Abstract
The emergence and spread of multidrug-resistant (MDR) Klebsiella pneumoniae strains have increased worldwide, posing a significant health threat by limiting the therapeutic options. This study aimed to investigate the antimicrobial potential of cinnamaldehyde against MDR-K. pneumoniae strains in vitro and in vivo assays. The presence of resistant genes in MDR- K. pneumoniae strains were evaluated by Polymerase Chain Reaction (PCR) and DNA sequencing. Carbapenem-resistant K. pneumoniae strains show the blaKPC-2 gene, while polymyxin-resistant K. pneumoniae presented blaKPC-2 and alterations in the mgrB gene. Cinnamaldehyde exhibited an inhibitory effect against all MDR- K. pneumoniae evaluated. An infected mice model was used to determine the in vivo effects against two K. pneumoniae strains, one carbapenem-resistant and another polymyxin-resistant. After 24 h of cinnamaldehyde treatment, the bacterial load in blood and peritoneal fluids decreased. Cinnamaldehyde showed potential effectiveness as an antibacterial agent by inhibiting the growth of MDR-K. pneumoniae strains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobial resistance represents a major threat to global health [1] since it is directly associated with delays in initiating the appropriate antimicrobial therapy, long-term hospitalization with increased medical expenditure costs, and high morbidity and mortality rates [2]. The resistance genes generally reside in the transposons transported by plasmids, which increases their dissemination possibility causing frequent hospital outbreaks. Klebsiella pneumoniae is a leading cause of nosocomial infections, and the emergence and spread of multidrug-resistant (MDR) strains have become a major public health concern worldwide [3] Infections caused by MDR K. pneumoniae have limited therapeutic options, resulting in increased use of polymyxin B as a therapeutic option of last resort, despite its nephrotoxicity and neurotoxicity potential [4].
The antimicrobial resistance of K. pneumoniae is primarily driven by the acquisition of mobile genetic elements carrying genes that encode antibiotic resistance, particularly to beta-lactams and carbapenems. Horizontal gene transfer, particularly via plasmids, has played a significant role in the spread of these resistance determinants [3]. However, for polymyxin resistance, the mechanism is mainly attributed to changes in the structure of lipopolysaccharide (LPS), mutations or insertion sequences in the mgrB gene, missense mutations, and the acquisition of mobile genetic elements that carry resistance genes, such as mcr [5].The emergence of MDR bacteria also resistant to polymyxin B reduces available therapeutic options, demonstrating the urgent need for developing alternative strategies to prevent its rapid spread [6].
Efforts to control infections caused by multidrug-resistant (MDR) strains of bacteria are hindered by the limited number of antibiotics currently available. According to the World Health Organization (WHO), only two out of the 50 antibiotics under study are currently being investigated specifically for use against MDR strains [7]. Therefore, more cumulative efforts are required to develop new and effective drug therapies. The bioactive potentials of various essential oils have been increasingly explored as a potential therapeutic strategy for fatal diseases caused by MDR bacteria [8]. Interestingly, cinnamon spices obtained from trees of the genus Cinnamomum contain a high amount of cinnamaldehyde (85.3–90.5%) [9] used as a natural flavorant and fragrance agent in kitchen and industry which has been identified as an active component with antimicrobial properties [10]. However, to the best of our knowledge, no study has described the activity of cinnamaldehyde against MDR K. pneumoniae to date. In this study, we investigated the antimicrobial performance of cinnamaldehyde in vitro and in vivo against K. pneumoniae strains resistant to carbapenem and polymyxin B along with the evaluation of the acute toxicity displayed by cinnamaldehyde.
Materials and methods
Reagents
Several antibiotics like amikacin (Lot 01774), aztreonam (Lot MKBW 2997 V), ceftazidime (Lot 1,004,245), ertapenem (Lot T024320) imipenem (Lot 1238–1), meropenem (Lot 037M4713), polymyxin B sulfate (Lot 027M400), tigecycline hydrate (Lot 0000023817), and cinnamaldehyde ≥ 95% (Lot MKBV4784V), were purchased from Sigma-Aldrich, St Louis, MO, USA.
Bacterial identification and antimicrobial susceptibility testing
The strains included in this study were previously isolated from infection sources of patients admitted to an intensive care unit of a tertiary hospital in Dourados, Mato Grosso do Sul, Brazil, and the resistance determinants were characterized as previously described [11, 12].
