Abstract
Carbapenem resistance observed in Klebsiella pneumoniae strains limits treatment options. Therefore, use of antibiotics combined with bioactive compounds may be an important strategy to control K. pneumoniae. The purpose of this study was to evaluate the activity of combination of carvacrol and meropenem on carbapenem-resistant K. pneumoniae (CRKP) strains. The presence of blaOXA-48 carbapenemase in all 25 CRKP strains was identified using the PCR technique. The combination of carvacrol and meropenem was tested for antimicrobial activity on CRKP strains. The minimum inhibitory concentrations of carvacrol and meropenem were detected within a range of 32–128 µg/mL using the broth microdilution method. Synergy between carvacrol and meropenem was observed on 8 of the 25 CRKP strains by checkerboard assay (FICI = 0.5) and confirmed by time-kill assay. According to the live-dead test results, the viability percentage of the cells exposed to synergistic combination was 35.47% at the end of 24 h. The membrane damage caused by the synergistic combination was spectrophotometrically measured (A = 0.21) and further confirmed by SEM analysis. According to the MTT assay, both carvacrol and meropenem did not show any statistically significant cytotoxic effect on Vero cells (p > 0.05). In conclusion, the results suggest that carvacrol and meropenem can act synergistically to inhibit the growth of CRKP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Klebsiella pneumoniae, which belongs to the Enterobacterales, is a bacterium normally living as a saprophyte in natural environments, such as soil and water (Tzouvelekis et al. 2012; Pitout et al. 2015; Doorduijn et al. 2016). This bacterium frequently colonizes in the gastrointestinal tract, skin, and nasopharynx in humans and causes severe infections, such as pneumonia and bacteremia with high mortality rates from mild urinary tract infections (García-Sureda et al. 2011; Pitout et al. 2015). K. pneumoniae is naturally resistant to penicillin and aminopenicillins (Bouza and Cercenado 2002; Ahmad et al. 2009). Because of chromosomal mutations and its ability to acquire multi drug-resistant plasmids, K. pneumoniae is now resistant to many antibiotics including broad spectrum beta-lactam antibiotics, aminoglycosides, and fluoroquinolones. This limits the treatment of infections caused by these strains (Tzouvelekis et al. 2012). Carbapenems (imipenem, ertapenem, meropenem, and doripenem) are the most recently developed group of β-lactam antibiotics. Because they are broad-spectrum antibiotics and are resistant to most β-lactamases, carbapenem antibiotics have become the first option for the treatment of K. pneumoniae (Nordmann et al. 2012; Tzouvelekis et al. 2012); however, their widespread use in this treatment has also led to the emergence of carbapenem-resistant species. This situation is extremely worrying because they are usually used as last resort antibiotics in the treatment of severe nosocomial infections that are often observed in transplantation, surgical, and intensive care units (Nordmann et al. 2009, 2012).
Carbapenem resistance depends on two basic mechanisms—acquisition of genes encoding carbapenem-degrading enzymes (carbapenemase) and the reduction uptake of antibiotics with a lack of porin expression in association with overproduction of β-lactamases. There are three most important carbapenemases types as follows: (1) K. pneumoniae carbapenemases (KPCs), (2) metallo-β-lactamases (verona integron-encoded metallo-β-lactamases [VIM], imipenemase [IMP], and New Delhi metallo-β-lactamases [NDM]), and (3) oxacillinase-48 (OXA-48) type enzymes (Nordmann et al. 2012; Pitout et al. 2015). Bacteria producing these enzymes are usually susceptible to only a few antibiotics, and this leads to prolonged hospital stays and increased mortality rates, especially in patients with immunodeficiency and bloodstream infections. Unfortunately, the use of new antibiotics cannot completely resolve this issue (Tzouvelekis et al. 2012; Munoz-Price et al. 2013). The potential strategies for resolving this issue include producing substances other than antibiotics and the development and/or discovery of various adjuvants. Combining antibiotics with antimicrobials selected from bioactive compounds present in nature is another option (Langeveld et al. 2014).
Based on ethnobotanical knowledge, a significant number of studies are being conducted worldwide on medicinal plants as alternative antimicrobial resources (Abdallah 2011; Chandra et al. 2017). The antimicrobial properties of medicinal plants depend on the presence of active compounds, such as quinolones, phenols, alkaloids, flavonoids, and terpenoids, as part of their contents. Terpenoids in plant essential oils play an important role in traditional herbal drugs and have been investigated for their many pharmacological properties (Chandra et al. 2017). Carvacrol (2-Methyl-5-(1-methylethyl)-phenol) is a monoterpenic phenolic component in the essential oils of Origanum, Thymus, Thymbra, Satureja species (Suntres et al. 2015). Carvacrol has many biological properties such as anti-inflammatory, antioxidant, antitumor, and insecticidal activities (Nostro and Papalia 2012). In addition, carvacrol, either alone or in combination with various antibiotics also exhibits very strong antimicrobial activity against many pathogens (Palaniappan and Holley 2010; Magi et al. 2015; Raei et al. 2017; El Atki et al. 2019). Based on this information, the aim of this study was to evaluate the combination activity of carvacrol with meropenem against carbapenem-resistant strains of K. pneumoniae (CRKP), investigate the effect of this combination on bacterial membrane, and detect its cytotoxic activity on eukaryotic cells.
