Abstract
Nitrogen (N) has been reported to act on both primary and specialized metabolism of plants. However, it is not clear how different N sources affect metabolism in species of the genus Annona. Thus, the aim of the work was to analyze how nitrate (NO3−) and ammonium (NH4+) influence photosynthesis and the production of alkaloids and leaf volatile compounds in Annona sylvatica A. St.-Hil. Plants were submitted to four treatments [NH4+, NO3−, NO3−:NH4+, and no N (W/N)], in hydroponic cultivation, and collected at 30, 60, and 90 days after the beginning of treatments. Plants maintained in NH4+ showed greater photosynthetic activity, high production of total alkaloids, in particular liriodenine, and increased production of leaf volatile compounds commonly related to stress situations. On the other hand, plants cultivated with NO3− showed lower photosynthetic activity and higher production of leaf volatile compounds related to plant resistance and defense. A. sylvatica seedlings are adapted to NH4+ with energy resources used to increase both primary and specialized metabolism, while using NO3−, the lower energy availability leads A. sylvatica plants to invest in leaf defense and not in photosynthesis. The individual use of NH4+ and NO3− increases the phytochemical potential of the species by stimulating the production of different groups of specialized metabolites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
N is widely reported in plants for maintaining metabolism (Kusano et al., 2011), being used in the synthesis of numerous molecules, such as amino acids, proteins, nitrogenous compounds, chlorophylls (Stitt et al., 2009), and the rubisco enzyme (Lawlor et al., 1989). For plants to be able to assimilate it, N must be available in the form of NO3− or NH4+ (Mur et al., 2016). N assimilation is energetically costly, requiring reducing agents, ATP and carbonic skeletons, especially when NO3− is the main N source. When absorbed as NO3−, the ion needs to be reduced into nitrite (NO2−) and then into NH4+ to be assimilated into a carbonic skeleton and, if absorption occurs in the form of NH4+, there is less energy expenditure because there is no reduction process involved (Mur et al., 2016). Thus, N metabolism is related to carbon (C) metabolism, being that both compete for reducing agents, since the reduction of NO3− into NO2− uses electrons from NAD (P) H + H + and the reduction of NO2− into NH4+ uses electrons from ferredoxin, thus interfering with the flow of electrons to reduce C during the photosynthetic process (Nunes-Nesi et al., 2010).
In addition to competition for reducing agents, N and C show codependence related to assimilation, where CO2 assimilation depends on adequate N supply to compose the photosynthetic apparatus, since N is present, for example, in the composition of chlorophyll (Nunes-Nesi et al., 2010) and the rubisco enzyme (Lawlor et al., 1989), and carbonic skeletons are necessary for N incorporation (Fritz et al., 2006), acting during its assimilation (Nunes-Nesi et al., 2010).
In relation to specialized metabolism, N is present in the structure of molecules such as alkaloids, which are nitrogenous compounds derived from amino acids (Evans, 2009; Wink, 2010). Among alkaloids, benzylisoquinolines (BIA) stand out, which are some of the most specialized metabolites found in the Annonaceae family (González-Esquinca et al., 2014; Lucio et al., 2015). BIA include aporphine and oxoaporphine alkaloids such as liriodenine, considered a chemotaxonomic marker in the Annonaceae family (González-Esquinca et al., 2014, Lucio et al., 2015). Liriodenine is an alkaloid of interest because it has medicinal properties, such as cytotoxic action against cancer cells (Chen et al., 2013; Lucio et al., 2015 Suresh et al., 2012) and properties against phytopathogens (De-la-Cruz-Chacón et al., 2019).
In addition to alkaloids, N also influences the biosynthesis of volatile compounds, which depends on C and N availability, as well as on energy provided by the primary metabolism (Dudareva et al., 2013). Volatile compounds act in the plant defense system (Alcântara et al., 2017; Erb, 2018) and in the medicinal field (Formagio et al., 2013), and are divided into classes including terpenoids, phenylpropanoids, benzenoids, fatty acids, and amino acids derivatives (Dudareva et al., 2013).
Based on the above, the effects of N concentrations and sources can be observed on the primary metabolism of several species (Cechin and Fumis, 2004; Imran et al., 2019; Liu et al., 2017; Zhao et al., 2005; Zhou et al., 2011). However, in the genus Annona, no studies were found assessing how different N sources (NH4+ and NO3−) affect photosynthetic variables and specialized metabolism. The literature only reports the effect of NO3− concentrations on primary and specialized metabolisms (leaf volatile compounds) in A. emarginata, indicating that at low NO3− concentrations (1.87 mM of N), there is low activity of the NO3− reductase enzyme and use of reducing agents and carbonic skeletons to increase volatile compounds and carbohydrates (Campos et al., 2019). In A. diversifolia, there are reports of increased production of alkaloids with the use of 30 mM of N in treatment with two N sources used together (NO3−:NH4+) (Orozco-Castillo et al., 2016). Thus, in both works, the effects of different N sources were not evaluated in isolation, which highlights the gap to be explored in relation to the individual supply of each N source (NH4+ and NO3−) on the primary and specialized metabolism of species of the genus Annona.
A. sylvatica (≡ Rollinia sylvatica) is an endemic and native species from Brazil, known as “araticum,” “araticum-do-mato,” “embira,” “cortiça,” and “cortiça-amarela” and inhabits the phytogeographic domains of Mata Atlântica and Pantanal (Lorenzi et al., 2005; Maas et al., 2015; Flora do Brasil, 2020). In addition, the species produces specialized metabolites of high pharmacological interest such as acetogenins, terpenes, and alkaloids (Andrade-Silva et al., 2020; Mikolajczak et al., 1990; Formagio et al., 2013; Gonçalves et al., 2015).
The work aimed to analyze how N sources (NH4+ and NO3−) impact the photosynthetic process and the production of specialized metabolites used to expand the phytochemical potential of A. sylvatica. The hypothesis is that the supply of NH4+ will increase the synthesis of alkaloids and volatiles with less energy expenditure. The results also provided physiological and chemical arguments for the conservation of this plant endemic and native to Brazil since the detection of an N source that increases the production and/or diversity of metabolites can be of great importance for the pharmaceutical area and future studies.