Bacterial species identification was performed using the BD Phoenix 100® automated system (BD Diagnostic Systems) and confirmed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) using the Microflex Spectrometer LT (Bruker Daltonics, Massachusetts, USA) [13]. The minimal inhibitory concentrations (MIC) were determined by broth microdilutions according to the medical laboratory standards of the Clinical and Laboratory Standards Institute (CLSI) [14], whereas the preliminary screening for carbapenemases was performed by ertapenem hydrolysis assay using mass spectrometry [11].
Molecular characterization of resistance genes
The presence of resistance genes (blaCTX-M-1-like, blaCTX-M-2-like, blaCTX-M-8-like, blaCTX-M-14-like, blaGES-like, blaGIM-like, blaIMP-10, blaIMP-like, blaKPC-2, blaNDM-like, blaOXA-23, blaOXA-48-like, blaSHV-like, blaSIM-like, blaSME-like, blaSPM-like, blaTEM-like, blaVIM-like, and mcr-1) was evaluated by polymerase chain reaction (PCR) and DNA sequencing. The nucleotide sequences of pmrA, pmrB, phoP, phoQ, crrB, and mgrB genes were amplified and sequenced to investigate the innate chromosomal polymyxin-resistance mechanism [12].
Antibacterial activity of cinnamaldehyde
Antimicrobial susceptibility tests were performed by a microdilution method using 96-well polystyrene microtiter plates in Mueller Hinton Broth (MHB) [14]. The cinnamaldehyde concentrations ranged from 72 to 0.035 mg/mL (supplemented with a final concentration of 0.5% Tween 80 v/v). The inoculum was adjusted to correspond to 0.5 McFarland standard (1 × 108 CFU/mL), using spectrophotometry, with an optical density ranging between 0.08 and 0.10. Each well was inoculated with a final bacterial concentration of 5 × 105 colony-forming units (CFU)/mL. The plates were incubated at 37 °C for 18–24 h. Two positive (Escherichia coli ATCC® 25,922 and K. pneumoniae ATCC® 700,603) and negative (MHB and Tween) controls were used. The tests were performed in triplicate.
Time-kill assay
The time-kill method was performed using the broth macrodilution technique [15] with approximately 5 × 105 CFU/mL in a final volume of 3.2 mL and verified with a spectrophotometer by Vitek® 2 (bioMerieux). The bacterial suspension was cultured at 37 °C, and the samples were obtained after 0, 2, 4, 6, and 12 h of incubation. At every designated sample time, 1 µL was withdrawn from each tube using a sterile loop and seeded in MHB agar plates. The plates were incubated for 24 h at 37 °C followed by the determination of the colony counts. MHB and saline were used as a negative and as a sterility control, respectively. Polymyxin B (Poly) and amikacin (Amik) were used as positive controls. Bacteria count log10 values were calculated as the mean of triplicate experiments.
Animals
Healthy female Swiss mice 8–12 weeks old and weighing 20–30 g were used for all in vivo experiments. The animals were kept in polypropylene cages with controlled temperature (22 ± 3 °C), humidity (40–60%), and light (12 h light–dark cycles), receiving standard commercial feed and water ad libitum. In vivo tests were performed according to the National Council for the Control of Animal Experimentation (CONCEA) [16]. This study was conducted with the approval of the Research Ethics Committee from Universidade Federal da Grande Dourados (UFGD) (number 877.292/2014 and 4.014.325/2020) by the Ethics Committee on Animal Use of UFGD (number 25/18) and the Centro Universitário da Grande Dourados (Unigran) (number 080/18).
The median lethal dose test
Determination of the median lethal dose (LD50) was performed as described previously [17], with few modifications. In brief, two independent experiments were carried out in the animals, one using carbapenem-resistant K. pneumoniae strain and another polymyxin-resistant K. pneumoniae strain. Seven groups of six animals were injected intraperitoneally (i.p.) using a needle in the right lower abdomen with bacterial suspensions (0.1 mL) of 2,8 × 108, 3.8 × 108, 4.8 × 108, 5.8 × 108, 6.8 × 108, 7.8 × 108, and 8.8 × 108 CFU/mL. After 24 h, the number of survivors was assessed.