Materials and methods
Bacterial strains and antimicrobial agents
Twenty-five CRKP strains used in this study were selected from those isolated from 2012 through 2015 in the Microbiology Department of Akdeniz University Hospital Central Laboratory, Turkey. Stock solutions of all strains were cultured in sheep-blood agar medium (Becton Dickinson, Franklin Lakes, NJ, USA). Colonies that grew after incubating at 35 ± 2 °C for 18–24 h were identified using the MALDI-TOF MS (Bruker Daltonics, Germany) method. The antibiotic susceptibilities of colonies identified as K. pneumoniae were analyzed using the BD Phoenix automated system (Becton Dickinson, Sparks, MD, USA). Klebsiella pneumoniae NCTC 13442 was used as the quality-control strain.
Carvacrol (W224511, 99% purity) and meropenem (M2574, ≥ 98% purity) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The stock solution of carvacrol was prepared using 10 mg/mL pure ethanol and preserved at − 20 °C until use. Meropenem was dissolved in distilled water, and 512 µg/mL stock solution was aliquoted and stored at − 80 °C.
Cell line and culture conditions
Vero cell line CCL-81-ATCC (African green monkey kidney cells) was kindly provided by Dr. Aydemir at Akdeniz University, Faculty of Science, Antalya, Turkey. The cells were grown in a monolayer culture in Dulbecco’s Modified Eagle’s Medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS), 10 μg/mL gentamicin, and 5% sodium pyruvate. The cells were then incubated in 5% CO2 with 95% humidity at 37 °C.
Real-time PCR with the BD MAX CRE assay
All strains were evaluated using the BD MAX CRE assay kit in the BD MAX real-time PCR fully automated system (Becton Dickinson, Sparks, MD, USA) for the presence of the blaKPC, blaNDM, and blaOXA-48 according to the manufacturer’s instructions (Ciftci et al. 2019).
Determining the minimum inhibitory concentration
To determine the minimum inhibitory concentration (MIC) values of meropenem and carvacrol, the broth microdilution method was studied according to the recommendations of the Clinical & Laboratory Standards Institute (CLSI) (CLSI 2018a). The double serial dilutions of antimicrobial agents were made using 96-well microplates with cation-adjusted Mueller Hinton Broth (MHB) (CAMHB, Merck KGaA, Darmstadt, Germany). The concentration ranges of carvacrol and meropenem were 0.25–512 and 0.0625–128 µg/mL, respectively. The bacterial suspension was adjusted according to the 0.5 McFarland standard and added to each well (final bacterial concentration: 5 × 105 colony forming units [CFU]/mL). In addition, the control of bacterial growth (CAMHB + bacteria) and medium sterility (CAMHB) were studied in each microdilution plate. Microdilution plates were incubated at 35 ± 2 °C for 18–24 h. The MIC values were determined by comparing the growth density in the wells containing antibiotics with those in the control wells used in each test set. The experiments were conducted in triplicate.
Checkerboard synergy test
To investigate the activity of combining meropenem and carvacrol, the checkerboard synergy test, which is based on microdilution, was conducted. The efficacy of the combination of the two antimicrobial agents was tested using a 96-well microplate for each strain. CAMHB was used as the medium. The activity of the two combined antimicrobial agents was studied within the dilution range of 4 × MIC and 0.03125 × MIC. Meropenem was added vertically and carvacrol was added horizontally to the wells. The bacterial suspension was prepared to produce a final inoculum of 5 × 105 CFU/mL and was added to each well. In addition, bacterial growth control (CAMHB + bacteria) and medium sterility control (CAMHB) for each plate were studied. The plates were incubated at 35 ± 2 °C for 18–24 h. Each experiment was conducted three times.
The fractional inhibition concentration (FIC) of both antimicrobial agents was calculated to be able to interpret the results according to the following formulas:
FICI ≤ 0.5 was interpreted as synergism, 0.5 ≤ FICI ≤ 4 was interpreted as indifference, and FICI > 4 was interpreted as antagonism (Moody 2010).
Time-kill assay
The time-kill assay was studied according to the method previously defined by Moody and Knapp (2010). The strains (CRKP-1, CRKP-3, CRKP-6, CRKP-12, CRKP-16, CRKP-17, CRKP-19, CRKP-22) in which a synergistic activity was detected using the checkerboard synergy test were reevaluated using the time-kill test. Carvacrol and meropenem alone (1/4 × MIC) and their synergistic combination (1/4 × MIC carvacrol + 1/4 × MIC meropenem) were prepared in test tubes containing CAMHB. The bacterial suspension prepared from mid-log phase bacteria was added to the test tubes with a final bacteria density of 6 × 105 CFU/mL. A tube containing bacteria and CAMHB was used as the growth control, and a tube with CAMHB alone was used as the sterility control. All tubes containing 10 mL were incubated at 35 ± 2 °C. Aliquots of 0.01 mL of each sample were taken from the tubes at intervals of 0, 2, 4, 8, and 24 h to determine the viable bacteria cell and were serially diluted in saline. The diluted samples were spread on Mueller–Hinton Agar (MHA, Merck KGaA) and incubated at 35 ± 2 °C for 18–24 h. The bacterial colonies between 30 and 300 CFU/mL were manually counted, averaged, and expressed as the log of CFU/mL (log10 CFU/mL). The time-kill assay was repeated three times to confirm the results. Bactericidal activity was defined as a ≥ 3 log10 decrease in CFU/mL according to the growth control. Synergistic activity was defined as a ≥ 2 log10 decrease in CFU/mL between the combination and its most active agents.