2 Material and Methods
The experiment was carried out using 180 A. sylvatica seedlings in hydroponic cultivation conducted in a paddy fan greenhouse with temperature maintained at ± 25.50 °C, air humidity at ± 50.05%, photosynthetic photon flux density (PPFD) at ± 300.00 µmol m−2 s−1, and CO2 environment at ± 420 PPM, located at 48° 240′ 3500″ W, 22° 49′ 1000″ S, and 800 m above sea level at the Biosciences Institute of the São Paulo State University, Campus of Botucatu, São Paulo, Brazil.
The experimental design was in randomized blocks in a 4 × 3 factorial scheme, with treatments consisting of four N sources (NO3−, NH4+, NO3−:NH4+, W/N) and three collection times (30, 60, and 90 days after the start of treatments (DAT)) with five replicates of three plants. The beginning of treatments was the moment in which plants were placed under different N conditions. Solutions were periodically changed and monitored as a function of pH and electrical conductivity, in order to keep nutrients as tools throughout the experiment. Plants were grown with Hoagland and Arnon (1950) nutrient solutions 1 and 2, with modifications, to supply the desired N source in amount equal to 0.105 g of N per liter, such as NO3−, NH4+, NO3−:NH4+, and W/N (Supplementary Fig. S1).
2.1 Gas Exchange and Chlorophyll a Fluorescence
Gas exchange and chlorophyll a fluorescence were simultaneously evaluated in one plant of each replicate for each treatment at 30, 60, and 90 days after the beginning of the experiment, between 09:00 am and 11:00 am, in leaves with fully expanded limbus. Open photosynthesis system equipment with CO2 and water vapor analyzer by infrared radiation and a coupled fluorometer (infrared gas analyzer (IRGA), model GSF 3000 FL, with Array/PAM-Fluorometer 3055-FL, Walz) was used.
For gas exchanges, saturating light of 1200 μmol m−2 s−1 was used, and net CO2 assimilation rate (Anet, μmol CO2 m−2 s−1), transpiration rate (E, mmol water vapor m−2 s−1), stomatal conductance (gs, mmol m−2 s−1), and internal CO2 concentration in the substomatal chamber (Ci, μmol CO2 mol−1) were evaluated. Water use efficiency (WUE, μmol CO2 (mmol H2O)−1) was calculated using the relationship between net CO2 assimilation rate (Anet, μmol CO2 m−2 s−1) and transpiration rate (E, mmol water vapor m−2 s−1); the instant carboxylation efficiency of the rubisco enzyme (Anet/Ci, mmol m−2 s−1 Pa−1) was calculated using the relationship between net CO2 assimilation rate (Anet, μmol CO2 m−2 s−1) and internal C concentration in the substomatal chamber (Ci, μmol CO2 mol−1) (Zhang et al., 2001).
For chlorophyll a fluorescence, actinic light pulse of 4,500 μmol m−2 s−1 was used, and maximum fluorescence (Fm '), potential quantum efficiency of photosystem II (Fv'/Fm '), effective quantum efficiency of photosystem II (ΦFSII), electron transport rate (ETR), photochemical dissipation (qP), fraction of light absorbed by the photosystem II antenna dissipated as heat (D), and fraction of excitation energy not dissipated in the antenna that cannot be used in photochemical reactions (Ex), calculated according to Demmig-Adams et al. (1996), were evaluated in light.
2.2 Extraction and Quantification of Alkaloids
To obtain the alkaloid extract, 5 replicates with a pool of 3 plants were used for each treatment.
Alkaloids were extracted from roots previously dried in a forced aeration oven at 30 °C, using the acid–base method. After thorough grinding, the plant material was moistened with a saturated sodium carbonate (Na2CO3) solution and left to dry for 48 h at room temperature. Alkaloids were extracted with chloroform (CHCl3) by constant stirring for 1 h and then filtered and washed with distilled water. The CHCl3 phases were extracted into a 1 M hydrochloric acid (HCl) solution before being alkalinized to pH 9.5 with a saturated solution of Na2CO3. The alkaline solution was then re-extracted with CHCl3, dried with anhydrous sodium sulfate (Na2SO4), filtered, and evaporated at approximately 25 °C to obtain total alkaloids (De-la-Cruz-Chacón and González-Esquinca, 2012; Sousa et al., 2019; Vinche et al., 2020).
The same extract was used for quantification of total alkaloid, HPLC alkaloids analysis, and liriodenine quantification. The quantification of total alkaloids was performed by spectrophotometry at 254 nm using liriodenine as reference for the elaboration of the standard curve (y = 0.0881x—0.0112, R2 = 0.9949). Alkaloid detection analysis and liriodenine quantification were performed by ultra-high-performance liquid chromatograph (UHPLC—Thermo Fisher-Scientific®, Waltham, MA, USA) with gradient pump and UVVIS detector using the C18 reverse phase column (150 × 4.6 mm and 5 μm particle diameter). The mobile phase was water (pH 3.5 with trifluoroacetic acid) and 30:70 gradient methanol with flow rate of 1 mm/min maintaining column temperature at 30 °C; detection was carried out in UV at 280 nm.
For liriodenine quantification, calibration curve was performed by analyzing the series of 100 mg mL−1 stock solutions (y = 0.3658x + 1.114; R2 = 0.9999), and for the alkaloid detection analysis, standards of reticulin, norpredicentrine, N-methyl-laurotetanine, norglaucine, discretine, xylopine, xylopinin, assimilobin, laurotetanine, liriodenine, oxoglaucine, and lanulinusin alkaloids were used (adapted from Sousa et al., 2019). Liriodenine was provided by Iván De-la-Cruz-Chacón. The methods for isolation and data identification of liriodenine were reported by De-la-Cruz-Chacón and González-Esquinca (2012). The presence identification of alkaloids norpredicentrine, reticuline, and discretine was also carried out by comparison to standards provided by Emmanoel Vilaça Costa and Jackson Roberto Guedes da Silva Almeida (spectrometric data were previously reported in Sousa et al., 2019).
