Abstract
Chromium (Cr) pollution is one of the primary environmental concerns because of its adverse impact on crop productivity. Cr translocate in plants from the rhizosphere and enters the food chain, where it poses a significant hazard to the health of humans. Glutathione (GSH) mediated regulation of the defense mechanism in pea under Cr toxicity has not been reported in the literature. Therefore, the current study was undertaken to evaluate the role of exogenous GSH in alleviation of Cr stress in Pisum sativum L. (pea). Pea seeds were primed with three different concentrations of GSH (25, 50, and 75 μmol L−1GSH). Cr-induced toxicity resulted in a notable reduction in germination rate, biomass production, stomatal conductance, transpiration rate, net photosynthesis and phenolic content in pea seedlings. The findings also revealed that Cr reduced chlorophyll, carotenoids and protein content in seedlings due to decreased uptake of mineral content (Na, K, Mg, Zn). Plants grown from GSH-primed seeds exhibited reduced oxidative damage caused by Cr, through accretion of non-enzymatic antioxidants such as proline and phenolic. Thus, GSH prevented the hampering of plant’s physiological activity, resulted in enhanced germination rate, biomass production, gas exchange attributes, net photosynthesis and protein content. Briefly, 50 μmol L− 1GSH priming provided best outcomes by increasing germination rate (33%), protein content (6.12 µg g−1 FW), proline content (18.01 µmol g−1 FW) and phenolic (0.493 mg g−1 FW). Consequently, GSH improved pea growth by enhanced nutrient uptake along with reduced Cr content in shoot. The current study reveals that GSH primed seeds minimizes Cr toxicity and makes it possible to cultivate pea in Cr-contaminated areas. Future studies will probably assist in elucidation of GSH intervened stress alleviation procedures in pea crop. Furthermore, research with reference to GSH for stress mitigation in other agronomic and horticultural crops will help in improvement of crop productivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Plant growth and development are greatly affected by heavy metal stress, which is a serious environmental problem (Dhalaria et al. 2020). Unchecked discharge of heavy metals into soil has been a major issue for the ecosystem because of its deleterious effects on plants (Khan et al. 2021). In the last few decades, air, water and soil have been contaminated due to increased levels of chromium (Cr) being used in various industries and emerging as a potential drastic pollutant in the environment (Vareda et al. 2019). It becomes a part of the environment either by natural activities (weathering of rocks) and anthropogenic practices (paints, chrome plating, alloys, fertilizers) (He and Li 2020). The chemical and physical degradation of soil induced by Cr contamination impairs soil fertility, as a consequence of soil infertility, biomass production and growth decreases (Vareda et al. 2019). The most prevalent and stable forms of Cr in soil are Cr (0), Cr (III), and Cr (VI) (Al-Huqail et al. 2020). It is readily available to plants because of its high solubility in water (Gupta et al. 2020). Cr (VI) causes changes in development, growth at different stages of plant life via alterations in hormonal homeostasis and assimilation of nutrients in plants (Bücker-Neto et al. 2017). As a redox metal, Cr provokes the production of reactive oxygen species (ROS), which in response disrupts the redox homeostasis balance within plant cells and causes oxidative damage to nucleic acids and proteins (Adhikari et al. 2018). As a non-essential element, Cr is often absorbed mostly in the roots. Excess Cr in plant tissues can have numerous deleterious impacts on physiology, biochemistry, and morphology (Uddin et al. 2015). According to earlier researches, Cr may slow down the rate at which seeds germinate and may have an impact on root elongation and growth (Kumari et al. 2016). In particular, Cr (VI) even at concentration considerably suppressed the germination of the seeds of Zea mays and Arabidopsis thaliana (Ramzan et al. 2023; Colzi et al. 2023). Furthermore, Cr can deactivate enzymes activity and photosynthetic pigments, obstruct the uptake of nutrients and water, decrease assimilation capacity, and produce excess ROS, which can cause membrane structure peroxidation (Ashraf et al. 2022). These harmful effects of Cr have resulted in reduced crop yield and quality (Askari et al. 2021). Plants subjected to heavy metal stress respond to exogenous antioxidant treatment, by boosting their resilience against stress (Nguyen et al. 2021). Furthermore, during heavy metal stress, the endogenous quantities of phytohormones also varies that regulate plants' adaptive behavior in stress (Bücker-Neto et al. 2017).
Seed priming is the process of exposing plants to an evoking stimulus in order to make them more resistant to stress in the germination process, speeds up the metabolic process that makes it easier for seeds to sprout early (Johnson and Puthur 2021). Recent studies have shown that exogenous applications of substances like sulphur, glutathione, abscisic acid and melatonin can enhance the tolerance of plants to toxic elements (Liu et al. 2020; Bamagoos et al. 2022; Cheng et al. 2022; Xie et al. 2021). Among them, glutathione (GSH) is a strong non-protein antioxidant and metabolite that contains sulphur that protects the cells from oxidative impairments and significantly contributes in plant oxidative damage and metal detoxification (Yao et al. 2021). By having thiol group (-SH) chelating processes, GSH can lead to the creation of Cr-GSH complexes, which lowers the amount of free Cr in cells (Hasan et al. 2016). Glutathione can act as an antioxidant through the ascorbate–glutathione cycle (ASA-GSH cycle) to prevent the Cr-mediated elevated formation of ROS from impairing plant physiological activity (Rasheed et al. 2022).Supplementation of GSH promotes growth and enhances chlorophyll content by inhibiting heavy metal stress (Yao et al. 2021). Glutathione reduces the accumulation of heavy metal in different parts of plants and keeps the structure of the thylakoid membrane and chloroplast intact, consequently improves the photosynthetic efficiency (Ahmed et al. 2023). Supplementation of 50 μmol L− 1GSH reduced the phytotoxic effects of heavy metal on Iris lacteal (Yuan et al. 2015). Exogenously applied GSH enhances antioxidant enzyme activity by upregulation of superoxide dismutase, ascorbic acid and catalase activity (Khan et al. 2016; Asgher et al. 2018).Hence, exogenous application of GSH is a cost-effective strategy which increases photosynthetic activity, growth and eventually increasing the yield of plants (Cao et al. 2015).
Pisum sativum L. (pea) belongs to the family Leguminosae, is a cool-weather legume, vital nutritive and economic crop that is often referred to as "poor man's meat" due to its high protein content (Powers and Thavarajah 2019). It is also enriched in minerals such as iron, zinc, vitamins, and carbohydrates and is still affordable for the poorest (Majeed et al. 2019). Despite the fact that peas can help alleviate invisible hunger around the world, little effort has been made to improve their production and yield under Cr stress. Cr concentration range from 1970 to 2980 mg Cr kg−1 of soil in some adjacent areas of our recent study (Afzal et al. 2014).These levels of Cr are higher than the permissible limit i.e. 3.8 mg kg−1 reported by Vodyanitskii (2016).Peas accumulate significant quantities of Cr in their shoots and roots under Cr toxicity (Faisal et al. 2022). Continuous detection investigations are required because of the significant harmful impact that Cr has on the viability of plants. There is almost no information about the physiological limits of GSH priming to eliminate Cr toxicity in pea. It hypothesized that seed priming of pea seeds with GSH will alleviate harmful impacts of Cr stress and resulted in improved growth. Therefore, the objective of the current study is to evaluate the influence of glutathione on P. sativum under Cr stress and to explore the potential of glutathione as a seed-priming agent in alleviation of Cr stress in P. sativum by improving nutrient orchestration, proline, and physiochemical attributes. Furthermore, the research was intended to reveal the possible beneficial role of glutathione on the growth, gas exchange characteristics, protein content, and DPPH activity of P. sativum grown in Cr-contaminated soil.
2 Materials and Methods
2.1 Seed Priming and Growth Conditions
Pisum sativum L. (pea) seeds were acquired from a local market in Lahore, Pakistan. Surface sterilization of pea seeds was accomplished by immersing them in a.