Antimicrobial therapy
The in vivo antibacterial activity of cinnamaldehyde was performed as described previously by Toledo et al. [17] with modifications. For this, two independent experiments were performed, one using carbapenem-resistant K. pneumoniae strain and the other with polymyxin-resistant K. pneumoniae strain. All animals were injected with a 0.1-mL i.p. aliquot of LD50 of each respective bacterial strain. Five groups with eight animals each experiment was used that were identified as follows: Naive (without infection to assess the baseline indexes), Control (infected without treatment), cinnamaldehyde at a dosage of 30 mg/kg (CEO 30), and cinnamaldehyde at a dosage of 100 mg/kg (Ceo 100). Polymyxin B at 2 mg/kg (Poly B) and amikacin at 7.5 mg/kg (Amik) were used as positive controls administered i.p. every 12 h, for carbapenem-and polymyxin-resistant K. pneumoniae strains, respectively. Treatments were performed 1 h after the bacterial inoculations. In both the tests, the cinnamaldehyde was administered by gavage after every 8 h. All surviving animals were anesthetized with a combination of xylazine and ketamine (10 and 60 mg/kg, i.p., respectively). After the euthanization of animals by exsanguination, organs were collected for analysis. Blood and pleural fluid samples were collected to assess the bacterial culture, followed by utilization of the blood for hematological analysis using an automated hematological analyzer (Sysmex Poch-100iV Diff). Peritoneal fluid was obtained by washing the incision, performed using the aseptic technique, with a Milli-Q lab water system.
Acute toxicity
The acute toxicity of cinnamaldehyde was determined according to the Bruce procedure adopted by the Organization for Economic Co-operation and Development (OECD) [18]. The test was performed by administering a single dose of cinnamaldehyde (2000 mg/kg) by intra-gastric gavage in only one animal based on its body weight. After the survival of the animal, within 48 h, four additional animals were dosed sequentially to obtain a total of five animals for the test. The animals were observed individually for the first 30 min after being dosed, followed by periodical observation for the first 24 h with particular attention provided during the first 4 h. After that, they were closely observed for 14 days for gross morphological, physiological, behavioral changes, and mortality. After the test was completed, the survived animals were weighed and then euthanized. The organs (heart, lung, spleen, liver, and kidney) were removed, weighed, and examined macroscopically.
Statistical analysis
The data were expressed as a percentage and mean ± standard error (SE). The differences among the groups were assessed using one-way and two-way analysis of variance (ANOVA). The significance test was performed using the GraphPad Prism software, considering the value of p < 0.05 (version 3.02; Graph-Pad Software Inc., San Diego, CA, USA).
Results
Susceptibility and molecular assays of MDR-K. pneumoniae strains
Five MDR-K. pneumoniae strains were evaluated. The results of antimicrobial susceptibility tests showed that carbapenem-resistant K. pneumoniae strains were resistant to amikacin (MIC, 64 mg/L), aztreonam (MIC > 32 mg/L), ceftazidime (MIC > 256 mg/L), imipenem (MIC > 16 mg/L), meropenem (MIC > 16 mg/L), and ertapenem (MIC, > 32 mg/L), though they were sensitive to polymyxin B and tigecycline (MIC < 0.5 mg/L) (Supplementary Table 1). While carbapenemase production was confirmed by MALDI-TOF MS, PCR and sequencing results displayed that the blaKPC-2 coding gene was present in carbapenem-resistant strains of K. pneumoniae that were resistant to polymyxin B and other antibiotics and sensitive to amikacin (MIC < 2 mg/L) and tigecycline (MIC < 0.5 mg/L) (Table 1). The presence of the blaKPC-2 gene and mutations in the mgrB gene were identified in polymyxin-resistant strains. In the carbapenem-resistant strains of K. pneumoniae studied, blaOXA-48, blaNDM, and blaCTX-M-8 were each detected in separate strains. The genes like blaCTX-M-1-like, blaCTX-M-2-like, blaCTX-M-14-like, blaGES-like, blaGIM-like, blaIMP-10, blaIMP-like, blaNDM-like, blaOXA-23, blaSHV-like, blaSIM-like, blaSME-like, blaSPM-like, blaTEM-like, blaVIM-like, and mcr-1, on the other hand, were not detected.
Antibacterial activity of cinnamaldehyde
Cinnamaldehyde exhibited significant inhibitory effects in the evaluated five MDR-K. pneumoniae strains, with MIC values ranging from 0.035 to 0.28 mg/mL (Table 1). In the time-kill assay, the minimum bactericidal concentration (MBC) was performed using K. pneumoniae strains, as described in Table 1. A decrease in the viable cell counts over a while was observed, while the total inhibition effects were displayed after 8 h of cinnamaldehyde treatment. Polymyxin B (1 µg/mL) and amikacin (64 µg/mL) were considered as positive controls, with successful inhibitory effects after 4 h. No bacterial growth was observed in the control MHB (Fig. 1).