2.3 Leaf Volatile Compounds
Leaf volatile compounds were captured from leaves dried in forced aeration oven at 30 °C until constant mass was obtained. Three replicates of 3 plants were used for each treatment, totaling 108 plants.
For each sample, 0.05 g of macerated dry leaves was placed in glass flask added of 10 mL of distilled water. The flask was sealed and placed in water bath for 1 h at temperature of 90 °C. After that period, the flask was removed from the water bath and the capture of volatiles was performed by headspace solid-phase microextraction (HS-SPME) using SPME Fiber Assembly 75, CarboxenTM-PDMS for Manual Holder-SUPELCO, for 30 min. Then, the chemical composition of volatiles was determined by gas chromatography coupled to mass spectrometry (GC–MS) in Shimadzu equipment model QP-5000 equipped with fused silica capillary column DB-5 (30 m × 0.25 mm × 0.25 μm); electron ionization (EI) was used at 70 eV, helium as carrier gas (flow rate of 1.0 mL min−1), injector at 220 °C, transfer line at 230 °C, and split ratio of 1:20, using the 60 °C to 240 °C, 3 °C min−1 temperature program.
To identify substances present in leaf volatile compounds, mass spectra of compounds were compared with those in the GC–MS system database (Nist. 62 Libr.) and linear retention indexes (LRI) found in literature (Adams, 2017). Linear retention indices were obtained from the injection of a standard mixture of n-alkanes (C9-C24), applying the equation of Van den Dool and Kratz (Van Den Dool, Kratz, 1963).
2.4 Statistical Analyses
Data obtained (gas exchange, fluorescence, total alkaloids, liriodenine, and relative percentage of leaf volatiles) were submitted to two-way analysis of variance (ANOVA). Two-way ANOVA was conducted to determine the effects of treatments (NO3−, NH4+, NO3−:NH4+, W/N) with time (30, 60, 90 DAT) and their interaction. Means were compared by the Tukey test at 5% significance level (p < 0.05), as mean with standard error (M ± SE) (Gomes, 1990).
For the heat map, hierarchical cluster analysis was performed, using the relative percentage of the leaf volatile profile after transforming data into log, distance measure using Euclidean, and clustering algorithm using ward.D and the MetaboAnalyst 4.0 statistical software (Chong et al., 2019).
3 Results
In the first 30 days of maintaining plants in different N sources, it was possible to observe mortality of some plants maintained in NH4+ and W/N. However, over time, plants adapted to NH4+, unlike those maintained in W/N (Table 1). This fact represents an important adaptive characteristic of the species as will be observed in the gas exchange and fluorescence results as well as in the production of alkaloids and leaf volatiles.
3.1 Gas Exchange
During the 90 days of plant cultivation, no variations in stomatal conductance (gs) and transpiration (E) were observed according to the different N sources (Fig. 1E, F). However, the instant carboxylation efficiency of the rubisco enzyme (Anet/Ci) was influenced by NO3− and NH4+ at different times (60 and 90 DAT, respectively).
Gas exchanges—net CO2 assimilation rate (Anet, μmol CO2 m−2 s−1) (a); internal CO2 concentration in the substomatal chamber (Ci, μmol CO2 mol−1 air) (b); water use efficiency (WUE, μmol CO2 (mmol H2O)−1) (c); instant carboxylation efficiency of the rubisco enzyme (Anet/Ci, μmol m−2 s−1 Pa−1) (d); stomatal conductance (gs, mmol m−2 s−1) (e); transpiration rate (E, mmol m−2 s−1) (f)—determined at 30, 60, and 90 days after the beginning of treatments (DAT) in Annona sylvatica plants grown with nitrate (NO3−), ammonium (NH4+), nitrate plus ammonium (NO3−: NH4+), and without nitrogen supply (W/N). Averages followed by the same letter, lower case letters compare the 4 treatments in the same collection period and upper case letters compare collection times for each treatment, do not differ by the Tukey test at 5% probability. Data are presented as mean ± SE (n = 5)
When NO3− was used, plants showed high net CO2 assimilation (Anet) at 60 DAT (Fig. 1a) and the lowest internal CO2 concentration in the substomatal chamber (Ci) (Fig. 1b), which resulted in the greatest instant carboxylation efficiency of the rubisco enzyme (Anet/Ci) (Fig. 1d). However, at 90 DAT, internal C accumulation (Ci) and reduction in CO2 assimilation (Anet) and consequently less carboxylation efficiency (Anet/Ci) were observed.
On the other hand, for plants maintained in NH4+, the greatest carboxylation efficiency was observed at 90 DAT (30 days later than plants maintained in NO3−), at which time there was high net CO2 assimilation (Anet) and low internal C concentration (Ci).
Treatment with combined N sources (NO3−:NH4+) decreased C assimilation (Anet) and the carboxylation efficiency of the rubisco enzyme (Anet/Ci). Likewise, the absence of N was also harmful to gas exchange, causing low efficiency in the photosynthetic process with lower carboxylation efficiency values (Anet/Ci) due to C assimilation values (Anet) and high internal C accumulation (Ci).
3.2 Chlorophyll a Fluorescence
At 60 and 90 days after the beginning of the experiment (DAT), no differences were observed in plants maintained in the various conditions in relation to the maximum efficiency of photosystem II (Fv'/Fm') (Fig. 2a).
