Abstract
This work aimed to investigate the effect of copper (Cu)-, iron (Fe)-, and nitrogen (N)-doped titanium dioxide nanoparticles (TiO2 NPs) toward cowpea plants as a promising approach in agriculture. TiO2 NPs were synthesized chemically and characterized in the light of X-ray diffraction (XRD), transmission electron microscope (TEM), ultraviolet–visible diffuse reflectance analyses (UV–Vis DRS), and energy dispersive spectroscopy (EDS). Foliar application of doped NPs on cowpea plants was achieved at the third leaf stage, then morphological and physiological studies were estimated. XRD results showed that phase structures of samples were a mixture of anatase and rutile phases. The incorporation of Cu, Fe, and N as dopants into the TiO2 induced changes in NP shapes and sizes. Cu-doped TiO2 NPs recorded the less particle size (5 nm) followed with Fe and N doped TiO2 NPs (11 and 18 nm) as compared to pure nano TiO2 (26 nm). Foliar application of TiO2 NPs on cowpea (Vigna unguiculata) as fertilizer was studied. Morphological studies revealed significant increments in plant lengths, fresh and dry weights in response to treatments. Shoot length increased by 14, 118, 86, and 68% while roots length recorded 16, 110, 85, and 51% increment in plants treated with pure nano TiO2, Cu-, Fe-, and N-doped TiO2 nanoparticles. Crop plant productivity enhanced in response to doped TiO2 NP treatment; the greatest values in pods number plant−1, seed number pod−1, and seed index were recorded in Cu-doped TiO2 NPs treatment. Phytohormone content changed in response to treatments as compared with untreated plants; indole acetic acid (IAA) and gibberellic acid (GA3) increased while abscisic acid (ABA) decreased engaging with plant growth enhancement. Amino acids, total soluble protein, and macro- and micro-nutrients increased; however, total soluble sugars decreased in plants that received doped TiO2 NPs. Oxidative stress in plant lipid peroxidation, hydrogen peroxide, and membrane leakage (MDA, H2O2, and EL) decreased combined with receiving Cu-, Fe-, and N-doped TiO2 NPs. Results showed that doping material XRD, TEM, and EDS results confirmed more uniform replacement and dispersion of Ti by Cu, Fe, and N in the TiO2 lattice structure. The DRS analysis results obviously presented the shift of absorption band gap towards the visible region upon doping TiO2 with Cu, Fe, and N, with the minimum value of 3.01 eV for Cu-doped TiO2. These results assumed that applying Cu-, Fe-, and N-doped TiO2 nanoparticles on cowpea plants could enhance plant growth, productivity, and alert physiological changes. Photosynthetic pigments and growth-activating hormone contents increased; however, lipid peroxidation and hydrogen peroxide content decreased. Amino acids and soluble protein content increased on the contrary soluble sugars decreased in treated plants. In addition, nitrogen, phosphorus, and potassium, contents increased in treated cowpea plants as compared with control plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, nanoparticle (NP) applications in agricultural research were studied by many researchers. NP applications such as iron oxide nanoparticles (Fe2O3 NPs), zinc oxide nanoparticle (ZnO NPs), silver oxide nanoparticles (Ag2O NPs), and titanium dioxide nanoparticles (TiO2 NPs) can alleviate the effects of plant pathogens and work as nano-fertilizers (Feidantsis et al., 2020). Nano-fertilizers could improve plant growth, photosynthetic ability and availability of nutrient (Irshad et al., 2021); between these nanoparticles, TiO2 NPs was used for enhancing fertilizers uptake and reducing abiotic stress on plants (Gul et al., 2020; Hu et al., 2020). The TiO2 NP application enhances the photosynthetic activity, plant biomass, and nutrient uptake (Lyu et al., 2017a, b). TiO2 and carbon nanotubes were classified by Liu and Lal (2015) as nano-fertilizers and plant-growth enhancers with unpredictable action, a contradictory performance of TiO2 nanoparticles application on the plant are previously reported by Irshad et al. (2021). According to Gohari et al. (2020), titanium oxide could affect a plant’s metabolic activity to induce physiological and morphological changes, overcoming oxidative stress, increasing chlorophyll, and plant productivity as well. However, other studies revealed TiO2 NPs also provoke cytotoxicity as well as genotoxicity in plants (Bo et al., 2014 and Picado et al., 2015). This effect may depend on TiO2 NPs concentration, crystal shape and size, method of application, and the plant species (Raliya et al., 2019 and Gallo et al., 2021).
Titanium dioxide (TiO2) NPs were recorded as the most produced NPs in the USA, almost 38,000 metric tons of TiO2 are produced yearly, and more production increment is expected (Hendren et al., 2011).