LIVE/DEAD BacLight bacterial viability assay
Effects of carvacrol, meropenem, and their synergistic combinations on bacterial viability were evaluated by LIVE/DEAD BacLight Bacterial Viability Kit (Molecular Probes, Eugene, OR, USA) assay following the manufacturer’s protocol (Latka and Drulis-Kawa 2020). The live⁄dead assay was conducted on the CRKP-6 strain, which had the lowest synergy value in the time-kill test.
Measuring cell membrane damage
Cell membrane damage was studied according to the method described by Devi et al. (2013) with minor modifications. Membrane damage measurements were conducted on the CRKP-6 strain, which had the lowest synergy value in the time-kill test. Initially, the bacteria were incubated overnight at 35 ± 2 °C in MHB (Merck KGaA). The bacterial culture was then centrifuged at 4000×g for 15 min, and the pellet was washed twice with PBS. Carvacrol and meropenem alone (1 × MIC, 1/2 × MIC, and 1/4 × MIC concentrations) and their synergistic combination (1/4 × MIC carvacrol + 1/4 × MIC meropenem) were added to the bacterial suspensions. The suspension containing only PBS and bacteria was used as the control. All samples were incubated at 35 ± 2 °C for 3 h. At the end of this period, all samples were centrifuged at 13,400×g for 15 min and the supernatant was removed. The absorbance (A)260 of the supernatant was measured using the Cary 60 UV–Vis spectrophotometer (Agilent Technologies, Santa Clara, CA, USA) to determine the amount of nucleic acids released from the cytoplasm. Measurements were conducted in triplicate.
Scanning electron microscopy
To observe the potential effect of the combination of carvacrol and meropenem on the cell morphology of the CRKP-6 strain, an analysis was conducted using SEM according to the method defined by Bendali et al. (2008). Bacteria were incubated overnight at 35 ± 2 °C in MH broth and then treated with the synergistic combination (1/4 × MIC carvacrol + 1/4 × MIC meropenem). Bacteria in MHB (without agents) were used as the control. The prepared samples were incubated at 35 ± 2 °C for 3 h, after which the samples were centrifuged at 4000×g for 10 min. The pellet was washed twice with PBS and fixed in 2.5% glutaraldehyde at 4 °C for 2 h. The bacterial pellet was again washed twice with PBS and fixed with 1% osmium tetroxide for 1 h. At the end of the process, the cells were washed twice with PBS and dehydrated using a graded ethanol series (30, 50, 70, 80, 90, and 100%). The ethanol was then replaced with 100% acetone. Finally, the samples were fixed on SEM support, and then sputter-coated with gold–palladium under vacuum, followed by microscopic examinations using a scanning electron microscope (Zeiss LEO 1430, Cambridge/England).
Cell proliferation assay
Cell proliferation was assessed using the CellTiter 96 aqueous nonradioactive cell proliferation assay kit (Promega, Madison, WI, USA), which is based on the cleavage of 3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfonyl)-2H-tetrazolium (MTT) into a soluble, yellow formasan salt (Akşit et al. 2020). The cells were seeded at 5 × 103 cells/well in 200 μL complete medium onto 96-well plates and allowed to attach for 24 h. After the cells had reached 80–90% confluency, the medium was removed, and the cells were washed with PBS. Subsequently, the cells were treated with various concentrations (8–2048 μg/mL) of meropenem, carvacrol, or their combinations prepared in 1% FBS containing the complete medium. Each treatment used eight well replicates. The cells were grown at 37 °C for 24 h, after which the medium was gently aspirated to terminate the experiment, and 180 μL serum-free complete medium and 20 μL MTT were added to each well for 4 h. The absorbance at 490 nm was measured using the Thermo Labsystem Multiscan Spectrum microplate reader (Thermolabsystem, Chantilly, VA, USA) and wells without cells as a background. The sample readings were calculated by subtracting the average of background absorbances. All experiments were conducted at least four times.
Statistical analyses
All values are expressed as the mean ± SEM. Analysis was performed using a professional statistics software program (Graph Pad InStat., San Diego, CA, USA). One-way analysis of variance (ANOVA) with Dunnett's multiple comparison post-test was used for comparing cell membrane damage, cell viability and live-dead bacterial viability tests between tested groups. p < 0.05 was considered to be statistically significant. The graphs were drawn using Sigma Plot version 10.0 (SPSS Inc., Chicago, IL, USA) software.
Results
Presence of carbapenemase genes
According to the results of real-time PCR assay conducted to determine the presence of carbapenemase genes, all 25 CRKP strains were determined to be blaOXA-48 positive (Table 1). None of the blaKPC or blaNDM genes were found in any of the strains studied.
Antibacterial susceptibility
The MIC values of carvacrol and meropenem against all bacterial strains are provided in Table 1. Carvacrol exhibited antibacterial activity with MIC values within the range of 32–128 µg/mL against all strains tested, including the control strain. The MIC values of the 25 clinical strains were 128 µg/mL for 11, 64 µg/mL for 9, and 32 µg/mL for 5 strains. Meropenem also exhibited an antibacterial effect with the same MIC values as carvacrol, and the MIC values of all the clinical strains were 128 µg/mL for 7, 64 µg/mL for 10, and 32 µg/mL for 8 strains. The MIC test results were evaluated based on CLSI criteria (CLSI 2018b). According to these results, all the tested clinical strains were found to be resistant to meropenem. The susceptibilities of all tested clinical strains to other antibiotics are given in Table 2.