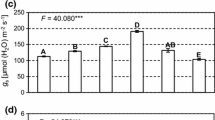
Chlorophyll a fluorescence—potential quantum efficiency of photosystem II (Fv'/Fm') (a), fraction of light absorbed by photosystem II antenna dissipated as heat (D) (b), fraction of excitation energy not dissipated in the antenna that cannot be used in photochemical reactions (Ex) (c), photochemical dissipation (qP) (d), electron transport rate (ETR) (e), and potential quantum efficiency of photosystem II (ΦFSII) (f)—determined at 30, 60, and 90 days after the beginning of treatments (DAT) in Annona sylvatica plants grown in nitrate (NO3−), ammonium (NH4+), nitrate plus ammonium (NO3−: NH4+), and without nitrogen supply (W/N). Averages followed by the same letter, lower case letters compare the 4 treatments in the same collection period and upper case letters compare collection times for each treatment, do not differ by the Tukey test at 5% probability. Data are presented as mean ± SE (n = 5)
At the end of the experiment (90 DAT), plants cultivated with NH4+ showed increase in photochemical dissipation (qP) (Fig. 2d) and in the electron transport rate (ETR) (Fig. 2e), resulting in higher effective quantum efficiency of photosystem II (ΦFSII) (Fig. 2f). The supply of NH4+ led plants to present low fraction of accumulated energy, which cannot be used in photochemical reactions (Ex), regardless of collection time (Fig. 2c).
On the other hand, plants cultivated with NO3− showed low photochemical dissipation (qP) (Fig. 2d), low electron transport rate (ETR), and effective quantum efficiency of photosystem II (ΦFSII) at the end of the experiment. The response of plants to NO3− in relation to energy deviated and not used in photochemical reactions (Ex) was intermediate in the other treatments.
In the absence of N, plants did not show fluorescence variations throughout the experiment. The use of NO3−:NH4+ led to increase in ETR and ΦFSII at 90 DAT.
3.3 Production of Total Alkaloids
The production of total alkaloids in A. sylvatica seedlings remained stable over time in plants submitted to treatment with mixture of N sources (NO3−:NH4+) and in those maintained in W/N (Fig. 3).
Concentration of total alkaloids (μg g−1 DM) determined at 30, 60, and 90 days after the beginning of treatments (DAT) in Annona sylvatica roots grown in nitrate (NO3−), ammonium (NH4+), nitrate plus ammonium (NO3−: NH4+), and without nitrogen supply (W/N). Averages followed by the same letter, lower case letters compare the 4 treatments in the same collection period and upper case letters compare collection times for each treatment, do not differ by the Tukey test at 5% probability. Data are presented as mean ± SE (n = 5)
Plants cultivated with NH4+ showed significant increase (DF: 6; f: 7.017; p < 0.001) in the concentration of alkaloids roots, being responsible for the highest production of total alkaloids at the end of the experiment (90 DAT) and the largest amount of the entire experiment. In plants cultivated with NO3−, increase in the concentration of alkaloids was also observed at 60 DAT, which did not differ from 90 DAT; however, concentration was lower in plants cultivated with NH4+ compared to those with NO3−:NH4+ (Fig. 3).
3.4 Alkaloid Detection and Liriodenine Production
Eight alkaloids were identified with the standard alkaloids used, and six of them are present in all treatments. Furthermore, discretine was not detected in plants grown with NO3−. Liriodenine appears as the main alkaloid, regardless of N sources used, and this main metabolite increased gradually over time in plants grown with NH4+, resulting in higher concentration at 90 DAT and throughout the experiment, which differed from plants grown with NO3− and W/N (Fig. 4, Table 2).
Liriodenine concentration (μg g−1 DM) determined at 30, 60, and 90 days after the beginning of treatments (DAT) in Annona sylvatica roots grown in nitrate (NO3−), ammonium (NH4+), nitrate plus ammonium (NO3−: NH4+), and without nitrogen supply (W/N). Averages followed by the same letter, lower case letters compare the 4 treatments in the same collection period and upper case letters compare collection times for each treatment, do not differ by the Tukey test at 5% probability. Data are presented as mean ± SE (n = 5)
3.5 Leaf Volatile Compounds
Forty-six volatile compounds were identified in A. sylvatica leaves grown with the different N sources, which correspond to approximately 69–99% of substances that make up the profile. Volatile profile is mainly composed of sesquiterpene (29 metabolites), followed by monoterpenes and fatty acids derivatives (13 and 4 compounds, respectively) (Tables 3, 4, and 5). The major volatile compounds were sesquiterpenes β-selinene with relative abundance varying from 13.74 to 40.4%, E-caryophyllene (3.23 to 14.64%), aromadendrene (2.25 to 9.69%), the monoterpenes α-thujene (2.31 to 18.85%), α-pinene (2.01 to 7.77%), and the fatty acid derivative 2-E-hexenal (0.15 to 10.00%). Correlations between leaf volatile compounds of A. sylvatica and treatments were evaluated using the heat map shown in Fig. 5.
Heat map indicating positive (red) and negative (blue) correlation between volatile compounds extracted from Annona sylvatica leaves and treatments with nitrate (NO3−), ammonium (NH4+), nitrate plus ammonium (NO3−:NH4+), and without nitrogen supply (W/N) at 30, 60, and 90 days after the beginning of treatments (DAT)
In plants cultivated with NO3−, α-thujene showed high relative percentage and positive correlation at 30 and 60 DAT (Tables 3, 4, and 5, Supplementary Fig. S3), as well as β-pinene, α-cubebene, γ-elemene, and germacrene D had higher relative percentage at 30 and/or 60 and 90 DAT. Among major compounds, β-selinene showed irregular responses to NO3− treatments with respect to recollection time, high relative percentage, and positive correlation compared to those grown with NH4+ at 30 DAT, while at 60 and 90 DAT, plants showed lower proportions (Tables 3, 4, and 5, Supplementary Fig. S1).
Hexanal and 2-E-hexenal were the main compounds that showed increase in the relative percentage when plants were grown with NH4+ 60 and 90 DAT, being 2-E-hexenal the treatment with the highest relative percentage and positive correlation at 90 DAT (Table 5, Supplementary Fig. S5). E-caryophyllene showed its highest proportion at 30 DAT but at 90 days, it decreased with respect to the other treatments. Trans-prenyl limonene and γ-gurgujene were other volatiles that showed their highest proportions with the NH4+ treatment.
In plants cultivated with NO3−:NH4+, limonene, α-phellandrene, β-bourbonene, and β-elemene showed higher relative percentage (Supplementary Fig. S4, Table 5).