0.5% sodium hypochlorite solution for 5 min (Sardar et al. 2022a, b) and then thoroughly washed them three times with distilled water. The sterilized seeds were primed with varying concentrations of glutathione; analytical grade employed for seed priming was purchased from Sigma-Aldrich (Saint-Louis, MO, USA). Seeds were kept in glutathione solutions (25 μmol L− 1, 50 μmol L− 1, 75 μmol L− 1) prepared in distilled water (Khan et al. 2016), were named as GSH 1, GSH 2 and GSH 3, respectively for 4 h at 25 oC under dim light (Shahid et al. 2011) following air drying at room temperature. Non-primed seed was designated as control.
The pot experiment was performed at the Botanical Garden, University of Punjab, Lahore (32,130′ 15′′ North, 7418′ 23′′ East). The soil samples were collected from a nearby study area's topsoil (0–20 cm). The soil was spiked with 100 mg of Cr kg−1while Cr-free soil was utilized as a control and was placed in plastic pots in a shady area for 15 days. Each pot had 7-inch length, 9-inch top diameter and filled with 4.5 kg of soil upto 6 inch height. Potassium dichromate (K2Cr2O7) was employed as a Cr source. Five GSH-primed and controlled seeds were sown uniformly in each pot. The pots were placed in conditions having a temperature of 18/25 °C at night and a dark–light cycle of 8/16 h. A randomized complete block design (RCBD) was used in the experiment, to arrange 32 pots for 8 treatments i.e. C = Control, Cr, GSH1, GSH2, GSH3, GSH1 + Cr, GSH2 + Cr, GSH3 + Cr, with four repetitions. After ten days, thinning was done and two seedlings per pot were allowed to grow. The 55-day old plants were carefully harvested to determine their physio-biochemical characteristics and non-enzymatic antioxidants. Attributes for morphology and biomass were noted at the time of harvest.
2.2 Measurement of Morphological and Biomass Attributes
All of the harvested plants were washed thrice with distilled water following dry on blotting paper. The length of the roots and shoots as well as the fresh weight of the roots and shoots, were measured. Leaf Area, No. of leaves, No. of nodules and No. of tendrils were also calculated. Subsequently, plant samples were oven dried at 70 0C for 2 days in order to assess their dry weights (Majeed et al. 2019).
2.3 Estimation of Photosynthetic Pigments
Fresh leaves of plant (0.5 g) were taken right after harvest and were cut into small fragments of 0.5 cm. In pestle and mortar, 10 ml of 80% acetone was added for the purpose of extraction. A test tube was taken to take some amount of extract and covered with aluminum foil. Then aliquots from the extract in test tube (3 mm) were shifted to cuvettes. Then supernatant was observed at 480 nm, 645 nm and 663 nm with the help of spectrophotometer (Shimadzu UV-1800).The amount of chl “a”, chl “b” and total chl was estimated by Arnon (1949) formula and carotenoid content was calculated according to Davies (1965).
2.4 Determination of Gas Exchange Parameters
Gas exchange characteristics i.e. stomatal conductance (Gs), transpiration rate (E) and net photosynthesis (A) were estimated during the day (10:00—11:00 a.m.) at 26˚C from the uppermost fully stretched leaves with the help a portable infra-red gas analyser LCA-4 system(ADC, Ltd)(Sardar et al. 2022a, b).
2.5 Assessment of Chromium Uptake
Pea leaves were cleaned and dried at 65˚C for two days in drying oven (Wiseven, Model WOF-105, Korea). Plant sample was powdered in pestle and mortar so that it can pass through 60 mesh screen. 0.5 g of sample was taken then 5 ml of 70% HNO3 and 1.5 ml of 60% HClO4 were added to it. This mixture was heated until the brown fumes disappeared. Then it was cooled and 5 ml of diluted (1:1) HCl was added. This digested material was filtered through whatman filter paper. Its volume was made up to 25 ml by adding distilled water (Moseley and Jones 1984). The concentration of Cr was assessed using Atomic Absorption Spectrometer (XPLOR AA-Dual) (Chapman and Dale 1976). Calculations were done according to standard curve of Cr. For the estimation of accumulation coefficient (AC), formula given by Al-Farraj et al. (2009) was used.
For approximation of metal tolerance index (MTI), the equation suggested by Balint et al. (2007) was applied:
2.6 Estimation of Mineral Content
To make an approximation of mineral content in pea plant, same extract was taken that was used for assessment of Cr content. The absorbance for Na and K was found through flame photometer (Model 410, Corning) (Sagner et al. 1998). The absorbance of Zn and Mg was observed using Atomic Absorption Spectrometer (XPLOR AA-Dual) (Chapman and Dale 1976). Concentration of these minerals were calculated through their standard curves.
2.7 Approximation of Proline and MDA Content
Prewashed ice chilled plant sample (0.25 g) was completely vortexed with 10 ml of sulfosalicylic acid (3%). Whatman filter paper was used to filter the mixture. 2 ml of this filtrate, 2 ml of glacial acetic acid and 2 ml of acid-ninhydrin (2.8 g Ninhydrin, 48.16 ml of 85% Phosphoric Acid, 72.8 ml of Acetic acid) was added in to it. This mixture was heated on 100˚C for 1 h on water bath (N.S Engineering concern XMTG-9000). After ice cooling, toluene (4 ml) was added and was kept at 25˚C for half an hour. Absorbance was taken at 520 nm (Bates et al. 1973). Proline concentration was found (µg/ml) from L-Proline standard curve and calculated on the bases of fresh mass by using following formula:
Melondialdehyde (MDA) content was determined by adopting methodology of Heath and Packer (1968).
2.8 Assessment of Soluble Protein
Protocol by Peterson et al. (1977) was used to estimate protein in pea plant. 1 g of plant sample was crushed with 2 ml of 1N phosphate buffer (17 g K2HPO4 in 1000 ml of distilled water) in pestle and mortar. After that it was centrifuged at 6000 rpm for 15 min. Supernatant (0.4 ml) was taken then 2 ml folin mixture (as used by Peterson et al. 1977) was added into it and left for 15 min. Then 0.5 ml Folin’s Reagent was added to each sample and shake well and kept at room temperature for 45 min. Absorbance was noticed at 750 nm by using spectrophotometer (Shimadzu UV-1800) and soluble protein content was approximated by using BSA (Bovine Serum Albumin) standard curve.
2.9 Determination of 2, 2- Diphenyl-1-picrylhydrazyl Free Radical Scavenging Activity
To determine DPPH free radical scavenging activity method described by Chen et al. (2008) was used. The ability of pea plant to remove free radical was estimated. To prepare methanolic extract, plant sample (1 g) was taken and 10 ml of methanol was added into it. From this methanolic extract, 1 ml was taken and completely mixed with 5 ml of freshly prepared 0.1 mM DPPH and placed in dark for 1 h. The absorbance was recorded at 517 nm by using spectrophotometer (Shimadzu, UV-1800). The blank was prepared by adding 1 ml of methanol in 5 ml of 0.1 mM DPPH solution. The free radical scavenging activity was evaluated with the help of following formula:
2.10 Determination of Phenolic Content
To quantify phenolic content, 2 g fresh leaf of the plant sample were cut into little pieces and extracted in 20 ml of 80% aqueous methanol at 65˚C for 15 min. 0.25 ml of 1N Folin–Ciocalteau reagent along with 5 ml of distilled water were added in 1 ml of previously obtained extract and stored at 30˚C. After this mixture had developed blue colour, the absorbance was observed at 725 nm. This absorbance was compared with the standard curve of gallic acid to estimate the quantity of phenolic content (Zieslin and Ben Zaken 1993).
3 Statistical Analysis
All obtained data were analyzed by applying two-way ANOVA, through software (IBM-SPSS Statistics Version 20). For splitting of mean values for significant treatment, Duncan’s multiple range test was applied at p ≤ 0.05. The specified values are average of 4 replicates ± SE. To assess relationships between the numerous studied variables, Pearson's correlation analysis was used. Rstudio software was used to calculate the principal component analysis (PCA) and Pearson correlation coefficients among the observed variables in P. sativum.