Time-kill curves of MDR-K. pneumoniae strains evaluated in this study. Carbapenem-resistant strains (A) polymyxin-resistant strains; (B) CR, carbapenem-resistant; PR, polymyxin-resistant; MHB, Mueller Hinton Broth. Differences among the groups were analyzed by a two-way ANOVA test, followed by the comparison of ***p < 0.001 and **p < 0.01 with the saline group. SD ± media
In vivo antibacterial assay of Cinnamaldehyde
LD50 survival curves were observed for 24 h in mice. LD50 value was 3.8 × 108 CFU/mL in both the evaluated strains. The in vivo antimicrobial activity of cinnamaldehyde was evaluated for 24 h using mice infections with carbapenem-resistant and polymyxin-resistant K. pneumoniae strains. Half of the control group (untreated) died within 24 h after getting infected (50% mortality). In the experiment using LD100, both K. pneumoniae strains presented 100% mortality in the Inf group (without treatment), while those treated with the reference drugs (poly B and Amik) had 100% survival. Treatment with 100 mg/kg cinnamaldehyde showed a 50% and 37% survival rate in carbapenem and polymyxin-resistant K. pneumoniae strains, respectively (Fig. 2A, B).
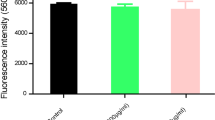
Treatment with cinnamaldehyde (100 mg/kg) showed a significant reduction in the log10 values of bacterial CFU/mL in the bloodstream (Figs. 3A, 4A) and pleural fluid (Figs. 3B, 4B) in mice infected with carbapenem-resistant and polymyxin-resistant K. pneumoniae strains. Mice infected with carbapenem-resistant K. pneumoniae strains and treated with Ceo (30 and 100 mg/kg, p < 0.001) showed a decrease in total leukocyte and neutrophil count (Fig. 3C, D). Regarding the response against polymyxin-resistant K. pneumoniae strain, all groups treated with Ceo (30 and 100 mg/kg), and amikacin had a significant decrease in total leukocyte count (Fig. 4C). A statistical difference in neutrophils was not observed in the Ceo-treated groups (Fig. 4D).
Effects of cinnamaldehyde in the number of cells of CFU in mice infected with MDR K. pneumoniae after 24 h of treatment. Blood (A) and pleural fluid (B) in animals infected with carbapenem-resistant K. pneumoniae. Total leukocytes (WBC) (C), neutrophils (D), and lymphocytes (E) in animals infected with carbapenem-resistant K. pneumoniae. ****p < 0.0001, ***p < 0.001, **p < 0.01, and *p < 0.05 as compared to the control group (Inf). Differences among the groups were analyzed by one-way ANOVA followed by the Newman-Keuls test. Among the groups were analyzed by one-way ANOVA followed by the Newman-Keuls test
Effects of cinnamaldehyde in the number of cells of CFU in mice infected with polymyxin-resistant K. pneumoniae after 24 h of treatment. Blood (A) and pleural fluid (B) in animals infected with carbapenem-resistant K. pneumoniae. Total leukocytes (WBC) (C), neutrophils (D), and lymphocytes (E) in animals infected with polymyxin-resistant K. pneumoniae. ****p < 0.0001, ***p < 0.001, **p < 0.01, and *p < 0.05 as compared to the control group (Inf). Differences among the groups were analyzed by one-way ANOVA followed by the Newman-Keuls test. Among the groups were analyzed by one-way ANOVA followed by the Newman-Keuls test
Acute toxicity
The animals did not show any signs of toxicity or death immediately after an experiment with cinnamaldehyde (2000 mg/kg) or during the observation period. No changes were observed in their behavior or water and food consumption. The macroscopic analysis showed no changes and differences in the organ weight.
Discussion
Due to the overuse of antibiotics, the emergence of resistant bacteria is now posing a serious public health threat worldwide. Therefore, the search for newer drugs to combat this grave situation is greatly needed to meet future demands [19]. This study described the inhibitory effect of cinnamaldehyde against K. pneumoniae strains resistant to carbapenem and polymyxin. Antibacterial assays showed the potential of action of cinnamaldehyde against carbapenem and polymyxin-resistant K. pneumoniae strains (MIC = 0.035–0.28 mg/mL). Similar results have been described for Cinnamomum cassia L. essential oil alone and in combination with antibiotics against carbapenemase-producing K. pneumoniae and Serratia marcescens [20].
