):1534–1540
Zhu C, Bao G, Huang S (2016) Optimization of laccase production in the white-rot fungus Pleurotus ostreatus (ACCC 52857) induced through yeast extract and copper. Biotechnol Biotechnol Equip 30(2):270–276
Author information
Authors and Affiliations
Contributions
Mohammadreza Ensani: investigation, visualization, and writing—original draft. Shideh Mojerlou: conceptualization, supervision, methodology, formal analysis, and writing—review and editing. Seyedeh Masoumeh Zamani: conceptualization, resources, data curation, and writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Julio Santos
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ensani, M., Mojerlou, S. & Zamani, S.M. Enhanced laccase activity in Trametes versicolor (L.: Fr.) Pilát by host substrate and copper. Braz J Microbiol 54, 1565–1572 (2023). https://doi.org/10.1007/s42770-023-01096-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01096-x