Evaluation of the checkerboard synergy test result
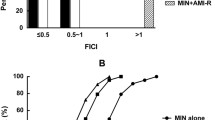
The checkerboard synergy test was conducted to evaluate the activity of the combined carvacrol and meropenem, and the FICI values were calculated (Table 1). According to these results, the synergistic effect was determined against eight of the clinical strains, and the FICI values were calculated to be 0.5. There was a fourfold decrease in the meropenem MIC values of the eight strains that displayed synergistic effect. The combination of these two agents exhibited an indifferent effect with FICI values within the range of 0.75–1 against the other 17 strains. No antagonistic effect was detected against any of the tested strains.
Evaluation of time-kill assay
The time-kill test was conducted to confirm the synergistic results obtained against the eight CRKP strains used in the checkerboard synergy test and to show the bactericidal activity of the combination of agents. According to these results, a decrease in the colony counts of > 2 log10 CFU/mL was detected for the strains exposed to the combination compared to that from the most active agent at the end of 24 h. In other words, the combination exhibited synergistic activity against eight strains. When the bactericidal activity was evaluated according to time-kill assay, no significant decrease was detected in the colony counts of the strains exposed to a concentration of 1/4 × MIC carvacrol throughout the entire incubation time. At the end of 24 h, a decrease of < 3 log10 CFU/mL was observed when bacterial colony counts were compared with that in the control. A concentration of 1/4 × MIC meropenem caused a decrease in the colony counts of the strains up to 4 h, but an increase in the colony counts was observed in the hours following that timespan. At the end of 24 h, a decrease of < 3 log10 CFU/mL was detected in the colony counts of the strains when compared with that of the control. In other words, by themselves, neither carvacrol nor meropenem exhibited any bactericidal activity on any CRKP strain. When the strains exposed to the synergistic combination (1/4 × MIC carvacrol + 1/4 × MIC meropenem) were evaluated for bactericidal activity, a decrease in the colony counts of > 3 log10 CFU/mL was recorded in the strains compared to that of the control, which indicated that the combination showed a bactericidal effect against all strains. The graph in Fig. 1 shows the time-kill test results for the CRKP strains that exhibited the synergistic activity.
LIVE/DEAD BacLight assay
The viability of the CRKP-6 strain exposed to carvacrol, meropenem, and their synergistic combination was evaluated using the LIVE/DEAD BacLight bacterial viability assay. According to the results, the viability percentage of the cells exposed to 1/4 × MIC carvacrol, 1/4 × MIC meropenem, and their synergistic combination (1/4 × MIC carvacrol + 1/4 × MIC meropenem) at the end of 24 h was 93.72, 71.46, and 35.47%, respectively. The decrease in the percentage of cell viability after administration of the combination was statistically significant when compared to the percentage of cell viability exposed to each agent alone. The fluorescence absorption measurements are provided in Table 3.
Measuring the membrane damage of the CRKP-6 strain
Cell membrane damage was determined by measuring the amount of nucleic acid leaking through the bacterial cell membrane at 260 nm using a UV spectrophotometer. The CRKP-6 strain was treated with different concentrations of carvacrol and meropenem (1/4 × MIC, 1/2 × MIC, or 1 × MIC) and with the synergistic combination (1/4 × MIC carvacrol + 1/4 × MIC meropenem). Bacteria not exposed to any antimicrobial agent were used as the control. Figure 2 shows the resulting absorbance values. Accordingly, as the concentration of carvacrol increased, the absorbance values also increased (0.14, 0.26, 0.52, respectively, for the above concentrations). This increase shows a statistically significant difference when compared with those of meropenem and the control (p < 0.01). The absorbance value obtained as a result of measurements made using the synergistic combination was 0.21. A statistically significant difference was found between this absorbance value and the absorbance values measured at the same concentration of each agent alone (p < 0.01).
Presence of 260-nm absorbing materials in the supernatants of CRKP-6 strain treated with different concentrations of carvacrol, meropenem, and their synergistic combination. The data are the average triplicates and *, **, and *** significance at the levels of p < 0.05, p < 0.01, and p < 0.001, respectively (B). CAR carvacrol, MEM meropenem
Scanning electron microscope analyses
SEM analyses were conducted to observe whether the synergistic combination caused any morphological changes in the CRKP-6 strain. Figure 3 shows SEM images of the CRKP-6 strain treated and untreated with the combined agents. The control cells have normal cell morphology. The characteristic rod-shaped, regular, and unchanged morphology of K. pneumoniae is provided in Fig. 3a1, a2. In contrast, serious damage was detected in the bacterial cells exposed to the synergistic combination. Deformations such as wrinkling, collapse of the bacterial cell surfaces, and cell structure disruptions were clearly visible (Fig. 3b1, b2).
Scanning electron microscopy images of the carbapenem-resistant Klebsiella pneumoniae (CRKP)-6 strain for a1 and a2 (original magnification, × 10,000, × 20,000) untreated bacteria have normal cell morphology with regular rod-shaped cells. The bacterial cells treated with the synergistic combination (1/4 × MIC carvacrol + 1/4 × MIC meropenem); b1 and b2 (original magnification, × 10,000, × 20,000) show cell structure disruptions such as wrinkling, collapsing cell surfaces
In vitro cytotoxicity against vero cells
The possible cytotoxic effects of various concentrations of carvacrol, meropenem and their combinations on Vero cells were determined in vitro. Neither carvacrol (Fig. 4a) nor meropenem (Fig. 4b) applied at the tested concentrations had any direct cytotoxicity against cells (p > 0.05). Results demonstrated that the combinations of carvacrol and meropenem is not toxic to Vero cells (p > 0.05) (Table 4) but rather specific against bacterial cells.