Finally, in W/N treatments, β-gurjunene and aromandendrene showed their most outstanding proportions.
4 Discussion
N source or absence of this element supplied to A. sylvatica generated different responses in the primary and specialized metabolism, which indicated the capacity of this species to adapt and survive to different nutritional conditions. Other Annona species, like A. macrophyllata (A. diversifolia) and A. emarginata, were able to grow in crops with various N concentrations either in NO3−:NH4+ combination or only in NO3−, respectively (Orozco et al., 2016; Campos et al., 2019). In A. sylvatica plants, NH4+ increased the photosynthesis, production of alkaloids, and specific leaf volatile compounds.
The supply of NH4+ resulted in higher photosynthetic efficiency (high instant carboxylation efficiency of the rubisco enzyme and high effective quantum efficiency of photosystem II—ΦFSII) and also the higher production of alkaloids. This fact may be related to the ready N availability for use in metabolic processes, which corroborates reports by Mur et al. (2016). Besides, the low Ex values suggest that the energy was destined for photosynthesis, since such N source is readily available, and there is no need to deviate electrons for reduction processes, as would occur with NO3− (Nunes-Nesi et al., 2010). The high photosynthetic efficiency with the use of NH4+ in A. sylvatica seedlings also shows that there was no excess energy dissipated in the form of heat or re-emitted as light, since according to Maxwell and Johnson (2000), these three processes occur in competition.
The results observed in A. sylvatica maintained in NH4+ suggest tolerance or acclimatization of this species to NH4+, since this ion is considered toxic to vegetables, as observed in species such as Cucumis sativus, where plants cultivated in NH4+ showed lower gas exchange rates and photosynthetic rate than plants cultivated in NO3− (Zhou et al., 2011), and in Catharanthus roseus plants, which showed low photosynthetic rate when submitted to high NH4+ concentrations (Guo et al., 2012). According to Marino and Moran (2019), tolerance to NH4+ is the way plant responds to this stress before suffering serious damage or even cell death. In this context, one of the ways to tolerate stress is NH4+ assimilation into organic compounds. Such organic compounds include amino acids and precursor alkaloid molecules (Wink, 2010). Thus, A. sylvatica plants cultivated in NH4+ showed high photosynthetic rate and directed possible NH4+ excess to produce alkaloids, such as the increase in total alkaloids and particularly that of oxoaporphine liriodenine (the main alkaloid of this species) (Table 2).
Treatment with NO3− led to decrease in photosynthetic efficiency, which can be seen by the reduction in the instant carboxylation efficiency of the rubisco enzyme (Anet/Ci) at the end of the experiment, reflecting in high internal C concentration and decreased net CO2 assimilation rate (Anet), and in lower photochemical dissipation (qP) and effective quantum efficiency of photosystem II values. This decrease in the photosynthetic process seems to be related to the energy deviation of this process to reduce NO3−. There is need to reduce NO3− into NO2−, which occurs in the cytosol using electrons from NAD (P) H + H + and then NO2− is transported to chloroplasts where it will be reduced into NH4+ by transfer of ferredoxin electrons (Miller and Cramer, 2004; Nunes-Nesi et al, 2010). Thus, A. sylvatica plants deviate the electron flow from photosynthesis to reduce NO3− when cultivated in the presence of this N source (alone or in combination), which can be proven by the increase in the accumulated energy fraction not used in photochemical reactions (Ex). Besides, in A. sylvatica, the deviation of electron flow from photosynthesis to reduce NO3− reduces not only photosynthesis, but also the synthesis of alkaloids.
The reduced photosynthetic efficiency observed by the gas exchange and fluorescence variables in A. sylvatica plants maintained without N supply confirms what is expected in relation to the importance of N in the various metabolic processes of plants (Lawlor et al., 1989; Stitt et al., 2009; Wang et al, 2014; Zhao et al., 2005).
In addition to the relationships between N sources in primary metabolism and alkaloids, the supply of different sources led to the differential synthesis of major leaf volatile compounds. In this context, NH4+ increased the synthesis of leaf volatile compounds related to plant responses to stress situations, such as 2-E-hexenal, emitted by plants under biotic stress caused by pathogens (Scala et al., 2013) and induced by herbivory (Goldberg et al., 2019). These components should also be highlighted because they have antimicrobial (Patrignani et al., 2008) and antifungal properties (Zhang et al., 2016).
The supply of NO3− led to higher production of leaf volatile compounds related to plant resistance and defense. Studies have shown that NO3− can cause changes in defense mechanisms related to pathogens, with increase in nitric oxide and polyamines and decrease in gamma-aminobutyric acid (GABA) (Mur et al., 2016). In A. emarginata (Campos et al., 2019), the application of NO3− at concentrations similar to those provided for A. sylvatica also induced plant defense mechanisms, with synthesis of mono- and sesquiterpenes. In A. sylvatica, NO3− promoted high relative percentage of β-selinene, which has antifungal properties (Ding et al., 2017), and of α-thujene, a compound that has cytotoxic properties (Blowman et al., 2018).
Treatment with NO3−:NH4+ resulted in high relative percentage of α-pinene in A. sylvatica, which may be interesting since this compound has antimicrobial (Leite et al., 2007), anti-inflammatory (Kim et al., 2015), and cytotoxic properties (Blowman et al., 2018). In addition, several other compounds, such as ρ-cymene, β-pinene, myrcene, limonene, 6-methyl-5-hepten-2-one, spathulenol, and α-humulene, also have cytotoxic properties (Blowman et al., 2018), which can also be explored from the pharmaceutical point of view.
A. sylvatica seems to adjust aspects of its specialized metabolism according to the N source, while NH4+ causes increases in alkaloids and to a lesser degree volatile derivatives of fatty acids (hexanal, 2-E-hexenal) and NO3− increases some important terpenic volatiles (β-selinene, α-thujene, β-pinene) and secondarily alkaloids, the combination of both salts expresses an intermediate behavior. Another aspect to be highlighted is that with the W/N treatment, the amount of total alkaloids and liriodenine is stable over the 3 months of experiment, which may be an indication of the relevance of these N molecules for A. sylvatica.