4 Results
4.1 Measurements of Morphological Attributes
Different morphological parameters of P. sativum were checked under Cr stress. Morphological growth was suppressed under Cr stress, whereas significantly higher growth was observed in glutathione treated plants. Table 1 demonstrates the influence of Cr stress and GSH priming on leaf area, number of leaves, tendrils, and root nodules, in addition to root length and shoot length. Pea plants under Cr stress exhibited 27.4%, 44.1%, 42%, and 50% decreases in shoot length, root length, leaf area, and number of nodules, respectively, as compared to control treatment. On the other hand, pea seeds primed with GSH2 under Cr stress developed seedlings with enhanced plant shoot length, root length, leaf area, and number of nodules by 10.2%, 17.11%, 8.4%, and 50%, respectively, as compared to Cr only treatment (Table 1; p ≤ 0.001).
4.2 Measurements of Biomass Attributes
Shoot and root fresh weight displayed a significant decline in plants grown in Cr-spiked soil as compared to control (Table 2; p ≤ 0.01).Plants developed from GSH primed seeds exhibited considerably more biomass than control. Table 2 shows that the shoot fresh weight of plants grown in Cr-spiked soil is 1.34 g, which is 60.5% less than that of control. The shoot fresh weight of plants grown from GSH2 primed seeds under Cr stress was 20% higher as compared to Cr alone treatment. The root fresh weight of Cr-treated plants is 0.24 g, producing 41.6% less biomass as compared to control. On the other hand, plants primed with GSH2 with and without Cr stress produced 16.2% and 59.5% more root fresh weight than Cr only treatment and control treatment respectively. Shoot dry weight (61.7%) and root dry weight (71%) also declined in Cr treated plants as compared to controls. A significant rise in shoot dry weight (68%) and root dry weight (11%) was observed in GSH2 primed seeds as compared to control treatment.
4.3 Estimation of Photosynthetic Pigments
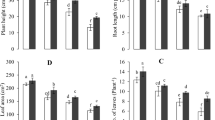
Cr remarkably impeded the synthesis of photosynthetic pigments in pea plants over control treatment (Figs. 1a, b, c and d);p ≤ 0.01).The GSH1 and GSH3 treatment under Cr stress had no definite effect on total photosynthetic content in comparison to Cr-alone treatment. Cr stress alone impeded chlorophyll a (32%), chlorophyll b (20.3%), total chlorophyll (30.8%), and carotenoids (35.3%) content compared to control conditions. Nevertheless, it was observed that treatment with GSH2 + Cr had a noticeable impact on the relief of the Cr-induced decline of chlorophyll content. The GSH2 + Cr treatment increased the content of Chl a (65%), Chl b (59.7%), total Chl (64%) and carotenoids (60.8%), compared to the Cr-only treatment.
Effect of glutathione (GSH) and chromium (Cr) on chl a (a), chl b (b), total chl (c), and carotenoid content (d) of P. sativum. The data is represented as a means ± SE of 4 replicates. Non-identical letters indicate significant difference between the treatments at P ≤ 0.01. C = Control, Cr = 100 mg kg− 1 Cr, GSH1 = 25 μmol L− 1 GSH, GSH2 = 50 μmol L− 1 GSH, GSH3 = 75 μmol L− 1 GSH
4.4 Determination of the Gas Exchange Parameters
The results disclosed a significant decline in gas exchange characteristics of pea plants under Cr regimes in comparison to control (Figs. 2a, b and c; p ≤ 0.001). Cr treatment reduced stomatal conductance (Gs), net photosynthesis (A) and transpiration rate (E) by 26.5%, 29.4% and 31.3%, respectively, when compared to the control. Additionally, when GSH was applied to the plants that had been exposed to Cr, the negative impacts of Cr were diminished, and the studied gas exchange attributes improved (Figs. 2a, b and c). The GSH2 application to plants grown in non-contaminated conditions increased Gs, A and E by 51.9%, 64.8%, and 33.33%, respectively, over control conditions.
Effect of glutathione (GSH) and chromium (Cr) on the stomatal conductance (a), photosynthetic rate (b), and rate of transpiration (c) of P. sativum. The data is represented as a means ± SE of 4 replicates. Non-identical letters display remarkable variance amongst the treatments at P ≤ 0.001. C = Control, Cr = 100 mg kg− 1 Cr, GSH1 = 25 μmol L− 1 GSH, GSH2 = 50 μmol L− 1 GSH, GSH3 = 75 μmol L− 1 GSH
4.5 Assessment of Chromium Uptake
The evaluation of Cr content in pea plants demonstrated that plants raised in Cr-spiked soil displayed 0.139 mg Cr g−1 DW in plant shoot. Both GSH1 and GSH2 proved to be effective in hindering the Cr (VI) uptake in plants grown in Cr-spiked soil. However, GSH2 had the most pronounced effect in reducing the Cr concentration in Cr-treated plants (Table 3; p ≤ 0.05).
4.6 The Metal Tolerance Index and Accumulation Coefficient
The AC value in the plants was relatively high in the Cr-alone treatment (Table 3; p ≤ 0.05). However, plants raised from GSH2 treated seeds under Cr stress had significantly improved MTI value, by 4.7 fold as compared to that of the Cr-only treatment. The AC value of plants grown from seeds treated with GSH2 under Cr toxicity decreased by 21.6% compared to plants treated with Cr.GSH3 also slightly reduced the AC value by 10.2%.
4.7 Assessment of Mineral Contents
It can be inferred from the data that Mg, Zn, K, and Na content was increased in GSH2 by 48.9%, 92%, 20.7%, and 26.8%, respectively, in comparison to control. A minimum nutrient content was found in Cr-affected plants not treated with GSH. GSH3 negligibly enhanced Mg and Zn content in comparison to GSH1 under Cr toxicity. Compared to GSH3, GSH1 had lesser enhancing effect on the amount of K and Na in plants that had been affected by Cr (Table 4; p ≤ 0.01).
4.8 Determination of Proline Content
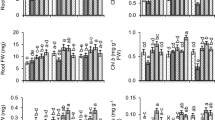
Plants affected by Cr toxicity exhibited, raised level of proline in comparison to control. The proline level in plants exposed to Cr toxicity was 64% higher than in control plants.GSH1, GSH2 and GSH3 applications to plants developed in non-contaminated conditions raised the level of proline by 71%, 85% and 64.4%, respectively, over control conditions. In comparison to GSH1 and GSH3, GSH2 priming significantly increased proline content in Cr stressed plants. Plants treated with GSH2 under Cr stress showed 47.8% increased levels of proline as compared to the Cr-only treatment (Fig. 3a; p ≤ 0.001).
Effect of glutathione (GSH) and chromium (Cr) on proline content (a), soluble protein (b), DPPH free radical scavenging activity (c) and phenolic content (d) of P. sativum. The data is represented as a means ± SE of 4 replicates. Non-identical letters indicate significant difference between the treatments at p ≤ 0.001. C = Control, Cr = 100 mg kg− 1 Cr, GSH1 = 25 μmol L− 1 GSH, GSH2 = 50 μmol L− 1 GSH, GSH3 = 75 μmol L− 1 GSH
4.9 Assessment of Soluble Protein
Phytotoxicity caused by Cr decreased the amount of protein in plants under the Cr-regime compared to the control condition. Cr reduced 26.5% of the protein content in pea plants as compared to control (Fig. 3b; p ≤ 0.001). The stress-alleviating capability of GSHs raised protein content in control and Cr-treated plants as well. Seeds treated with GSH1 and GSH2 with Cr-spiked soil magnified the protein content by 37.7% and 40.8% relative to Cr-only treatment. Likewise, GSH3 supplementation to Cr-spiked plants showed a 32.12% increase in protein content as compared to Cr sole treatment.
4.10 DPPH Free Radical Scavenging Activity
Plants affected by Cr stress exhibited a reduced level of free radical scavenging activity in comparison to control. Cr Stress reduced this activity by 31.1% in plants grown in Cr-spiked soil compared to control plants (Fig. 3c; p ≤ 0.001). GSH2 priming enhanced it by 63.5% relative to control. GSH1 and GSH2 treated plants grown in Cr-affected soil enhanced the DPPH free radical scavenging activity by 63.7% and 89%, respectively, as compared to Cr-sole treatment.