Discussion
Carbapenemases are known to be the most important enzymes by which K. pneumoniae strains become resistant to a bactericidal agent. The main problem is that the genes encoding these enzymes are located on the transferable plasmids and transposons, and thus resistance can be rapidly transferred between the same and/or different species; therefore, resistant strains producing carbapenemases are spreading rapidly throughout the world (Nordmann et al. 2012). OXA-48 carbapenemase was initially identified in Turkey in a CRKP isolate from Istanbul (Poirel et al. 2004). Since then, OXA-48-producing strains have been extensively reported as sources of nosocomial outbreaks in Turkey (Carrër et al. 2008; Azap et al. 2013). Apart from the OXA-48 producers, isolates producing other types of carbapenemases (NDM, IMP, VIM, and KPC-2) were recently identified in Turkey as well (Alp et al. 2013; Genc et al. 2016; Labarca et al. 2014). Su et al. (2020) reported that 105 strains of 113 carbapenem-resistant isolates were detected as OXA-48 (69.7%) and followed by VIM, NDM, and IMP in 3 strains, 3 strains, and 1 strain, respectively. Similarly, in this study OXA-48 carbapenemase was detected in all randomly selected 25 clinical CRKP strains; none of the other enzymes were found in them.
The carbapenem resistance revealed using PCR analyses in all clinical strains was also confirmed by the MIC results for meropenem. According to the MIC values obtained against the 25 CRKP strains, all were found to be resistant to meropenem. Carvacrol exhibited antibacterial activity equivalent to that of meropenem on these strains. In another study investigating the antibacterial activity of carvacrol against carbapenemase producing K. pneumoniae strains, the MIC of carvacrol against K. pneumoniae (NDM), K. pneumoniae (VIM-1), K. pneumoniae (OXA-48), K. pneumoniae (KPC) strains were found as 250, 125, 125, 125 µg/mL, respectively (Raei et al. 2017). de Souza et al. (2021) also found that carvacrol showed significant antibacterial activity against KPC-producing K. pneumoniae strains with MIC values in the range of 130–260 mg/L. Several studies have shown the antibacterial activity of carvacrol on different microorganisms. In some of these, the antibacterial activity of carvacrol against Bacillus cereus (Ultee et al. 1999), Shigella sonnei and Shigella flexneri (Bagamboula et al. 2004), Escherichia coli, Pseudomonas fluorescens, Staphylococcus aureus, Lactobacillus plantarum, and Bacillus subtilis (Ben Arfa et al. 2006), Salmonella typhimurium (Zhou et al. 2007), and E. coli (Pei et al. 2009; Khan et al. 2017) has been demonstrated.
In this study, the effect of the combination of carvacrol and meropenem was first assessed using the checkerboard synergy test. While synergistic effect was observed in a minority of 25 isolates with eight CRKP strains, the others were found to be indifferent. Based on the literature, there has been no study that has investigated the effect of the combination of carvacrol and meropenem on strains of CRKP. However, combination activities of various antibiotics with carvacrol against many bacterial strains have been investigated in previous studies. Cordeiro et al. (2020) found that combination of carvacrol with both ceftazidime and cefepime against all tested K. pneumoniae strains showed additive effect. The combination of nalidixic acid with carvacrol has been determined to exhibit synergistic (62.5%) and indifference effect (37.5%) on nalidixic acid-resistant pathogenic bacteria by Choi et al. (2009). Palaniappan and Holley (2010) indicated that the combinations of carvacrol with various antibiotics (ampicillin, tetracycline, penicillin, bacitracin, erythromycin and novobiocin) showed synergistic effect on resistant S. typhimurium, Streptococcus pyogenes, E. coli and S. aureus. Maggi et al. (2015) found that the combination of carvacrol and erythromycin had synergistic effect against 21 of 32 erythromycin resistant group A Streptococci. On the other hand, Wijesundara et al. (2021) reported that the combination of carvacrol and erythromycin did not exhibit any synergistic effect on S. pyogenes. There are also studies investigating the combination effects of different phytochemicals against K. pneumoniae in the literature. Baicalein has been found to exhibit synergistic activity against extended spectrum β-lactamase (+) K. pneumoniae when combined with cefotaxime (Cai et al. 2016). Dhara and Tripathi (2020) determined that the combination of cinnamaldehide with cefotaxime and ciprofloxacin against ESBL-producing quinolone-resistant K. pneumoniae showed 60.6% and 42.4% synergistic effect, respectively.
The time-kill assay is a reliable test used to evaluate the synergistic effect between antibacterial agents. Therefore, we used the time-kill test to confirm our checkerboard synergy results. In line with the results of our checkerboard synergy test, the time-kill test also showed that the combination of carvacrol and meropenem was synergistically effective against 8 of 25 strains. Moreover, the time-kill test results also indicated that carvacrol and meropenem alone had no bactericidal effect against the 8 strains tested; however, their synergistic combinations exhibited bactericidal effect on these strains at 24 h of exposure. de Souza et al. (2021) found that carvacrol at a concentration of 1 × MBC eradicated all bacterial cells within 4 h. This difference between results may be due to the fact that carvacrol was tested at a lower concentration (1/4 × MIC) in our study. According to the results of the live/dead test conducted to support the results of the time-kill assay, the mortality rate was very low in the CRKP-6 strain treated with either carvacrol or meropenem alone; however, a high mortality rate was observed after applying the combination of these two agents.