Antibacterial action of cinnamaldehyde and its in vitro antibacterial effects against polymyxin-resistant Klebsiella aerogenes [21], MDR-Pseudomonas aeruginosa [10], and fluoroquinolone-resistant extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-PE) were described [22]. Its antimicrobial activity involves the disruption of the bacterial cell membrane’s integrity by increasing the cell membrane permeability, thereby causing seepage of intracellular contents [23]. In K. aerogenes, the cinnamaldehyde carbonyl group binds to proteins preventing the decarboxylase activity [24, 25] and getting access to the periplasmic space as well as the inner cellular contents of Gram-negative bacteria, through the outer membrane of porous proteins [26].
Although antimicrobial effects of cinnamaldehyde against MDR-K. pneumoniae has been described in previous studies [10, 21, 27, 28], to our knowledge, in vivo antimicrobial activity of cinnamaldehyde has not been reported to date. Our study described the models of peritoneal inoculums of carbapenem and polymyxin-resistant K. pneumoniae strains to achieve a median-lethal dose of sepsis. Our results revealed that cinnamaldehyde treatment significantly increased the survival rate of mice infected with carbapenem and polymyxin-resistant K. pneumoniae strains as compared to the control group (untreated). Additionally, a significant decrease in the number of CFU in blood and pleural fluid with cinnamaldehyde (100 mg/kg) was observed. These results were similar to the commercial drugs used as a positive control.
It was evident that only tigecycline, polymyxin, and some aminoglycosides had favorable antibacterial activities against MDR bacterial strains. However, these drugs have varied side effects that limit their usage. According to the Food and Drug Administration (FDA), tigecycline is not indicated for treating diabetic foot infection, hospital pneumonia, or mechanical ventilation [29]. Moreover, the global spread of carbapenem-resistant K. pneumoniae has increased the use of polymyxin B with an inevitable risk of emerging drug resistance [30]. As polymyxins have several neurotoxic and nephrotoxic effects, and their pharmacokinetics are still poorly understood [4] such therapies resulted in higher mortality of patients when compared to other described antibiotics [31]. Unfortunately, aminoglycosides are also known to cause ototoxicity [32]. Thus, alternative therapies to replace these antibiotics for combating the increased antibiotic resistance are the need of the hour for which cinnamaldehyde might be considered as a promising candidate.
During the spread of infection, the leukocytes initiate the immune cascade by enhancing the expression of immunosuppressive pathways, such as interleukin 10 (IL-10), and early activation of innate inflammatory response systems [33]. In our study, untreated animals had a higher total leukocyte count as compared to the treated and non-infected groups (NAIVE); thus, suggesting a functional infectious or inflammatory process in these animals. Our results depicted that the groups treated with cinnamaldehyde showed a decrease in total leukocyte count, suggesting that it was efficient in controlling the initial surge of infection, being the first defense against the pathogens [34]. Another study reported a similar result in mice infected by KPC-producing K. pneumoniae and treated with carvacrol [35].
The concentration of the neutrophils in infected mice with P. aeruginosa was higher than in the control animals without infection [36]. In our study, differences in the neutrophil count between the two evaluated K. pneumoniae strains were observed. The group treated with cinnamaldehyde (100 mg/kg) displayed a statistically significant decrease in neutrophils, similar to the group treated with the reference drug. However, groups treated with cinnamaldehyde and infected by polymyxin-resistant strain did not show any statistical differences. Despite the fact that K. pneumoniae strains may vary in genome size, capsule composition, and expression of resistance genes, it is still unclear whether immunological defenses against different K. pneumoniae strains may differ according to their genetic characteristics [30]. Although the strains evaluated in this study had different resistance mechanisms, it is difficult to predict that this reason might be responsible for the observed difference in neutrophils. Thus, more experimental studies are required to have a lucid understanding of the contribution of cinnamaldehyde in initiating the immune response during the infection of MDR K. pneumoniae. Hence, it might provide additional helpful information regarding the severity of the disease and its acuity or effective response to anti-inflammatory treatment.