4.11 Determination of Phenolic Content
Cr prompted phytotoxicity and decreased phenolic content in plants under the Cr effect as compared to the control condition. The total phenolic content of seedlings decreased by 22.2% under the impact of Cr as compared to control. Maximum phenolic content (0.493 mg g−1 FW) was noticed in the case of GSH2 sole treatment. Under Cr toxicity, GSH2 pre-treated plants had 44.4% greater phenolic content relative to the Cr alone treatment. In contrast to Cr-sole treatment, GSH1 under Cr stress also increased the amount of phenolic by 25.12% (Fig. 3d; p ≤ 0.001).Cr stress leads to a significant increase in lipid peroxidation (MDA) of pea seedlings (Table 3; p ≤ 0.05).Seed primed with GSH reduced MDA content under normal and Cr stress conditions. However, application of GSH2 decreased MDA under normal and Cr stress conditions (Fig. 4).
4.12 Correlation between Various Growth and Physio-biochemical Attributes with Cr Uptake and Accumulation
A Pearson’s correlation reckoned a relationship with the growth and physio-biochemical attributes of P. sativum grown in Cr-contaminated soil with exogenously applied glutathione (Fig. 5; p ≤ 0.01).The Cr concentration in the shoot was positively correlated with Cr accumulation coefficient in the shoot and proline content in pea plants. GSH1, GSH2 and GSH3 application is negatively correlated with Cr accumulation coefficient and positively correlated with proline content. On the other hand, Cr concentration in the shoot was negatively correlated with the metal tolerance index, potassium, magnesium, sodium, zinc, root dry weight, total phenol content, stomatal conductance, leaf area, and germination percentage, total chlorophyll, transpiration rate, shoot dry weight, photosynthetic rate, number of tendrils, soluble protein content, root fresh weight, DPPH free radical scavenging activity, shoot fresh weight, root length, carotenoids, number of leaves, and number of root nodules. GSH2 application is positively correlated to metal tolerance index, potassium, magnesium, sodium, zinc, root dry weight, total phenol content, stomatal conductance, leaf area, germination percentage, total chlorophyll, transpiration rate, shoot dry weight, photosynthetic rate, number of tendrils, soluble protein content, root fresh weight, DPPH free radical scavenging activity, shoot fresh weight, root length, carotenoids, number of leaves, and number of root nodules. This relationship expressed a close relationship between growth and Cr uptake in P. sativum.
Correlation between growth and physio-biochemical attributes of P. sativum grown in chromium (Cr) contaminated soil with exogenously applied glutathione. Different abbreviations used in the figure are as follows: Pro (proline content in pea plants), MTI (metal tolerance index), Cr (chromium concentration in shoot), AC (accumulation coefficient), K (potassium), Mg (magnesium), Na (sodium), Zn (zinc), RDW (root dry weight), Phe (total phenol content), SC (stomatal conductance), LA (leaf area), GP (germination percentage), SL (shoot length), TC (total chlorophyll), T C (transpiration rate), SDW (shoot dry weight), PR (photosynthetic rate), NOT (number of tendrils), SP (soluble protein content), RFW (root fresh weight), DPPH (DPPH free radical scavenging activity), SFW (shoot fresh weight), RL (root length), Car (carotenoids), NOL (number of leaves),NRN (number of root nodules). This corelation expressed a close relation between growth and Cr uptake in P. sativum
4.13 Principal Component Analysis
P. sativum cultivated on Cr-contaminated soil with exogenously supplied glutathione was shown to have a connection between growth and physio-biochemical characteristics using loading plots of principal component analysis (Fig. 6; p ≤ 0.01).The first two primary components, Dim1 and Dim2, make up the largest percentage of all components and account for more than 91.2% of the entire database.
Loading plots of principal component analysis demonstrating a connection betweengrowth and physio-biochemical attributes of P. sativum grown in chromium contaminated soil with exogenously applied glutathione. Different abbreviations used in the figure are as follows: Pro (proline content in pea plants), MTI (metal tolerance index), Cr (chromium concentration in shoot), AC (accumulation coefficient), K (potassium), Mg (magnesium), Na (sodium), Zn (zinc), RDW (root dry weight), Phe (total phenol content), SC (stomatal conductance), LA (leaf area), GP (germination percentage), SL (shoot length), TC (total chlorophyll), T C (transpiration rate), SDW (shoot dry weight), PR (photosynthetic rate), NOT (number of tendrils), SP (soluble protein content), RFW (root fresh weight), DPPH (DPPH free radical scavenging activity), SFW (shoot fresh weight), RL (root length), Car (carotenoids), NOL (number of leaves), NRN (number of root nodules)
Dim1 makes up 77.1% of this dataset, whereas Dim2 makes up 14.1% of it. The first set of variables; PC1 has a positive correlation with potassium, magnesium, sodium, zinc, root dry weight, total phenol content, stomatal conductance, leaf area, germination percentage, shoot length, total chlorophyll, transpiration rate, shoot dry weight, photosynthetic rate, number of tendrils, soluble protein content, root fresh weight, DPPH free radical scavenging activity, shoot fresh weight, root length, carotenoids, number of leaves, Number of root nodules. The factors related to proline content, metal tolerance index, Cr concentration in shoots, and accumulation coefficient were found to have a strong negative relationship with PC1 variables.
5 Discussion
Heavy metals are the toxic bioavailable elements in the rhizosphere, causing serious harms to plants. After their successful entrance into the plant system, the metal ions bind to the sulfhydryl group of cellular proteins and misplace the vital cations from the binding sites of enzymes, making them inactive, which consequently disrupts the cellular processes. Chromium being redox metal, activates reactive oxygen species (ROS) formation, that damages the membrane and disturbs the redox homeostasis balance of plant cell, subsequently causing oxidative harms to lipids, nucleic acids and proteins (Adhikari et al. 2018). Cr being toxic in soil is a serious threat to the existence of mankind, plants and animals on this planet (Askari et al. 2021). The current study explores the chief responses of pea plants to Cr vulnerability consisted of a remarkable decline in biomass (fresh and dry weight basis), with a massive assemblage of Cr in seedlings. In maize, a similar elevated level of Cr was reported that reduced fresh and dry weight of shoot and root (Adhikari et al. 2020). Cr-induced toxicity hinders plant growth (Jabeen et al. 2015) and biomass production (Zhao et al. 2019), similar to our present findings. During current study, a strong negative correlation of Cr with shoot length, root length, number of leaves, leaf area, fresh and dry weight of shoot and root was observed (Fig. 5). Ahmed et al. (2020) reported that Cr may have made it harder for the plants to take in essential nutrients. Decreased nutrient uptake leads to a decline in growth attributes (shoot length, root length, number of leaves, leaf area) and biomass. The reduction in root length in plants grown under the Cr regime was because of the assemblage of Cr in root cells that damaged the cellular structure of root (Ali et al. 2013). The eventual decrease in shoot length is due to Cr-instigated impairment of mesophyll cells (Gillet al. 2015). However, application of glutathione (GSH) enhanced plant morphological growth and biomass as well by mitigating Cr stress through Cr sequestration. Earlier researchers have also reported growth improvement because of GSH supplementation in cotton because of raised amount of DNA and RNA (Khan et al. 2016).The GSH level in nucleus increases and add more no. of nucleotide that maintain a proper redox environment to synthesize or repair DNA (Garcia-Gimenez et al. 2013). Evidence shows that meristematic cells proliferate and differentiate at a greater rate after getting stimulation from exogenously applied GSH, leading to an increase in shoot length, root length, and leaf length, and consequently leaf area (Pasternak et al. 2014).
Pigment content and rate of photosynthesis significantly reduced under Cr stress. Cr primarily affects the structure of plastids, electron transport chain and photosynthetic phosphorylation and CO2 fixation (Ma et al. 2016).Some ultrastructural alterations occur in chloroplasts that caused lower photosynthetic pigment formation (Gill et al. 2015). Unbalanced ROS formation under Cr toxicity caused these ultrastructural changes in structure of chloroplast (Ahmad et al. 2019). Cr inhibits biosynthesis of chlorophyll or may interrupt the normal functioning of enzymes that degrade chlorophyll. Cr-reduced changes in the volume and auto-fluorescence of chloroplasts, altered thylakoid arrangements, chloroplast membrane deformation, and negatively affected light/dark reactions (Shahid et al. 2017), consequently declining rate of photosynthesis. Exogenously applied GSH enhanced chlorophyll content and uptake of different mineral nutrients raised along with decreased uptake of heavy metal (Ur Rehman et al. 2021). Treatment with GSH encouraged the maintenance of more plastids and mitochondria and improved their ultrastructure. Plastids exhibit well-developed thylakoid membrane characteristics, parallel lamellar patterns, and more unfolded starch grains. Additionally, GSH maintains well-structured mitochondrial cristae and homogeneous chromatin distribution in nuclei (Ahmed et al 2023), and assist plant to actively produce chlorophyll content.