The primary antibacterial mechanism of carvacrol is that it causes damage to the bacteria cell membrane. Because of its hydrophobic properties, it increases the permeability and fluidity of the cell membrane structure by interacting with fatty acids. Moreover, it has also been demonstrated that carvacrol causes structural changes in the bacterial cell wall and disrupts the outer membrane of Gram-negative bacteria. Thus, carvacrol causes the leakage of vital intracellular components, nucleic acids, ions, and ATP out of the cell, thereby leading to cell death. (Lambert et al. 2001; Burt 2004; Nostro and Papalia 2012). In addition, the amount of ATP was decreased in cells exposed to carvacrol. This effect is thought to occur with a decrease in ATP synthesis rate or an increase in ATP hydrolysis rate. This effect is believed to be achieved by a reduction in the ATP rate of the synthesis or an increase in the rate of ATP hydrolysis (Ultee et al. 1999). All these mechanisms are believed to depend on the chemical structure of carvacrol. In addition to its hydrophobic character, having a free hydroxyl group and a delocalized electron system makes carvacrol a more effective antibacterial component (Ben Arfa et al. 2006). Based on this information, we investigated whether carvacrol alone and in combination with meropenem caused damage to the bacterial cell membrane. According to the results of the UV spectrophotometer measurements, carvacrol was determined to cause damage to the cell membrane of the CRKP-6 strain. As the concentration of carvacrol increased, the amount of substances leaking out of the cell membrane also increased. The synergistic combination caused more membrane damage than carvacrol alone. Since the synergistic combination has a greater effect on cell membrane damage than carvacrol alone, we speculate that carvacrol reduces the resistance to meropenem and thereby causes more membrane damage. However, further studies are needed to exactly reveal the mechanism of the combination’s effect.
In SEM observations of the cells exposed to the synergistic combination of the agents, images of disintegrated and lysed cells were observed. According to these images, the synergistic combination of carvacrol and meropenem caused severe damage to the cell wall and membrane of the CRKP-6 strain. Thus, the bactericidal effect detected by the time-kill and live-dead assays, and the membrane damage observed with the UV spectrophotometer measurements were also confirmed by SEM observations. Based on these results, we suggest that the combination of carvacrol and meropenem demonstrated a detrimental effect on the CRKP strain by causing both membrane damage and cell wall disintegration.
According to the results of our study, although carvacrol alone did not exhibit strong antibacterial activity against CRKP strains, it did decrease the MIC values of meropenem when it was combined with meropenem. In other words, the combination of carvacrol with meropenem increased the activity of meropenem against CRKP species. β-lactamase inhibitors, such as clavulanic acid, have been used for years as a codrug to enhance the effectiveness of an antibiotic (Hemaiswarya et al. 2008). Based on the results of the synergy test, we speculate that carvacrol could also be used for this purpose.
Carvacrol has been reported to be safe and exert minimal toxicity on eukaryotic cells and also listed as a generally recognized safe (GRAS) food additive by the United States Food and Drug Administration (Wijesundara et al. 2021). It is very important when using any antibacterial agent that the agent has selective inhibition against bacteria with less or no cytotoxic effects on normal healthy cells. Herein, we confirmed that either carvacrol alone or in combination with meropenem exhibits high selective cytotoxicity towards bacterial cells over normal epithelial cells. Hence, carvacrol is an effective terpenoid compound and exerts selective inhibitory effects on bacterial infections. Its use is quite safe in preventing or treating bacterial infections because it does not cause any cytotoxic side effects in healthy tissues.
Conclusion
The results of the present study showed that the combination of carvacrol and meropenem had synergistic effect in minority of CRKP strains. Carvacrol caused a decrease in the bacteria’s resistance to meropenem in the strains tested. The synergistic combination has shown bactericidal effect by causing cell membrane damage. Carvacrol could be an important resource in new antimicrobial researches against resistant K. pneumoniae. Further studies are needed to confirm efficacy of this synergistic combination in clinical practice.
Data availability
All material and data are stored at Akdeniz University, Vocational School of Health Services, and can be shared upon request directed to the corresponding author.