In our study, animals treated with cinnamaldehyde at a dose of 2000 mg/kg did not exhibit any signs of acute toxicity. This was further supported by the absence of clinical findings in toxicological screening and the earlier reported mortality [22]. The acute toxicity and oral LD50 values for cinnamaldehyde in rats and guinea pigs have been reported as 2220 mg/kg and 1160 mg/kg, respectively [37].
There was no evidence of increased unscheduled DNA synthesis when rats were administered cinnamaldehyde 500 mg/kg of the body weight by intraperitoneal injection [38]. Cinnamaldehyde is completely absorbed in mice and humans, rapidly converted to cinnamic acid, and eliminated from the body [39]. Essential oils and their primary compounds, which are considered flavoring substances by the European Regulation 1999/217/CE and Generally Recognized As Safe (GRAS) substances by the Food and Drug Administration (FDA), have been extensively studied for their antimicrobial properties [40]. The lower dose of cinnamaldehyde (100 mg/kg), demonstrated in our study, which was able to inhibit the growth of the evaluated MDR strains, is lower than the levels described by previous studies as safe concentrations of cinnamaldehyde for animals.
However, it is important to recognize the limitations of our study, including the fact that we evaluated only a limited number of K. pneumoniae strains. Future studies should expand the range of microorganisms being tested, as well as its pharmacokinetics and pharmacodynamics. Furthermore, exploring new techniques such as cytometry assay and scanning electron microscopy may provide more information about cinnamaldehyde’s mechanism of action and its potential as a clinical treatment. On the other hand, the in vitro and in vivo antimicrobial activity of cinnamaldehyde were promising, without toxicity. Thus, its therapeutic potential may be more explored.
Conclusion
Our results provided evidence on the potential of cinnamaldehyde as a promising antimicrobial agent against multidrug-resistant K. pneumoniae, a clinically important Gram-negative bacteria. This study provides a starting point for further research on cinnamaldehyde as an antimicrobial agent. Thus, this findings contribute to ongoing efforts to identify new treatments for bacterial infections resistant to conventional antibiotics.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Yap PSX, Lim SHE, Hu CP, Yiap BC (2013) Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine 20(8–9):710–713. https://doi.org/10.1016/j.phymed.2013.02.013
Pouch SM, Patel G (2019) The AST infectious diseases community of practice. Multidrug‐resistant Gram‐negative bacterial infections in solid organ transplant recipients—guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 33(9). https://doi.org/10.1111/ctr.13594
Navon-Venezia S, Kondratyeva K, Carattoli A (2017) Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev 41(3):252–275. https://doi.org/10.1093/femsre/fux013
Chung JH, Bhat A, Kim CJ, Yong D, Ryu CM (2016) Combination therapy with polymyxin B and netropsin against clinical isolates of multidrug-resistant Acinetobacter baumannii. Sci Rep 6(1):28168. https://doi.org/10.1038/srep28168
Conceição-Neto OC, da Costa BS, da Pontes LS et al (2022) Polymyxin resistance in clinical isolates of K. pneumoniae in Brazil: update on molecular mechanisms, clonal dissemination and relationship with KPC-producing strains. Front Cell Infect Microbiol 12:898125. https://doi.org/10.3389/fcimb.2022.898125
Da Silva MO, Aquino S (2018) Resistência aos antimicrobianos: uma revisão dos desafios na busca por novas alternativas de tratamento. Rev Epidemiol Control Infect. 8(4):472–482. https://doi.org/10.17058/reci.v8i4.11580
WHO WHO. Lack of new antibiotics threatens global efforts to contain drug-resistant, infections. Published online 2020. Accessed June 4, 2021. https://www.who.int/news/item/17-01-2020-lack-of-new-antibiotics-threatens-global-efforts-to-contain-drug-resistant-infections#:~:text=Lack%20of%20new%20antibiotics%20threatens%20global%20efforts%20to%20contain%20drug%2Dresistant%20infections,-17%20January%202020&text=Declining%20private%20investment%20and%20lack,World%20Health%20Organization%20(WHO).