Plants developed in the Cr-regime reduced gas exchange parameters in Brassica napus (Gill et al. 2015) and in Brassica oleracea (Ahmed et al. 2020), similar to our recent findings. Cr uptake and its assemblage in plants have also been reported in wheat (Ali et al. 2015), that renders plants incapable of assimilating other vital nutrients, disturbs normal growth pattern, development, ultra-cellular configuration and eventually disturbs transpiration (Maqbool et al. 2015), photosynthesis (Afshan et al. 2015), and causes chlorosis (Gill et al. 2014). Our findings are parallel with the results of Farid et al. (2018) that showed that Cr toxicity adversely affects the growth of sunflower. Because of the extremely high generation of ROS caused by Cr toxicity, cellular proteins and plasma membranes may be damaged (Kaur and Zhawar 2017). This damage can be repaired by glutathione that eradicates excessive ROS (Wen et al. 2022). This damage repair plays a role in preventing Cr entry and improving nutrient uptake (Ahmad et al. 2019). That’s why Cr uptake is reduced because it has to compete with other essential nutrients. GSH plays its role in avoiding oxidative damage to photosynthetic apparatus in Brassica oleracea (Ahmed et al. 2020), and is capable of improving photosynthetic rate. Changes in guard cell aperture or stomatal opening due to GSH supplementation in Cr-affected plants have improved stomatal conductance, rate of transpiration, and net photosynthesis.
The reduced mineral content under Cr stress is the consequence of increased Cr build-up, which pushes mineral nutrients away from their binding sites and consequently restricts their absorption (Gupta et al. 2019). Decreased K+ level is linked to xylem blockage and a reduction in plant root growth (Javed et al. 2021). As a result, lower K+ levels found in pea plants in the current study caused stomatal dysfunction, inadequate stomatal control of water loss, and decreased photosynthesis, which impaired pea plants' ability to thrive in the presence of Cr toxicity. Mg2+ plays a role in the production of chlorophyll and is a crucial component of the chloroplast's normal structure (Hafeez et al. 2022). Yan and Hou (2018) studied that Mg2+ encourages ATP or ADP breakdown, which yields phosphoric acid and energy. Additionally, it stimulates phosphorylation, ATP synthesis, and ATPase activity (Chen et al. 2018). Tobacco roots and shoots under Cr stress had poor Mg2+ uptake, which reduced chlorophyll production (Hayat et al. 2012). This decline in Mg2+ was exhibited primarily in our recent study as poor root development, decreased chlorophyll biosynthesis, and decreased ATP synthesis, resulting in poor growth. According to Sohag et al. (2020), GSH application improved K+ levels in rice seedlings under abiotic stress. In higher K+ levels in pea plants led to better stomatal functionality, proper stomatal control of water loss, and improved photosynthesis, which enhanced pea plants' ability to thrive in the presence of Cr. The regulation of genes governing nutrient absorption and transport increased the level of important nutrient ions in Medicago scutellata under drought stress after GSH treatment (Gheshlaghi et al. 2020). It seems that complexes of GSH and Cr may produce and transported by certain transporters into insensitive organelles, such as heavy-metal ATPase (HMA), natural resistance-associated macrophage protein (NRAMP), and ATP binding Cassette (ABC) transporter protein. They have become immobilized in the root cell wall (Zeng et al. 2012a), which eventually lead to decreased Cr content in the shoot and reduced Cr accumulation coefficient as well.
Proline is a stress-associated metabolite that acts as an indicator of oxidative stress (Bashir et al. 2020). In both biotic and abiotic stress, proline act as an effective antioxidant and interact with other signaling compounds to maintain metabolism in cell. In normal conditions, proline makes up less than 5% of total free amino acids in plants, whereas in stressed plants its concentration increases to up to 80% of the total amino acid pool (Meena et al. 2019). This high assemblage of proline in plants exposed to hexavalent Cr is a strategic adaptation by the plants to survive oxidative damage. We observed a strong positive correlation of Cr concentration in shoot with proline content (Fig. 6). This outcome is in line with other findings (Adhikari et al. 2020) that reported that endogenous levels of free proline rise when plants are exposed to Cr than their threshold level to cope up with the oxidative stress by scavenging the ROS. Nevertheless, in our present study, exogenous GSH application to Cr spiked plants raised endogenous proline levels in comparison to the Cr treated seedlings without GSH. It is because GSH being a significant non-enzymatic phytohormone, improves the activity of other antioxidants (Hasanuzzaman et al. 2017). Proline being a non-enzymatic antioxidant, contributes in maintaining subcellular structures (such as membranes and proteins), scavenging free radicals, and buffering cellular redox potential during stressful circumstances in addition to serving as an osmolyte for osmotic adjustment (Ashraf and Foolad 2007).
Decreased protein level in pea seedlings under Cr toxicity in current investigation are in accordance with previous work demonstrating that Cr toxicity impedes the protein content (Ashraf et al. 2022). This reduction in protein content is due to decreased protein synthesis or a heightened degree of protein degradation, or both could be the reason during abiotic stress (Bashir et al. 2020). We found a negative correlation of Cr concentration with protein content (Fig. 6).Whereas it was observed that protein formation was enhanced after GSH application. Glutathione controls gene expression and positively takes part in signal transduction (Li et al. 2021). This improved signal transduction is attributed to protein formation and function, providing solid evidence of improved protein synthesis in the presence of GSH. The rate of translation in plants increases after treating plants with GSH (Cheng et al. 2015). These translational alterations due to GSH supplementation target various hormones and stress-signaling compounds, which is a major contributor to improve the stress tolerance capability of plants.
The DPPH free radical scavenging activity is a key to check the antioxidant potential. Our results are in line with the results of Osama et al. (2019), who displayed a considerable reduction in DPPH activity on exposure to Cr toxicity in red beans. This decline is due to the overproduction of free radicals. Plants produce different secondary metabolites during stress that can perform as antioxidants (Baslam et al. 2013), but these antioxidants are not incredibly effective at quenching high levels of free radicals. Qureshi et al. (2020) reported heightened antioxidant potential in Ricinus communis in Cr-contamination after exogenous antioxidant application. This efficiently high DPPH activity is because of the high phenolic content, since phenols have the ability to get rid of free radicals by giving up an electron (Rahman et al. 2015).It was formerly found that total phenolic compounds in tomatoes and grape leaves also decreased significantly under Cr (Gupta et al. 2020), support our present findings (Fig. 3). The reduction in the total phenolic content in our study is due to the decline in specific phenolic compounds in the leaves of pea plants subjected to Cr metal. This reduction is also because of changes in secondary metabolites that are dependent upon soil (growth medium) and environmental conditions. Wen et al. (2022) reported a slight increase in phenolic content after GSH supplementation similar to our current outcomes. Phenolic are antioxidants that can chelate metal ions and reduce the noxious impact of metal ions (Ahmed et al. 2023). Thus, antioxidant potential in GSH primed Pea seedlings improved through increased DPPH activity and Phenolic content, assuaged Cr toxicity and enhanced growth.
6 Conclusion
Chromium toxicity significantly decreased pea growth, biomass production, gas exchange characteristics, photosynthetic pigments, and protein content by inflicting oxidative damage and reduced uptake of nutrients uptake. Seed priming of pea seeds with glutathione successfully alleviated harmful impacts of chromium stress and resulted in improved growth. Through enhanced gas exchange characteristics, reduced chromium accumulation in plant shoots, adjustment of the soluble phenol and proline for osmotic pressure regulation, and reducing oxidative damage induced by chromium toxicity, glutathione supplementation lowers Cr translocation and toxicity in peas. Exogenous 50 µmolL−1 GSH supplementation was ultimately found to be the most effective treatment for reducing chromium toxicity. As a priming agent, glutathione can assist farmers in better establishing peas in challenging environmental circumstances to reduce world hunger by producing less expensive protein for people. It is recommended that more study be done on the use of glutathione in field situations to better understand the chromium stress tolerance mechanism. In the future, other significant agronomic and horticultural crops may be grown by using glutathione priming as a stress mitigation strategy under abiotic stress conditions.