In summary, the main advances obtained with this research refer to the ability of different N sources to influence the primary and specialized metabolism of A. sylvatica, in addition to the fact that the species is adapted to possible toxicity caused by NH4+. Thus, although the different N sources present similar responses at the beginning of treatments (30 DAT), over time, NH4+ (source more readily available and with the lowest energy expenditure for the plant) leads A. sylvatica to a stress condition with reduction in photosynthesis, but without altering the synthesis of alkaloids (60 DAT), and later, the maintenance of plants in NH4+ (90 DAT) leads to acclimatization, with increase in photosynthesis and production of alkaloids, transferring to the specialized metabolism, the probable excess that would be toxic to the primary metabolism (Britto and Kronzucker, 2002). The N availability can influence the dynamics of specialized metabolite expression and the release of defense compounds, an important condition in plant-animal relationships (Nagegowda, 2010; Campos et al., 2019). In this context, variations in leaf volatile profiles were also observed depending on the source used, with increase in compounds related to plant defense (antifungal and cytotoxic substances) when using NO3−, while with NH4+, there is production of volatiles related to biotic stress (pathogens and herbivory). Such characteristics of A. sylvatica also suggest that the species can be used as an alternative to obtain bioactive molecules of interest, whose production can be modulated with specific treatments such as the N sources evaluated in this study.
5 Conclusion
Variations of N sources have distinct impact on the primary and specialized metabolism of A. sylvatica. Photosynthesis reduction caused by the deviation of the electronic flow for NO3− reduction also leads to reduction in the synthesis of alkaloids, while there is increase in the production of volatile defense compounds. On the other hand, A. sylvatica plants show acclimation to NH4+, with increase in both photosynthesis and alkaloids and leaf volatiles compounds related to stress situations. It could be concluded that plants maintained in NO3− mobilize their energy more towards specialized metabolism, while plants maintained in NH4+ invest in both primary and specialized metabolism, which increases the phytochemical potential of the species by stimulating the production of different groups of metabolites.
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- N:
-
Nitrogen
- NO2 − :
-
Nitrite
- NO3 − :
-
Nitrate
- NH4 + :
-
Ammonium
- NO3 −:NH4 + :
-
Nitrate plus ammonium
- W/N:
-
Without nitrogen
- C:
-
Carbon
- DAT:
-
Days after the start of treatments
References
Adams RP (2017) Identification of essential oil components by gas chromatography/mass spectrometry. Biology Department Baylor University Ed 4:1
Alcântara JM, Lucena JMVM, Facanali R, Marques MOM, Lima MP (2017) Chemical Composition and Bactericidal Activity of the Essential Oils of Four Species of Annonaceae Growing in Brazilian Amazon. Nat Prod Commun 12:619–622. https://doi.org/10.1177/1934578X1701200437
Andrade-Silva M, Carvalher-Machado SC, Formagio ASN, Maris RS, de Lima Junior OM, de Mello SBV, Henriques MGMO, Arena AC, Kassuya CAL (2020) Anti-inflammatory and anti-allergic activity of the methanolic extract from Annona sylvatica (Annonaceae). Genet Mol Res 19:3. https://doi.org/10.4238/gmr18661
Blowman, K., Magalhães, M., Lemos, M. F. L., Cabral, C., Pires, I. M., 2018. Anticancer Properties of Essential Oils and Other Natural Products. Evid Based Complement Alternat Med, 12. https://doi.org/10.1155/2018/3149362
Britto DT, Kronzucker HJ (2002) NH4+ toxicity in higher plants: a critical review. J Plant Physiol 15:567–584. https://doi.org/10.1078/0176-1617-0774
Campos, F. G., Vieira, M. A. R., Amaro, A. C. E., De-La-Cruz-Chacón, I., Marques, M. O. M., Ferreira, G., Boaro, C. S. F., 2019. Nitrogen in the defense system of Annona emarginata (Schltdl.) H. Rainer. PLoS One, 14. https://doi.org/10.1371/journal. pone.0217930
Cechin I, Fumis TF (2004) Effect of nitrogen supply on growth and photosynthesis of sunflower plants grown in the greenhouse. Plant Sci 166:1379–1385. https://doi.org/10.1016/j.plantsci.2004.01.020
Chen C, Wu H, Chao W, Lee C (2013) Review on pharmacological activities of liriodenine. Afr J Pharm Pharmacol 7:067–1070. https://doi.org/10.5897/AJPP2013.3477
Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J (2019) MetaboAnalyst 40: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46(W1):W486–W494. https://doi.org/10.1093/nar/gky310
De-la-Cruz-Chacón I, González-Esquinca AR (2012) Liriodenine alkaloid in Annona diversifolia during early development. Nat Prod Res 26:42–49. https://doi.org/10.1080/14786419.2010.533373
De-la-Cruz-Chacón I, López-Fernández NY, Riley-Saldaña CA, Castro-Moreno M, González-Esquinca AR (2019) Antifungal activity in vitro of Sapranthusmicrocarpus (Annonaceae) against phytopathogenus. Acta Bot Mex 126:1420. https://doi.org/10.21829/abm127.2019.1420
Demmig-Adams B, Adams WW, Barker DH, Logan BA, Bowling DR, Verhoeven AS (1996) Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol Plant 98:253–264. https://doi.org/10.1034/j.1399-3054.1996.980206.x
Ding Y, Huffaker A, Kollner TG, Weckwerth P, Robert CAM, Spencer JL, Lipka AE, Schmelz EA (2017) Selinene Volatiles Are Essential Precursors for Maize Defense Promoting Fungal Pathogen Resistance. Plant Physiol 175:1455–1468. https://doi.org/10.1104/pp.17.00879
Dudareva N, Klempien A, Muhlemann JK, Kaplan I (2013) Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol 198:16–32. https://doi.org/10.1111/nph.12145
Erb M (2018) Volatiles as inducers and suppressors of plant defenseand immunity — origins, specificity, perception andsignaling. Curr Opin Plant Biol 44:117–121. https://doi.org/10.1016/j.pbi.2018.03.008
Evans, W. C. Trease And Evans Pharmacognosy, 2009. Ed. 16, p. 353–415. ISBN: 9780702029349
Flora do Brasil, 2020, 2017. Flora do Brasil 2020 em contrução [WWW Document]. Jard. Botânico do Rio Janeiro. URL http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB110219 (Consultado 07.31.17).