Doping technique based on the introduction of specific elements in the vacant crystal lattice of another element resulted in improved properties that can be employed for different purposes and improve the efficiency of NPs by manipulating the band gap energy (Carofiglio et al., 2020). Doping different elements into the titanium oxide NPs and the effect this has on photocatalysis (Nah et al., 2010 and Reszczynska et al., 2014) and photoelectric conversion (Duan et al., 2012 and Elghniji et al., 2012) were widely studied. Doping TiO2 with selected elements such as Cu, Fe, and N may generate oxygen vacancies, create grain boundaries, introduce surface, and bulk defects for efficient charge trapping and reduced recombination rates (Zhang et al., 2010), increase dye adsorption leading to higher charge carrier injection (Zhang et al., 2010), cause a reduction in particle size (Xie et al., 2013), induce phase transitions (Ghanbari Niaki et al., 2014), and decrease band gaps for absorption in the visible or near-infrared (Zhang et al., 2019). Application of doped nutrients to plants provides slow controlled release of needed nutrients for plant growth and development (Lateef et al., 2016). Few reports about nanoparticles surface coated and doped are available while an additional investigation is needed.
Cowpea (Vigna unguiculata) belongs to the family Papilionaceae (Fabaceae) order Leguminosae and genus Vigna (Singh et al., 1997). It is widely grown in Africa and India and used for human consumption, as green manure and organic material source. Cowpea has been introduced to Egypt agriculture as both human and animal diet (Hamd Alla et al., 2014). Dry seeds are rich in proteins (25%) and carbohydrates (64%) (Odedeji and Oyeleke, 2011). The effects of N-, Fe-, and Cu-doped TiO2 NPs on Vigna unguiculata plants as new nano-fertilizer generation have not been studied yet.
The present study was conducted to examine the effects of the chemical and bioactive differences between pure titanium oxide NPs and Cu-, Fe-, and N-doped titanium oxide NPs toward Vigna unguiculata plants as new nano-fertilizer generation.
2 Material and Methods
All chemicals were analytical grade and utilized without further purification. Titanium tetraisopropoxide (TTiP) (97.0%), copper nitrate (Cu (NO3)2·3H2O), ferric nitrate (Fe(NO3)3·9H2O), and urea (NH2CONH2). Nitric acid (HNO3) was supplied from Sigma-Aldrich. Plant seeds were provided by Field Crops Research Institute, Agricultural Research Center (ARC), Giza, Egypt.
2.1 Synthesis and Characterization of Nanoparticles
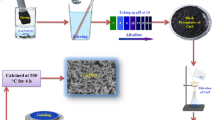
Nitrogen-, ferric-, and copper-doped TiO2 were prepared through the sol–gel method using titanium tetraisopropoxide (TTIP), and urea, ferric nitrate, and copper nitrate as sources of Ti, N, Fe, and Cu, respectively. In a typical experiment, 0.042 mol of TTIP was dissolved in 9 mL of absolute ethanol and stirred using a magnetic stirrer for 1 h. A total of 2 wt% of (urea or ferric nitrate or copper nitrate) was dissolved in 10 ml of distilled water and then added to the mixture and stirred continuously for 1 h. The pH of the solution was adjusted to 2.0 by the addition of HNO3 at room temperature. Next, the prepared solution was heated to 50 °C, followed by drop-wise addition of 2 mL distilled water to the solution until the gel was formed. Finally, the obtained gel was dried in the electric oven for 2 h at 70 °C and then calcined at 300 °C for 1 h.
The crystallographic structure of the samples was examined by X-ray diffraction (XRD) at room temperature using an X’pert Philips diffractometer (PW-3710) equipped with Cu Kα radiation (λ = 1.5404 Å), 40 kV and 30 mA and scan rate 2.5° min−1. Transmission electron microscopy (TEM) images were obtained using JEM-2000 EX (JEOL, Japan) at an accelerating voltage of 200 kV. Ultraviolet–visible diffuse reflectance analyses (UV–Vis DRS) of the samples were carried out at room temperature using a JASCO V-550 spectrometer (Japan), in the range of 200 − 750 nm. Energy dispersive spectroscopy (EDS) (S-3400 N II, Hitachi, Japan) was used.
2.2 Green House Experiment
The study was conducted at the greenhouse of the faculty of education, Ain Shams University, Cairo, Egypt. Cowpea (Vigna unguiculata) seeds were obtained from the agriculture research center (ARC). Homogenous seeds were selected and surface sterilized using 1% sodium hypochlorite solution, washed thoroughly with distilled water, and then left to dry in the air. The seeds were sown in pots 25 cm in diameter filled with 1.5 kg air-dried mixture of sand, salt, and clay (24.2, 29.5, and 46.3%) the potential of hydrogen ions (pH) was 7.7.5 ± 0.03 while the electrical conductivity (EC) was 1.72 ± 0.02 dSm−1. The growth conditions maintained during the experiments, light duration 12 h, temperature 23 ± 4 °C, and relative air humidity 50 ± 2%.