References
Adhikari A, Adhikari S, Ghosh S, Azahar I, Shaw AK, Roy D, Hossain Z (2020) Imbalance of redox homeostasis and antioxidant defense status in maize under Cr (VI) stress. Environ Exp Bot 169:103873. https://doi.org/10.1016/2Fjenvexpbot.2019.10387
Adhikari S, Ghosh S, Azahar I, Adhikari A, Shaw AK, Konar S, Hossain Z (2018) Sulfate improves cadmium tolerance by limiting cadmium accumulation, modulation of sulfur metabolism and antioxidant defense system in maize. Environ Exp Bot 153:143–162. https://doi.org/10.1016/2Fj.envexpbot.2018.05.008
Afshan S, Ali S, Bharwana SA, Rizwan M, Farid M, Abbas F, Ibrahim M, Mehmood MA, Abbasi GH (2015) Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ Sci Pollut Res 22:11679–11689. https://doi.org/10.1007/2Fs11356-015-4396-8
Afzal M, Shabir G, Iqbal S, Mustafa T, Khan QM, Khalid ZM (2014) Assessment of heavy metal contamination in soil and groundwater at leather industrial area of Kasur, Pakistan. Soil Air Water 42:1133–1139. https://doi.org/10.1007/2Fs11356-015-4396-8
Ahmad R, Ali S, Rizwan M, Dawood M, Farid M, Hussain A, Wijaya L, Alyemeni MN, Ahmad P (2019) Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol Plant. https://doi.org/10.1111/2Fppl.13001
Ahmed KBS, Aoues A, Kharoubi O, Hetraf I (2020) Lead-induced changes in germination behavior, growth and inhibition of-aminolevulinic acid dehydratase activity in Raphanus sativus L. African J Plant Sci 14:254–261. https://doi.org/10.5897/2Fajps2019.1899
Ahmed S, Khan M, Sardar R (2023) Glutathione primed seed improved lead-stress tolerance in Brassica rapa L. through modulation of physio-biochemical attributes and nutrient uptake. Inter J Phytoremediation 1–11. https://doi.org/10.1080/2F15226514.2023.2178380
Al-Farraj AS, Al-Otabi TG, Al-Wabel MI (2009) Accumulation coefficient and translocation factor of heavy metals through Ochradenus baccatus plant grown on mining area at Mahad AD’Dahab, Saudi Arabia. WIT Trans Ecol Environ 122:459–468. https://doi.org/10.2495/eco090421
Al-Huqail AA, Ali HM, Kushwaha BK, Al-Huqail AA, Singh VP, Siddiqui MH (2020) Ascorbic acid is essential for inducing chromium (VI) toxicity tolerance in tomato roots. J Biotech 322:66–73. https://doi.org/10.1016/j.jbiotec.2020.07.011
Ali S, Chaudhary A, Rizwan M, Anwar HT, Adrees M, Farid M, Irshad MK, Hayat T, Anjum SA (2015) Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ Sci Pollut Res 22:10669–10678. https://doi.org/10.1007/s11356-015-4193-4
Ali S, Farooq MA, Hussain S, Yasmeen T, Abbasi GH, Zhang G (2013) Alleviation of chromium toxicity by hydrogen sulfide in barley. Environ Toxicol Chem 32:2234–2239. https://doi.org/10.1002/etc.2309
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1. https://doi.org/10.1104/pp.24.1.1
Ashraf MFMR, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216. https://doi.org/10.1016/j.envexpbot.2005.12.006
Asgher M, Per TS, Verma S, Pandith SA, Masood A, Khan NA (2018) Ethylene supplementation increases PSII efficiency and alleviates chromium-inhibited photosynthesis through increased nitrogen and sulfur assimilation in mustard. J Plant Growth Regul 37:1300–1317
Ashraf MA, Rasheed R, Hussain I, Iqbal M, Farooq MU, Saleem MH, Ali S (2022) Taurine modulates dynamics of oxidative defence, secondary metabolism, and nutrient relation to mitigate boron and chromium toxicity in Triticum aestivum L. plants. Environ Sci Pollut Res 1–22. https://doi.org/10.1007/s11356-022-19066-5
Askari SH, Ashraf MA, Ali S, Rizwan M, Rasheed R (2021) Menadione sodium bisulfite alleviated chromium effects on wheat by regulating oxidative defense, chromium speciation, and ion homeostasis. Environ Sci Pollu Res 28:36205–36225. https://doi.org/10.1007/s11356-021-13221-0
Balint AF, Röder MS, Hell R, Galiba G, Börner A (2007) Mapping of QTLs affecting copper tolerance and the Cu, Fe, Mn and Zn contents in the shoots of wheat seedlings. Biol Plant 51:129–134. https://doi.org/10.1007/s10535-007-0025-9
Bamagoos AA, Mallhi ZI, El-Esawi MA, Rizwan M, Ahmad A, Hussain A, Ali S (2022) Alleviating lead-induced phytotoxicity and enhancing the phytoremediation of castor bean (Ricinus communis L.) by glutathione application: new insights into the mechanisms regulating antioxidants, gas exchange and lead uptake. Int J Phytore 24:933–944. https://doi.org/10.1080/15226514.2021.1985959
Bashir MA, Naveed M, Ahmad Z, Gao B, Mustafa A, Núñez-Delgado A (2020) Combined application of biochar and sulfur regulated growth, physiological, antioxidant responses and Cr removal capacity of maize (Zea mays L.) in tannery polluted soils. J Environ Manage 259:110051. https://doi.org/10.1016/j.jenvman.2019.110051
Baslam M, Garmendia I, Goicoechea N (2013) Enhanced accumulation of vitamins, nutraceuticals and minerals in lettuces associated with arbuscular mycorrhizal fungi (AMF): a question of interest for both vegetables and humans. Agriculture 3:188–209. https://doi.org/10.3390/agriculture3010188
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/bf00018060
Bücker-Neto L, Paiva ALS, Machado RD, Arenhart RA, Margis-Pinheiro M (2017) Interactions between plant hormones and heavy metals responses. Genet Mol Bio 40:373–386. https://doi.org/10.1590/1678-4685-gmb-2016-0087
Cao F, Cai Y, Liu L, Zhang M, He X, Zhang G, Wu F (2015) Differences in photosynthesis, yield and grain cadmium accumulation as affected by exogenous cadmium and glutathione in the two rice genotypes. Plant Growth Regul 75:715–723. https://doi.org/10.1007/s10725-014-9973-1
Chapman JF, Dale LS (1976) The determination of lithium isotope abundances with a dual-beam atomic absorption spectrometer. Anal Chim Acta 87:91–95. https://doi.org/10.1016/s0003-2670(01)83123-8
Chen IN, Chang CC, Ng CC, Wang CY, Shyu YT, Chang TL (2008) Antioxidant and antimicrobial activity of Zingiberaceae plants in Taiwan. Plant Food Hum Nutr 63:15–20. https://doi.org/10.1007/s11130-007-0063-7
Chen ZC, Peng WT, Li J, Liao H (2018) Functional dissection and transport mechanism of magnesium in plants. Semin Cell Dev Biol 74:142–152. https://doi.org/10.1016/j.semcdb.2017.08.005
Cheng MC, Ko K, Chang WL, Kuo WC, Chen GH, Lin TP (2015) Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. Plant J 83:926–939. https://doi.org/10.1111/tpj.12940
Cheng L, Pu L, Li A, Zhu X, Zhao P, Xu X, Chen J (2022) Implication of exogenous abscisic acid (ABA) application on phytoremediation: plants grown in co-contaminated soil. Environ Sci Pollut Res 29:8684–8693. https://doi.org/10.1007/s11356-021-16241-y
Colzi I, Gonnelli C, Vergata C, Golia G, Coppi A, Castellani MB, Martinelli F (2023) Transgenerational effects of chromium stress at the phenotypic and molecular level in Arabidopsis thaliana. J Hazard Mater 442:130092. https://doi.org/10.2139/ssrn.4043454
Davies B H (1965) Analysis of carotenoid pigments. In Goodwin TW (ed.) Chemistry and biochemistry of plant pigments Academic Press 489–532. https://doi.org/10.1016/b978-1-4832-3243-0.50028-9