References
Abdallah EM (2011) Plants: an alternative source for antimicrobials. J Appl Pharm Sci 1(6):16–20
Ahmad S, Al-Juaid NF, Alenzi FQ, Mattar EH, Oel-S B (2009) Prevalence, antibiotic susceptibility pattern and production of extended-spectrum beta-lactamases amongst clinical isolates of Klebsiella pneumoniae at Armed Forces Hospital in Saudi Arabia. J Coll Phys Surg Pak 19(4):264–265
Akşit NN, Gürdap S, İşoğlu SD, İşoğlu İA (2020) Preparation of antibacterial electrospun poly (D, L-lactide-co-glycolide)/gelatin blend membranes containing Hypericum capitatum var. capitatum. Int J Polym Mater. https://doi.org/10.1080/00914037.2020.1765354
Alp E, Percin D, Colakoğlu S, Durmaz S, Kürkcü CA, Ekincioğlu P, Güneş T (2013) Molecular characterization of carbapenem-resistant Klebsiella pneumoniae in a tertiary university hospital in Turkey. J Hosp Infect 84(2):178–180. https://doi.org/10.1016/j.jhin.2013.03.002
Azap Ö, Otlu B, Yeşilkaya A, Yakupoğulları Y (2013) Detection of OXA-48-like carbapenemase-producing Klebsiella pneumoniae in a tertiary care center in Turkey: molecular characterization and epidemiology. Balkan Med J 30(2):259–260. https://doi.org/10.5152/balkanmedj.2013.7499
Bagamboula CF, Uyttendaele M, Debevere J (2004) Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol 21(1):33–42. https://doi.org/10.1016/S0740-0020(03)00046-7
Ben Arfa A, Combes S, Preziosi-Belloy L, Gontard N, Chalier P (2006) Antimicrobial activity of carvacrol related to its chemical structure. Lett Appl Microbiol 43(2):149–154. https://doi.org/10.1111/j.1472-765X.2006.01938.x
Bendali F, Gaillard-Martinie B, Hebraud M, Sadoun D (2008) Kinetic of production and mode of action of the Lactobacillus paracasei subsp. paracasei anti-listerial bacteriocin, an Algerian isolate. LWT - Food Sci Technol 41(10):1784–1792. https://doi.org/10.1016/j.lwt.2008.02.010
Bouza E, Cercenado E (2002) Klebsiella and enterobacter: antibiotic resistance and treatment implications. Semin Respir Infect 17(3):215–230. https://doi.org/10.1053/srin.2002.34693
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods-a review. Int J Food Microbiol 94:223–253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022
Cai W, Fu Y, Zhang W et al (2016) Synergistic effects of baicalein with cefotaxime against Klebsiella pneumoniae through inhibiting CTX-M-1 gene expression. BMC Microbiol 16(1):1–9. https://doi.org/10.1186/s12866-016-0797-1
Carrër A, Poirel L, Eraksoy H, Cagatay AA, Badur S, Nordmann P (2008) Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob Agents Chemother 52(8):2950–2954. https://doi.org/10.1128/AAC.01672-07
Chandra H, Bishnoi P, Yadav A, Patni B, Mishra AP, Nautiyal AR (2017) Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials-a review. Plants 6(2):16. https://doi.org/10.3390/plants6020016
Choi JG, Kang OH, Lee YS, Oh YC, Chae HS, Jang HJ, Shin DW, Kwon DY (2009) Antibacterial activity of methyl gallate isolated from Galla Rhois or carvacrol combined with nalidixic acid against nalidixic acid resistant bacteria. Molecules 14(5):1773–1780. https://doi.org/10.3390/molecules14051773
Ciftci E, Cetin ES, Us E, Kutlu HH, Arıdogan BC (2019) Investigation of carbapenem resistance mechanisms in Klebsiella pneumoniae by using phenotypic tests and a molecular assay. J Infect Dev 13(11):992–1000. https://doi.org/10.3855/jidc.10783
Clinical and Laboratory Standards Institute (CLSI) (2018a) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th edn. CLSI Standard M07, Wayne
Clinical and Laboratory Standards Institute (CLSI) (2018b) Performance standards for antimicrobial susceptibility testing, 28th edn. CLSI Supplement M100, Wayne
Cordeiro LV, Figueiredo PTR, Sousa AP, Andrade FP Jr, Souza HDS, Araújo DL, Sobreira ALC, Lima EO (2020) Association of carvacrol with ceftazidime and cefepime against Klebsiella pneumoniae. Res Soc Dev 9(7):1–10. https://doi.org/10.33448/rsd-v9i7.4089
de Souza GHdA, dos Santos Radai JA, Mattos Vaz MS, Esther da Silva K, Fraga TL, Barbosa LS, Simionatto S (2021) In vitro and in vivo antibacterial activity assays of carvacrol: a candidate for development of innovative treatments against KPC-producing Klebsiella pneumoniae. PLoS ONE 16(2):e0246003. https://doi.org/10.1371/journal.pone.0246003
Devi KP, Sakthivel R, Nisha SA, Suganthy N, Pandian SK (2013) Eugenol alters the integrity of cell membrane and acts against the nosocomial pathogen Proteus mirabilis. Arch Pharm Res 36(3):282–292. https://doi.org/10.1007/s12272-013-0028-3
Dhara L, Tripathi A (2020) Cinnamaldehyde: a compound with antimicrobial and synergistic activity against ESBL-producing quinolone-resistant pathogenic Enterobacteriaceae. Eur J Clin Microbiol Infect Dis 39(1):65–73. https://doi.org/10.1007/s10096-019-03692-y
Doorduijn DJ, Rooijakkers SH, van Schaik W, Bardoel BW (2016) Complement resistance mechanisms of Klebsiella pneumoniae. Immunobiology 221(10):1102–1109. https://doi.org/10.1016/j.imbio.2016.06.014
El Atki Y, Aouam I, El Kamari F, Taroq A, Gourch A, Lyoussi B, Abdellaoui A (2019) Antibacterial efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of nosocomial infection-bacteria. J Pharm Sci Res 11(2):306–309
García-Sureda L, Doménech-Sánchez A, Barbier M, Juan C, Gascó J, Albertí S (2011) OmpK26, a novel porin associated with carbapenem resistance in Klebsiella pneumoniae. Antimicrob Agents Chemother 55(10):4742–4747. https://doi.org/10.1128/AAC.00309-11
Genc O, Aksu E, Gulcan A (2016) The identification of carbapenemase types in Enterobacteriaceae by using molecular assay and phenotyping confirmation tests. J Microbiol Methods 125:8–11. https://doi.org/10.1016/j.mimet.2016.03.010