Fritz C, Palacios-Rojas N, Feil R, Stitt M (2006) Regulation of secondary metabolism by the carbon–nitrogen status in tobacco: nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J 46:533–548. https://doi.org/10.1111/j.1365-313X.2006.02715.x
Formagio ASN, Vieira MC, Santos LAC, Cardoso CAL, Foglio MA, Carvalho JE, Silva MA, Kassuya C (2013) Composition and Evaluation of the Anti- Inflammatory and Anticancer Activities of the Essential Oil from Annona sylvatica A. St.-Hil. J Med Food 16:20–25. https://doi.org/10.1089/jmf.2011.0303
Goldberg JK, Pintel G, Weiss SL, Martins EP (2019) Predatory lizards perceive plant-derived volatile odorants. Ecol Evol 9:4733–4738. https://doi.org/10.1002/ece3.5076
Gomes, F.P., 1990. Estatística experimental. 13 ed. Piracicaba: Nobel, 467 p.
Gonçalvez GLP, Ribeiro LP, Gimenes L, Vieira PC, Silva MFGF, Forim MR, Fernandes JB, Vendramim JD (2015) Lethal and sublethal toxicities of Annona sylvatica (Magnoliales: Annonaceae) extracts to Zabrotessubfasciatus (Coleoptera: Chrysomelidae: Bruchinae). Fla Entomol 98:921–928. https://doi.org/10.1653/024.098.0317
González-Esquinca AR, De-La-Cruz-Chacón I, Castro-Moreno M, Orozco-Castillo JA, Riley-Saldaña CA (2014) Alkaloids and acetogenins in Annonaceae development: biological considerations. Rev Bras Frutic 36:001–016. https://doi.org/10.1590/S0100-29452014000500001
Guo X, Zu Y, Tang Z (2012) Physiological responses of Catharanthusroseus to different nitrogen forms. Acta Physiol Plant 34:589–598. https://doi.org/10.1007/s11738-011-0859-9
Hoagland, D. R., Arnon, D. I., 1950. The water: culture method for growing plants without soil. Berkeley: California Agricultural Experiment Station, 347, 1–32. citeulike-article-id:9455435
Imran M, Hu C, Hussain S, Rana MS, Riaz M, Afzal J, Aziz O, Elyamine AM, Ismael MAF, Sun X (2019) Molybdenum-induced effects on photosynthetic efficacy of winter wheat (Triticumaestivum L.) under different nitrogen sources are associated with nitrogen assimilation. Plant Physiol Biochem 141:154–163. https://doi.org/10.1016/j.plaphy.2019.05.024
Kim D, Lee H, Jeon Y, Han Y, Kee J, Kim H, Shin H, Kang J, Lee BS, Kim S, Kim S, Park S, Choi B, Park S, Um J, Hong S (2015) Alpha-Pinene Exhibits Anti- Inflammatory Activity Through the Suppression of MAPKs and the NF-κB Pathway in Mouse Peritoneal Macrophages. Am J Chinese Med 43:731–742. https://doi.org/10.1142/S0192415X15500457
Kusano M, Fukushima A, Redestig H, Saito K (2011) Metabolomic approaches toward understanding nitrogen metabolism in plants. J Exp Bot 62:1439–1453. https://doi.org/10.1093/jxb/erq417
Lawlor DW, Kontturi M, Young AT (1989) Photosynthesis by flag leaves of wheat in relation to protein, ribulose bisphosphate carboxylase activity and nitrogen supply. J Exp Bot 40:43–52. https://doi.org/10.1093/jxb/40.1.43
Leite AM, Lima EO, Souza EL, Diniz MFFM, Trajano VN, Medeiros IA (2007) Inhibitory effect of β-pinene, α-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Rev Bras Cienc Farm 43:121–126. https://doi.org/10.1590/S1516-93322007000100015
Liu G, Du Q, Li J (2017) Interactive effects of nitrate-ammonium ratios and temperatures on growth, photosynthesis, and nitrogen metabolism of tomato seedlings. Sci Hortic 214:41–50. https://doi.org/10.1016/j.scienta.2016.09.006
Lorenzi AQ, Araujo DSD, Kurtz BC (2005) Annonaceae das restingas do estado do Rio de Janeiro. Rodriguésia 56:85–96
Lúcio ASSC, Almeida JRGDS, Vasconcelos Leitão da-Cunha E, Tavares JF, Filho JMB (2015) Alkaloids of the Annonaceae: Occurrence and a Compilation of Their Biological Activities. Alkaloids Chem Biol 74:233–409. https://doi.org/10.1016/bs.alkal.2014.09.002
Maas P, Lobão A, Rainer H (2015) Annonaceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. Rodriguésia 66:1085–1113
Marino D, Moran JF (2019) Can Ammonium Stress Be Positive for Plant Performance? Front Plant Sci 10:1103. https://doi.org/10.3389/fpls.2019.01103
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence – a practical guide. J Exp Bot 51:659–668. https://doi.org/10.1093/jexbot/51.345.659
Mikolajczak KJ, Madrigal RV, Rupprecht JK, Hui YH, Liu YM, Smith DL, McLaughlin JL (1990) Sylvaticin: a new cytotoxic and insecticidal acetogenin from Rollinia sylvatica (Annonaceae). Experientia 46:324–327. https://doi.org/10.1007/BF01951779
Miller AJ, Cramer MD (2004) Root nitrogen acquisition and assimilation. Plant Soil 274:1–36. https://doi.org/10.1007/1-4020-4099-7_1
Mur LAJ, Simpson C, Kumari A, Gupta AK, Gupta KJ (2016) Moving nitrogen to the centre of plant defence against pathogens. Ann Bot 119:703–709. https://doi.org/10.1093/aob/mcw179
Nagegowda DA (2010) Plant volatile terpenoid metabolism: biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett 584:2965–2973. https://doi.org/10.1016/j.febslet.2010.05.045
Nunes-Nesi A, Fernie AR, Stitt M (2010) Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol Plant 3:973–996. https://doi.org/10.1093/mp/ssq049