Dhalaria R, Kumar D, Kumar H, Nepovimova E, Kuča K, Torequl Islam M, Verma R (2020) Arbuscular mycorrhizal fungi as potential agents in ameliorating heavy metal stress in plants. Agronomy 10:815. https://doi.org/10.3390/agronomy10060815
Faisal M, Imran K, Muhammad BC, Rizwan M, Athar M, Rashid WK, Sameer HQ (2022) Exogenously applied Glycine betain alleviates chromium toxicity in pea by reducing CR uptake and improving antioxidant defense system. Appl Eco Environ Res 20: 4295–4307. https://doi.org/10.15666/aeer/2005_42954307
Farid M, Ali S, Rizwan M, Ali Q, Saeed R, Nasir T, Abbasi GH, Rehmani MIA, Ata-Ul-Karim ST, Bukhari SAH, Ahmad T (2018) Phyto-management of chromium contaminated soils through sunflower under exogenously applied 5-aminolevulinic acid. Ecotoxicol Environ Saf 151:255–265. https://doi.org/10.1016/j.ecoenv.2018.01.017
Garcia-Gimenez JL, Markovic J, Dasi F, Queval G, Schnaubelt D, Foyer CH, Pallardo FV (2013) Nuclear glutathione. Biochim Biophys Acta 1830:3304–3316. https://doi.org/10.1016/j.bbagen.2012.10.005
Gill RA, Hu XQ, Ali B, Yang C, Shou JY, Wu YY, Zhou WJ (2014) Genotypic variation of the responses to chromium toxicity in four oilseed rape cultivars. Biol Plant 58:539–550. https://doi.org/10.1007/s10535-014-0430-9
Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Zhou WJ (2015) Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere 120:154–164. https://doi.org/10.1016/j.chemosphere.2014.06.029
Gupta P, Kumar V, Usmani Z, Rani R, Chandra A, Gupta VK (2020) Implications of plant growth promoting Klebsiella sp. CPSB4 and Enterobacter sp. CPSB49 in luxuriant growth of tomato plants under chromium stress. Chemosphere 240:124944. https://doi.org/10.1016/j.chemosphere.2019.124944
Gheshlaghi Z, Khorassani R, Abadía J, Alvarez-Fernández A, Luis-Villarroya A, Fotovat A, Kafi M (2020) Glutathione supplementation prevents iron deficiency in Medicago scutellata grown in rock sand under different levels of bicarbonate. Plant and Soil 446:43–63. https://doi.org/10.1007/s11104-019-04314-4
Gupta P, Kumar V, Usmani Z, Rani R, Chandra A, Gupta VK (2019) A comparative evaluation towards the potential of Klebsiella sp. and Enterobacter sp. in plant growth promotion, oxidative stress tolerance and chromium uptake in Helianthus annuus (L.). J Hazard Mate 377:391–398. https://doi.org/10.1016/j.jhazmat.2019.05.054
Hafeez A, Rasheed R, Ashraf MA, Rizwan M, Ali S (2022) Effects of exogenous taurine on growth, photosynthesis, oxidative stress, antioxidant enzymes and nutrient accumulation by Trifolium alexandrinum plants under manganese stress. Chemosphere 308:136523
Hasan MK, Liu C, Wang F, Ahammed GJ, Zhou J, Xu MX, Yu JQ, Xia XJ (2016) Glutathione-mediated regulation of nitric oxide, S-nitrosothiol and redox homeostasis confers cadmium tolerance by inducing transcription factors and stress response genes in tomato. Chemosphere 161:536–545. https://doi.org/10.1016/j.chemosphere.2016.07.053
Hasanuzzaman M, Nahar K, Fujita M (2017) Glutathione in plants: biosynthesis and physiological role in abiotic stress tolerance. Physiol Mol Biol Plants 23:249–268. https://doi.org/10.1007/s12298-017-0422-2
Hayat S, Khalique G, Irfan M, Wani AS, Tripathi BN, Ahmad A (2012) Physiological changes induced by chromium stress in plants: an overview. Protoplasma 249:599–611. https://doi.org/10.1007/s00709-011-0331-0
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archi. Biochem. Biophy., 125(1), 189–198.
He X, Li P (2020) Surface water pollution in the middle Chinese Loess Plateau with special focus on hexavalent chromium (Cr6+): occurrence, sources and health risks. Expos Health 1–17. https://doi.org/10.1007/s12403-020-00344-x
Jabeen N, Abbas Z, Iqbal M, Rizwan M, Jabbar A, Farid M, Ali S, Ibrahim M, Abbas F (2015) Glycinebetaine mediates chromium tolerance in mung bean through lowering of C uptake and improved antioxidant system. Arch Agron Soil Sci 62:648–662. https://doi.org/10.1080/03650340.2015.1082032
Javed MT, Tanwir K, Abbas S, Saleem MH, Iqbal R, Chaudhary HJ (2021) Chromium retention potential of two contrasting Solanum lycopersicum Mill. cultivars as deciphered by altered pH dynamics, growth, and organic acid exudation under Cr stress. Environ Sci Pollut Res 28:27542–27554. https://doi.org/10.1007/s11356-020-12269-8
Johnson R, Puthur JT (2021) Seed priming as a cost effective technique for developing plants with cross tolerance to salinity stress. Plant Physiol Biochem 162:247–257. https://doi.org/10.1016/j.plaphy.2021.02.034
Kaur R, Zhawar VK (2017) Hydrogen peroxide and nitric oxide regulation of phenolic metabolism under water stress and ABA in wheat. Acta Biol Hung 68:162–174. https://doi.org/10.1556/018.68.2017.2.4
Khan MIR, Chopra P, Chhillar H, Ahanger MA, Hussain SJ, Maheshwari C (2021) Regulatory hubs and strategies for improving heavy metal tolerance in plants: chemical messengers, omics and genetic engineering. Plant Physiol Biochem 164:260–278. https://doi.org/10.1016/j.plaphy.2021.05.006
Khan M, Daud MK, Basharat A, Khan MJ, Azizullah A, Muhammad N, Zhu SJ (2016) Alleviation of lead-induced physiological, metabolic, and ultramorphological changes in leaves of upland cotton through glutathione. Environ Sci Pollut Res 22:8431–8440. https://doi.org/10.1007/s11356-015-5959-4
Kumari V, Yadav A, Haq I, Kumar S, Bharagava RN, Singh SK, Raj A (2016) Genotoxicity evaluation of tannery effluent treated with newly isolated hexavalent chromium reducing Bacillus cereus. J Environ Manage 183:204–211. https://doi.org/10.1016/j.jenvman.2016.08.017
Liu Z, Chen B, Wang LA, Urbanovich O, Nagorskaya L, Li X, Tang L (2020) A review on phytoremediation of mercury contaminated soils. J Hazard Matt 400:123138. https://doi.org/10.1016/j.jhazmat.2020.123138
Li GZ, Chen SJ, Li NY, Wang YY, Kang GZ (2021) Exogenous glutathione alleviates cadmium toxicity in wheat by influencing the absorption and translocation of cadmium. Bull Environ Contam Toxicol 107(2):320–326. https://doi.org/10.1007/s11104-019-04314-4
Ma J, Lv C, Xu M, Chen G, Lv C, Gao Z (2016) Photosynthesis performance, antioxidant enzymes, and ultrastructural analyses of rice seedlings under chromium stress. Environ Sci Pollu Res 23:1768–1778. https://doi.org/10.1007/s11356-015-5439-x
Majeed A, Muhammad Z, Siyar S (2019) Assessment of heavy metal induced stress responses in pea (Pisum sativum L.). Acta Ecol Sin 39(4):284–288. https://doi.org/10.1016/j.chnaes.2018.12.002
Maqbool Z, Asghar HN, Shahzad T, Hussain S, Riaz M, Ali S, Arif MS, Maqsood M (2015) Isolating, screening and applying chromium reducing bacteria to promote growth and yield of okra (Hibiscus esculentus L.) in chromium contaminated soils. Ecotoxicol Environ Saf 114:343–349. https://doi.org/10.1016/j.ecoenv.2014.07.007
Meena M, Divyanshu K, Kumar S, Swapnil P, Zehra A, Shukla V, Upadhyay RS (2019) Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 5:e02952. https://doi.org/10.1016/j.heliyon.2019.e02952