After 2 weeks, seedlings were thinned to five per pot. The pots were divided into four groups each consists of five pots. The first group worked as a control group, the second group received copper doped on titanium nanoparticles (Cu + TiO2) treatment, the third group received iron doped on titanium nanoparticles (Fe + TiO2) treatment, and the fourth group received nitrogen doped on titanium nanoparticles (N + TiO2) treatment. All treatments were applied at 10 ppm concentration as exogenous foliar on plant leaves after 15 days after emergence.
2.3 Morphological and Physiological Studies
-
Morphological criteria were determined as root and shoot lengths, root and shoot fresh and dry weights, number of pods plants−1, number of seeds pods−1, number of seeds plant−1, and weight of 100 seeds.
-
Photosynthetic pigment contents of fresh leaves chlorophyll a and b (Chl a and Chl b), carotenoids, and total pigments contents in fresh leaves were extracted by 85% acetone, estimated using the method of Lichtenthaler and Buschmann (2001). Using spectrophotometer at 470-, 649-, and 665-nm wavelengths.
-
Total free amino acids (TF amino acids) were extracted by 70% boiling ethanol according to Sugano et al. (1975) and estimated using 1% Ninhydrin reagent according to Yemm and Cocking (1955).
-
Total soluble sugars (TSS) were extracted in 80% ethanol and determined spectrophotometry following the phenol sulphuric method of Homme et al. (1992) and Yemm and Willis (1954).
-
Total soluble protein content was measured by using Folin-Ciocalteu reagent after extracting using phosphate buffer pH 6.5 according to Lowry et al. (1951).
-
Phytohormones gibberellic acid (GA3), abscisic acid (ABA), and indole acetic acid (IAA) were determined using HPLC according to the method described by Vogel (1975).
-
Mineral content: Nitrogen (N) concentration in the plant tissue was determined using the Kjeldahl procedure (AOAC 1995). Phosphorous (P) was determined by using a spectrophotometer (Chapman and Pratt 1978). Potassium (K), copper (Cu), and iron (Fe) were assayed using an atomic absorption spectrophotometer (Ieggli et al., 2010). Titanium (Ti) content was determined by using inductively coupled plasma mass spectroscopy (ICP-MS).
-
Oxidative stress: Lipid peroxidation was determined as malondialdehyde (MDA) content using 6% trichloroacetic acid and determined spectrophotometry according to Jiang and Zhang (2002), hydrogen peroxide (H2O2) was extracted by phosphate buffer and determined according to Velikova et al. (2000), and the percentage of membrane leakage (EL) was assayed according to Lutt et al. (1996).
-
Statistical analysis: The study was designed in a complete randomized block; three replicates were used. All results are presented as mean ± standard deviation. Data were analyzed using one-way analysis of variance (ANOVA) using SPSS 20.0 (SPSS Inc., Chicago, IL, USA) followed by an LSD test.
-
3 Results
3.1 Crystal Structure, Morphology, and UV–Vis Diffuse Reflectance Spectroscopic Analysis
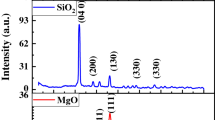
X-ray diffraction (XRD) patterns of pure TiO2 and Cu-, Fe-, and N-doped TiO2 NPs are displayed in Fig. 1. All the diffraction peaks are assigned to tetragonal anatase (JPCDS No. 84–1285) and rutile (JPCDS No. 87–0920) TiO2 which shows that the prepared samples have high purity and do not observe any peaks of copper, iron, and nitrogen doping in the XRD pattern of the samples. These give the suggestion that doping may be incorporated into the TiO2 lattice. The diffraction peaks at 2θ values 25.27°, 38.02°, and 48.06° can be indexed to (101), (004), and (200) crystal planes of anatase, while peaks at 2θ = 27.35, 36.26, and 53.99° can be attributed to (110), (101), and (211) crystal planes of rutile. A (101) and R (110) peaks slightly shift to higher angles with the dopants due to occurring distortions in structure upon replacing Ti ions with dopants nanoparticles.
The average crystallite size of pure TiO2 and Cu-, Fe-, and N-doped TiO2 nanoparticles is determined using Scherrer’s formula (Chen et al., 2012a, b).
where λ is the wavelength of the incident X-ray (1.5406 Å), β is full width at half maximum (FWHM) in radiance, and θ is the angle of diffraction. From the XRD data, the average crystallite size of pure TiO2 and Cu-, Fe-, and N-doped TiO2 nanoparticles was estimated, and it was found to be 30.20, 9.50, 15.50, and 23.59 nm respectively. The decreased average crystallite size with doping into the TiO2 lattice may be due to inhibiting the TiO2 formation and growth (Santos et al., 2015 and Ahmed et al., 2018).