Orozco-Castillo JA, Cruz-Ortega R, Martinez-Vázquez M, González-Esquinca AR (2016) Aporphine alkaloid contents increase with moderate nitrogen supply in Annona diversifolia Saff. (Annonaceae) seedlings during diurnal periods. Nat Prod Res 30:2209–2214. https://doi.org/10.1080/14786419.2016.1143826
Patrignani F, Iucci L, Belletti N, Gardini F, Guerzoni ME, Lanciotti R (2008) Effects of sub-lethal concentrations of hexanal and 2-(E)-hexenal on membrane fatty acid composition and volatile compounds of Listeria monocytogenes, Staphylococcus aureus, Salmonella enteritidis and Escherichia coli. Int J Food Microbiol 123:1–8. https://doi.org/10.1016/j.ijfoodmicro.2007.09.009
Scala A, Allman S, Mirabella R, Haring MA, Schuurink RC (2013) Green leaf volatiles: a plant’s multifunctional weapon against herbivores and pathogens. Int J Mol Sci 14:17781–17811. https://doi.org/10.3390/ijms140917781
Sousa MC, Bronzatto AC, González-Esquinca AR, Campos FG, Dalanhol SJ, Boaro CSF, Martins AL, Almeida JRGS, Costa EV, De-la-Cruz-Chacón I, Ferreira G (2019) The production of alkaloids in Annona cacans seedlings is affected by the application of GA4+7+6-bezyladenine. Biochem Syst Ecol 84:47–51. https://doi.org/10.1016/j.bse.2019.03.007
Stitt M, Sulpice R, Keurentjes J (2009) Metabolic Networks: How to Identify Key Components in the Regulation of Metabolism and Growth. Plant Physiol 152:428–444. https://doi.org/10.1104/pp.109.150821
Suresh HM, Shivakumar B, Shivakumar SI (2012) Cytotoxicity of aporphine alkaloids from the roots of Annona Reticulata on Human Cancer Cell Lines. Int J Plant Res 2:57–60. https://doi.org/10.5923/j.plant.20120203.02
Van Den Dool H, Kratz P (1963) A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J Chromatogr A 11:463–471. https://doi.org/10.1016/S0021-9673(01)80947-X
Vinche, A. D. L., De-la-Cruz-Chacón, I., González-Esquinca, A. R., Silva, J. F., Ferreira, G., Santos, D. C., Garces, H. G., Oliveira, D. V. M., Marçon, C., Cavalcante, R. S., Mendes, R. P., 2020. Antifungal activity of liriodenine on agentes of systemic mycoses, with emphasis on the genus Paracoccidioides. J Venom Anim Toxins incl Trop Dis., 26. https://doi.org/10.1590/1678-9199-JVATITD-2020-0023
Wang M, Shen Q, Xu G, Guo S (2014) New insight into the strategy for nitrogen metabolism in plant cells. Int Rev Cell Mol Biol 310:1–37. https://doi.org/10.1016/B978-0-12-800180-6.00001-3
Wink, M, 2010. Biochemistry of plant secondary metabolism. Annual Plant Reviews, vol. 40, 2nd ed Publisher: Wiley Blackwell Publishing Ltd 464 Pages. https://doi.org/10.1002/9781444320503
Zhang J, Tian H, Sun H, Wang X (2016) Antifungal activity of trans-2-hexenal against Penicilliumcyclopium by a membrane damage mechanism. J Biochem 41:1–9. https://doi.org/10.1111/jfbc.12289
Zhang S, Li Q, Ma K, Chen L (2001) Temperature-dependent gas exchange and stomatal/non-stomatal limitation to CO2 assimilation of QuercusLiaotungensis under Midday High Irradiance. Photosynth Res 39:383–388. https://doi.org/10.1023/A:1015130326683
Zhao D, Reddy KR, Kakani VG, Reddy VR (2005) Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur J Agron 22:391–403. https://doi.org/10.1016/j.eja.2004.06.005
Zhou Y, Zhang Y, Wang X, Cui J, Xia X, Shi K, Yu J (2011) Effects of nitrogen form on growth, CO2 assimilation, chlorophyll fluorescence, and photosynthetic electron allocation in cucumber and rice plants. J Zhejiang Univ Sci B 12:126–134. https://doi.org/10.1631/jzus.B1000059
Acknowledgements
We would like to acknowledge to Professor Magali Ribeiro da Silva, together with the Department of Forestry Science at the Faculty of Agronomic Sciences, UNESP, Campus of Botucatu/SP, for providing Annona sylvatica seedlings used in the present work.
Funding
This work was supported by the National Council for Scientific and Technological Development (CNPq) (169222/2017–9), financed in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) - Finance Code 001 and CAPES-Print - Unesp.
Author information
Authors and Affiliations
Contributions
PLCC and GF contributed equally to this manuscript. CSFB assisted in the elaboration and development of the work, in addition to making laboratory resources available. IDCC carried out the extraction and identification of total alkaloids and liriodenine. MOMM and MARV contributed with the analysis of leaf volatiles compounds. MCS and FGC contributed with all the work processes. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Corrêa, P.L.C., De-la-Cruz-Chacón, I., Sousa, M.C. et al. Effect of Nitrogen Sources on Photosynthesis and Biosynthesis of Alkaloids and Leaf Volatile Compounds in Annona sylvatica A. St.-Hil. J Soil Sci Plant Nutr 22, 956–970 (2022). https://doi.org/10.1007/s42729-021-00705-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-021-00705-8