Moseley G, Jones JR (1984) The physical digestion of perennial ryegrass (Lolium perenne) and white clover (Trifolium repens) in the foregut of sheep. British J Nutri 52:381–390. https://doi.org/10.1079/bjn19840104
Nguyen TQ, Sesin V, Kisiala A, Emery RN (2021) Phytohormonal roles in plant responses to heavy metal stress: implications for using macrophytes in phytoremediation of aquatic ecosystems. Environ Toxicol Chem 40:7–22. https://doi.org/10.1002/etc.4909
Osama S, El Sherei M, Al-Mahdy DA, Bishr M, Salama O (2019) Effect of salicylic acid foliar spraying on growth parameters, γ-pyrones, phenolic content and radical scavenging activity of drought stressed Ammi visnaga L. plant. Ind Crops Prod 134:1–10. https://doi.org/10.1016/j.indcrop.2019.03.035
Pasternak T, Asard H, Potters G, Jansen MAK (2014) The thiol compounds glutathione and homoglutathione differentially affect cell development in alfalfa (Medicago sativa L.). Plant Physiol Biochem 74:16–23. https://doi.org/10.1016/j.plaphy.2013.10.028
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356. https://doi.org/10.1016/0003-2697(77)90043-4
Powers SE, Thavarajah D (2019) Checking agriculture’s pulse: field pea (Pisum Sativum L.), sustainability, and phosphorus use efficiency. Front Plant Sci 10:1489. https://doi.org/10.3389/fpls.2019.01489
Qureshi FF, Ashraf MA, Rasheed R, Ali S, Hussain I, Ahmed A, Iqbal M (2020) Organic chelates decrease phytotoxic effects and enhance chromium uptake by regulating chromium-speciation in castor bean (Ricinus communis L.). Sci Total Environ 716:137061. https://doi.org/10.1016/j.scitotenv.2020.137061
Rasheed R, Ashraf MA, Ahmad SJN, Parveen N, Hussain I, Bashir R (2022) Taurine regulates ROS metabolism, osmotic adjustment, and nutrient uptake to lessen the effects of alkaline stress on Trifolium alexandrinum L. plants. South Afri J Bot 148:482–498
Rahman MM, Islam MB, Biswas M, Alam AK (2015) In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes 8:1–9. https://doi.org/10.1186/s13104-015-1618-6
Ramzan M, Naz G, Parveen M, Jamil M, Gill S, Sharif HMA (2023) Synthesis of phytostabilized zinc oxide nanoparticles and their effects on physiological and anti-oxidative responses of Zea mays (L.) under chromium stress. Plant Physiol Biochem 196:130–138. https://doi.org/10.1016/j.plaphy.2023.01.015
Sagner S, Kneer R, Wanner G, Cosson JP, Deus-Neumann B, Zenk MH (1998) Hyperaccumulation, complexation and distribution of nickel in Sebertia acuminata. Phytochemistry 47:339–347. https://doi.org/10.1016/s0031-9422(97)00593-1
Sardar R, Ahmed S, Akbar M, Yasin NA, Li G (2022a) Alleviation of cadmium phytotoxicity in triacontanol treated Coriandrum sativum L. by modulation of physiochemical attributes, oxidative stress biomarkers and antioxidative system. Chemosphere 295:133924. https://doi.org/10.2139/ssrn.3995303
Sardar R, Ahmed S, Shah AA, Yasin NA (2022b) Selenium nanoparticles reduced cadmium uptake, regulated nutritional homeostasis and antioxidative system in Coriandrum sativum grown in cadmium toxic conditions. Chemosphere 287:132332. https://doi.org/10.1016/j.chemosphere.2021.132332
Shahid MA, Pervez MA, Balal RM, Mattson NS, Rashid A, Ahmad R, Abbas T (2011) Brassinosteroid (24-epibrassinolide) enhances growth and alleviates the deleterious effects induced by salt stress in pea (Pisum sativum L.). Austr J Crop Sci 5:500–510. https://doi.org/10.1007/s11738-019-2901-2
Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Rashid MI (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere 178:513–533. https://doi.org/10.1016/j.chemosphere.2017.03.074
Sohag AAM, Tahjib-Ul-Arif M, Polash MAS, Belal Chowdhury M, Afrin S, Burritt DJ, Afzal Hossain M (2020) Exogenous glutathione-mediated drought stress tolerance in rice (Oryza sativa L.) is associated with lower oxidative damage and favorable ionic homeostasis. Iran J Sci Technol Trans A Sci 44:955–971. https://doi.org/10.1007/s40995-020-00917-0
UdDin I, Bano A, Masood S (2015) Chromium toxicity tolerance of Solanum nigrum L. and Parthenium hysterophorus L. plants with reference to ion pattern, antioxidation activity and root exudation. Ecotox Environ Saf 113:271–278. https://doi.org/10.1016/j.ecoenv.2014.12.014
Ur Rehman H, Alharby HF, Bamagoos AA, Abdelhamid MT, Rady MM (2021) Sequenced application of glutathione as an antioxidant with an organic biostimulant improves physiological and metabolic adaptation to salinity in wheat. Plant Physiol Biochem 158:43–52. https://doi.org/10.1016/j.plaphy.2020.11.041
Vareda JP, Valente AJ, Durães L (2019) Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: a review. J Environ Manage 246:101–118. https://doi.org/10.1016/j.jenvman.2019.05.126
Vodyanitskii YN (2016) Standards for the contents of heavy metals in soils of some states. Ann Agrar Sci 14:257–263. https://doi.org/10.1016/j.aasci.2016.08.011
Wen K, Li X, Huang R, Nian H (2022) Application of exogenous glutathione decreases chromium translocation and alleviates its toxicity in soybean (Glycine max L.). Ecotox Environ Saf 234:113405. https://doi.org/10.1016/j.ecoenv.2022.113405
Xie C, Pu S, Xiong X, Chen S, Peng L, Fu J, Sun L, Guo B, Jiang M, Li X (2021) Melatonin-assisted phytoremediation of Pb-contaminated soil using bermudagrass. Environ Sci Pollut Res 28:44374–44388. https://doi.org/10.1007/s11356-021-13790-0
Yan B, Hou Y (2018) Effect of soil magnesium on plants: a review. IOP Conf Ser Earth Environ Sci 170:022168. https://doi.org/10.1088/1755-1315/170/2/022168
Yao M, Ge W, Zhou Q, Zhou X, Luo M, Zhao Y, Ji S (2021) Food Chem 352:129458. https://doi.org/10.1016/j.foodchem.2021.129458
Yuan H, Zhang Y, Huang S, Yang Y, Gu C (2015) Effects of exogenous glutathione and cysteine on growth, lead accumulation, and tolerance of Iris lactea var. chinensis. Environ Sci Pollu Res 22:2808–2816. https://doi.org/10.1007/s11356-014-3535-y
Zeng Q Y, Yang C Y, Ma Q B, Li X P, Dong W W, Nian A (2012a) Identification of wild soybean miRNAs and their target genes responsive to aluminum stress. BMC Plant Biol 12(1):1–16. https://www.biomedcentral.com/1471-2229/12/182
Zhao Y, Hu C, Wang X, Qing X, Wang P, Zhang Y, Zhang X, Zhao X (2019) Selenium alleviated chromium stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotox Environ Safe 173:314–321. https://doi.org/10.1016/j.ecoenv.2019.01.090
Zieslin N, Ben Zaken R (1993) Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol Biochem 31:333–339
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, S., Umar, I. & Sardar, R. Glutathione Alleviates Chromium Stress and Promotes Growth of Pisum sativum L. by Improving Nutrient Orchestration, Proline, and Physiochemical Attributes. J Soil Sci Plant Nutr 23, 3780–3796 (2023). https://doi.org/10.1007/s42729-023-01299-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01299-z