Hemaiswarya S, Kruthiventi AK, Doble M (2008) Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15(8):639–652. https://doi.org/10.1016/j.phymed.2008.06.008
Khan I, Bahuguna A, Kumar P, Bajpai VK, Kang SC (2017) Antimicrobial potential of carvacrol against uropathogenic Escherichia coli via membrane disruption, depolarization, and reactive oxygen species generation. Front Microbiol 8:2421. https://doi.org/10.3389/fmicb.2017.02421
Labarca J, Poirel L, Ozdamar M, Turkoglü S, Hakko E, Nordmann P (2014) KPC-producing Klebsiella pneumoniae, finally targeting Turkey. New Microbes New Infect 2(2):50–51. https://doi.org/10.1002/nmi2.42
Lambert RJW, Skandamis PN, Coote PJ, Nychas GJ (2001) A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 91(3):453–462. https://doi.org/10.1046/j.1365-2672.2001.01428.x
Langeveld WT, Veldhuizen EJ, Burt SA (2014) Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol 40(1):76–94. https://doi.org/10.3109/1040841X.2013.763219
Latka A, Drulis-Kawa Z (2020) Advantages and limitations of microtiter biofilm assays in the model of antibiofilm activity of Klebsiella phage KP34 and its depolymerase. Sci Rep 10(1):1–12. https://doi.org/10.1038/s41598-020-77198-5
Magi G, Marini E, Facinelli B (2015) Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin resistant Group A Streptococci. Front Microbiol 6:165. https://doi.org/10.3389/fmicb.2015.00165
Moody J (2010) Synergism testing: broth microdilution checkerboard and broth macrodilution methods. In: Garcia LS (ed) Clinical microbiology procedures handbook. ASM Press, Washington DC
Moody J, Knapp C (2010) Tests to assess bactericidal activity. In: Garcia LS (ed) Clinical microbiology procedures handbook. ASM Press, Washington DC
Munoz-Price LS, Poirel L, Bonomo RA et al (2013) Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13(9):785–796. https://doi.org/10.1016/S1473-3099(13)70190-7
Nordmann P, Cuzon G, Naas T (2009) The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 9(4):228–236. https://doi.org/10.1016/S1473-3099(09)70054-4
Nordmann P, Dortet L, Poirel L (2012) Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med 18(5):263–272. https://doi.org/10.1016/j.molmed.2012.03.003
Nostro A, Papalia T (2012) Antimicrobial activity of carvacrol: current progress and future prospectives. Recent Pat Antiinfect Drug Discov 7(1):28–35. https://doi.org/10.2174/157489112799829684
Palaniappan K, Holley RA (2010) Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int J Food Microbiol 140(2–3):164–168. https://doi.org/10.1016/j.ijfoodmicro.2010.04.001
Pei RS, Zhou F, Ji BP, Xu J (2009) Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J Food Sci 74(7):379–383. https://doi.org/10.1111/j.1750-3841.2009.01287.x
Pitout JD, Nordmann P, Poirel L (2015) Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 59(10):5873–5884. https://doi.org/10.1128/AAC.01019-15
Poirel L, Héritier C, Tolün V, Nordmann P (2004) Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother 48(1):15–22. https://doi.org/10.1128/AAC.48.1.15-22.2004
Raei P, Pourlak T, Memar MY, Alizadeh N, Aghamali M, Zeinalzadeh E, Asgharzadeh M, Kafil HS (2017) Thymol and carvacrol strongly inhibit biofilm formation and growth of carbapenemase-producing Gram negative bacilli. Cell Mol Biol 63(5):108–112. https://doi.org/10.14715/cmb/2017.63.5.20
Su HR, Turhan Ö, Erman Daloğlu CA, Öğünç MD, Özhak B, Öngüt G, Kuşkucu MA, Midilli K, Mamıkoğlu L (2020) Molecular epidemiology of carbapenem-resistant enterobacterales strains isolated from blood cultures in Antalya, Turkey. Lab Med 51(6):601–605. https://doi.org/10.1093/labmed/lmaa017
Suntres ZE, Coccimiglio J, Alipour M (2015) The bioactivity and toxicological actions of carvacrol. Crit Rev Food Sci Nutr 55(3):304–318. https://doi.org/10.1080/10408398.2011.653458
Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL (2012) Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25(4):682–707. https://doi.org/10.1128/CMR.05035-11
Ultee A, Kets EPW, Smid EJ (1999) Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol 65(10):4606–4610. https://doi.org/10.1128/AEM.65.10.4606-4610.1999
Wijesundara NM, Lee SF, Cheng Z, Davidson R, Rupasinghe HV (2021) Carvacrol exhibits rapid bactericidal activity against Streptococcus pyogenes through cell membrane damage. Sci Rep 11(1):1–14. https://doi.org/10.1038/s41598-020-79713-0
Zhou F, Ji B, Zhang H, Jiang HUI, Yang Z, Li J, Li J, Yan W (2007) The antibacterial effect of cinnamaldehyde, thymol, carvacrol and their combinations against the foodborne pathogen Salmonella typhimurium. J Food Saf 27(2):124–133. https://doi.org/10.1111/j.1745-4565.2007.00064.x
Acknowledgements
The author thanks Prof. Dilara Öğünç and Assist. Prof. Özlem Koyuncu Özyurt from the Department of Medical Microbiology, the Faculty of Medicine, Akdeniz University, Turkey, for their valuable help in providing the bacteria strains and PCR testing; Assist. Prof. Hakan ER from the Electron Microscopy and Image Analyzing Unit, the Faculty of Medicine, Akdeniz University, Turkey, for his valuable help in making SEM observations; and Assoc. Prof. Esra Aydemir from the Department of Biology, Faculty of Science, Akdeniz University, Turkey, for her help with the statistical analyses of the tests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Köse, E.O. In vitro activity of carvacrol in combination with meropenem against carbapenem-resistant Klebsiella pneumoniae. Folia Microbiol 67, 143–156 (2022). https://doi.org/10.1007/s12223-021-00908-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-021-00908-7