Gadisa E, Usman H (2021) Evaluation of antibacterial activity of essential oils and their combination against multidrug-resistant bacteria isolated from skin ulcer. Comi G, ed. Int J Microbiol 2021:1–8. https://doi.org/10.1155/2021/6680668
Doyle AA, Stephens JC (2019) A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 139:104405. https://doi.org/10.1016/j.fitote.2019.104405
Utchariyakiat I, Surassmo S, Jaturanpinyo M, Khuntayaporn P, Chomnawang MT (2016) Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement Altern Med 16(1):158. https://doi.org/10.1186/s12906-016-1134-9
Da Silva KE, Maciel WG, Sacchi FPC et al (2016) Risk factors for KPC-producing Klebsiella pneumoniae: watch out for surgery. J Med Microbiol 65(6):547–553. https://doi.org/10.1099/jmm.0.000254
Da Silva KE, Thi Nguyen TN, Boinett CJ, Baker S, Simionatto S (2020) Molecular and epidemiological surveillance of polymyxin-resistant Klebsiella pneumoniae strains isolated from Brazil with multiple mgrB gene mutations. Int J Med Microbiol 310(7):151448. https://doi.org/10.1016/j.ijmm.2020.151448
Fehlberg LCC, da Nogueira KS, Cayô da Silva R et al (2014) Detection of PER-2-producing Enterobacter cloacae in a Brazilian liver transplantation unit. Antimicrob Agents Chemother 58(3):1831–1832. https://doi.org/10.1128/AAC.01260-13
CLSI C& LSI. Performance Standards for antimicrobial susceptibility testing (M100). Published online 2020. Accessed February 2, 2022. https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf
Morinaka A, Tsutsumi Y, Yamada K et al (2016) In vitro and in vivo activities of OP0595, a new diazabicyclooctane, against CTX-M-15-positive Escherichia coli and KPC-positive Klebsiella pneumoniae. Antimicrob Agents Chemother 60(5):3001–3006. https://doi.org/10.1128/AAC.02704-15
CONCEA. National Council for Animal Experimentation Control. Brazilian guideline for the care and use of animals in teaching or scientific research activities. http://bit.ly/2VEvkG9. Published 2016. Accessed April 20, 2021.
Toledo PVM, Tuon FF, Bail L, Manente F, Arruda P, Aranha-Junior AA (2014) Experimental model for treatment of extended spectrum betalactamase producing-Klebsiella pneumoniae. ABCD, arq bras cir dig 27(3):168–171. https://doi.org/10.1590/S0102-67202014000300002
OECD O for EC and D. Guidance document on acute oral toxicity. Environmental Health and Safety Monograph Series on Testing and Assessment. https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd-gd24.pdf. Published 2001. Accessed June 20, 2021.
Parimala M, Shoba FG (2014) In vitro antimicrobial activity and HPTLC analysis of hydroalcoholic seed extract of Nymphaea nouchali Burm. f. BMC Complement Altern Med 14(1):361. https://doi.org/10.1186/1472-6882-14-361
Vasconcelos NG, de Queiroz JHFS, da Silva KE, de Vasconcelos PC, P, Croda J, Simionatto S, (2020) Synergistic effects of Cinnamomum cassia L essential oil in combination with polymyxin B against carbapenemase-producing Klebsiella pneumoniae and Serratia marcescens. PLoS One. 15(7):e0236505. https://doi.org/10.1371/journal.pone.0236505
Vasconcelos NG, Silva KE, Croda J, Simionatto S (2020) Antibacterial activity of Cinnamomum cassia L. essential oil in a carbapenem- and polymyxin-resistant Klebsiella aerogenes strain. Rev Soc Bras Med Trop 53:e20200032. https://doi.org/10.1590/0037-8682-0032-2020
Dhara L, Tripathi A (2020) Sub-acute toxicological and behavioural effects of two candidate therapeutics, cinnamaldehyde and eugenol, for treatment of ESBL producing-quinolone resistant pathogenic Enterobacteriaceae. Clin Exp Pharmacol Physiol 47(6):977–988. https://doi.org/10.1111/1440-1681.13276
Gill AO, Holley RA (2006) Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int J Food Microbiol 111(2):170–174. https://doi.org/10.1016/j.ijfoodmicro.2006.04.046
Wendakoon CN, Sakaguchi M (1995) Inhibition of amino acid decarboxylase activity of Enterobacter aerogenes by active components in spices. J Food Prot 58(3):280–283. https://doi.org/10.4315/0362-028X-58.3.280
Helander IM, Alakomi HL, Latva-Kala K et al (1998) Characterization of the action of selected essential oil components on Gram-negative bacteria. J Agric Food Chem 46(9):3590–3595. https://doi.org/10.1021/jf980154m
Nikaido H (1994) Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264(5157):382–388. https://doi.org/10.1126/science.8153625
Oliva A, Costantini S, De Angelis M et al (2018) High potency of Melaleuca alternifolia essential oil against multi-drug resistant Gram-negative bacteria and methicillin-resistant Staphylococcus aureus. Molecules 23(10):2584. https://doi.org/10.3390/molecules23102584
Muntean D, Licker M, Alexa E et al (2019) Evaluation of essential oil obtained from Mentha×piperita L. against multidrug-resistant strains. IDR. 12:2905–2914. https://doi.org/10.2147/IDR.S218141
FDA F and DA. FDA Drug Safety Communication: increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. Published online 2017. Accessed June 20, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-increased-risk-death-tygacil-tigecycline-compared-other-antibiotics
Bengoechea JA, Sa PJ (2019) Klebsiella pneumoniae infection biology: living to counteract host defences. FEMS Microbiol Rev 43(2):123–144. https://doi.org/10.1093/femsre/fuy043
Wang J, Pan Y, Shen J, Xu Y (2017) The efficacy and safety of tigecycline for the treatment of bloodstream infections: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob 16(1):24. https://doi.org/10.1186/s12941-017-0199-8
Kros CJ, Steyger PS (2019) Aminoglycoside- and cisplatin-induced ototoxicity: mechanisms and otoprotective strategies. Cold Spring Harb Perspect Med 9(11):a033548. https://doi.org/10.1101/cshperspect.a033548
Gentile LF, Nacionales DC, Lopez MC et al (2014) Host responses to sepsis vary in different low-lethality murine models Caldwell CC, ed. PLoS ONE. 9(5):e94404. https://doi.org/10.1371/journal.pone.0094404
da Lima MS, Quintans-Júnior LJ, de Santana WA, Martins Kaneto C, Pereira Soares MB, Villarreal CF (2013) Anti-inflammatory effects of carvacrol: evidence for a key role of interleukin-10. Eur J Pharmacol 699(1–3):112–117. https://doi.org/10.1016/j.ejphar.2012.11.040
de Souza GH de A, dos Santos Radai JA, Mattos Vaz MS, et al. 2021 In vitro and in vivo antibacterial activity assays of carvacrol: a candidate for development of innovative treatments against KPC-producing Klebsiella pneumoniae Galdiero M, ed. PLoS ONE. 16(2):e0246003. https://doi.org/10.1371/journal.pone.0246003
Murdoch EL, Karavitis J, Deburghgraeve C, Ramirez L, Kovacs EJ (2011) Prolonged chemokine expression and excessive neutrophil infiltration in the lungs of burn-injured mice exposed to ethanol and pulmonary infection. Shock 35(4):403–410. https://doi.org/10.1097/SHK.0b013e31820217c9
Bickers D, Calow P, Greim H et al (2005) A toxicologic and dermatologic assessment of cinnamyl alcohol, cinnamaldehyde and cinnamic acid when used as fragrance ingredients. Food Chem Toxicol 43(6):799–836. https://doi.org/10.1016/j.fct.2004.09.013
Hayashi M, Kishi M, Sofuni T, Ishidate M (1988) Micronucleus tests in mice on 39 food additives and eight miscellaneous chemicals. Food Chem Toxicol 26(6):487–500. https://doi.org/10.1016/0278-6915(88)90001-4
Peters MMCG, Caldwell J (1994) 1994 Studies on trans-cinnamaldehyde I The influence of dose size and sex on its disposition in the rat and mouse. Food and Chem Toxicol 32(10):869–876. https://doi.org/10.1016/0278-6915(94)90084-1
Requena R, Vargas M, Chiralt A (2019) Eugenol and carvacrol migration from PHBV films and antibacterial action in different food matrices. Food Chem 277:38–45. https://doi.org/10.1016/j.foodchem.2018.10.093
Acknowledgements
The authors are grateful for financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (408778/2022-9 and 307946/2022-3), Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (325/2022, 76/2023 and 113/2023) and Coordenação de Aperfeicoamento de Pessoal de Nível Superior (001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vaz, M.S.M., de Almeida de Souza, G.H., dos Santos Radai, J.A. et al. Antimicrobial activity of cinnamaldehyde against multidrug-resistant Klebsiella pneumoniae: an in vitro and in vivo study. Braz J Microbiol 54, 1655–1664 (2023). https://doi.org/10.1007/s42770-023-01040-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01040-z