The shape and average particle size of the prepared NPs were investigated using transmission electron microscopy. TEM images of pure TiO2 and N-, Fe-, and Cu-doped TiO2 NPs are shown in Fig. 2. The TEM image of the pure TiO2 sample (Fig. 2a) shows the aggregation of spherical structures and hexagonal forms with an average particle size of 26 nm. While the morphology of Cu-, Fe-, and N-doped TiO2 nanoparticles (Fig. 2b–d) shows the aggregation of nearly spherical-shaped particles, the sizes of the particles were in the nanometer range with average diameters 5, 11, and 18 nm, respectively.
To explore the elemental composition of the samples obtained from EDS measurements, Fig. 3 (a, b, and c) displays the EDS spectra of Cu-, Fe-, and N-doped TiO2. The EDS analysis clearly presented the presence of Cu, Fe, and N dopants in the powder and indicated that the doping concentrations were 1.86, 1.79, and 1.84 wt% for Cu, Fe, and N, respectively, close to the value of 2 wt% (the inset table).
UV–vis diffuse reflectance spectroscopic of all samples are present in Fig. 4(a). All the samples had significant adsorption in the UV region. However, there was no adsorption in the visible light region. It is observed that by doping, the prepared samples were expanded to the visible light range and exhibit a redshift to the higher wavelengths which means the band gaps decreased. The band gap energies (Eg) of the samples are determined by the Tauc relation (Arunachalam et al., 2015):
where α is the absorption coefficient, hν is the energy of the incident photon, Eg is the optical band gap, and B is a proportional constant. the power coefficient m = 1/2, 2, 3/2, or 3 corresponds to direct allowed, indirect allowed, direct forbidden, and indirect forbidden transition, respectively. For direct optical band gap, we have taken m = 1/2 and plotted the curves of (αhv)2 versus hv. Then the values of Eg are obtained by extracting the linear portion of curves to (αhv)2 = 0 (Fig. 4b). This figure shows that the incorporation of Cu, Fe, and N ions into the TiO2 lattice leads to the decrease of band gap energy and is found to be 3.21, 3.01, 3.07, and 3.14 eV for TiO2, Cu-, Fe-, and N-doped TiO2 nanoparticles, respectively. Therefore, the incorporation of Cu, Fe, and N ions into the TiO2 lattice leads to the decrease of band gap energy. This diminution in band gap due to introducing new energy levels between the valence band and the conduction band (Sean et al., 2015). It was observed that the Cu-doped TiO2 nanopowder showed a minimum band gap of 3.01 eV. Hence, it has the potential to be activated by visible light and improving the photosensitivity in the visible light region.
3.2 Plant Morphological and Physiological Studies and Yield Attributes
The results in Fig. 5 show that cowpea treated with pure TiO2 NPs showed non-significant changes in roots and shoots lengths Fig. 5 (a and b) while fresh/dry weights Fig. 5 (c, d, e, and f) increased significantly as compared with control plants. Nano Cu-, Fe-, and N-doped TiO2-treated plants showed the greatest values; almost 118, 86, and 68% increment in the shoot length and 110, 85, and 51% in the root length were recorded respectively as compared with control plants. Also, root and shoot fresh weights increased in response to Cu-doped Ti NPs (150 and 109%) as compared to untreated cowpea plants.
Effect of doped titanium NPs on morphological characters of cowpea plants. Root length (a), shoot length (b), root fresh weight (c), shoot fresh weight (d), root dry weight (e), and shoot dry weight (f). Values are the mean of three replicates. Means followed by the same letters are not significantly different at P ≤ 0.05 according to the least significant difference (LSD) test
Plant productivity represented in pod number plant−1, seeds number pod−1, and weight of 1000 seeds enhanced in cowpea plants treated with Cu-, Fe-, and N-doped TiO2 NPs; however, no significant increment was recorded for pure TiO2 NP application compared with untreated plants (Fig. 6 a–d). Among the treatments, Cu-doped titanium oxide NP-treated plants recorded the greatest values, and pods produced plant−1 increased almost onefold while the number of seeds plants−1 showed a fivefold increment as compared with other control groups.
Effect of doped titanium NPs on yield characters of cowpea plants. Pods number plant −1 (a), seed number pod −1 (b), seed number plant −1 (c), and weight of 100 seed (d). Values are the mean of three replicates. Means followed by the same letters are not significantly different at P ≤ 0.05 according to the least significant difference (LSD) test
The effect of different TiO2 NPs on cowpea photosynthetic pigments content is demonstrated in Table 1. Pure TiO2 NPs showed nonsignificant changes in chl a and chl b contents (2.17 and 1.02 mg g−1) as compared with control plants (2.17 and 1.22 mg g−1) while Cu-, Fe-, and N-doped NPs induced significant increment (4.79 and 2.98 mg g−1, 4.45 and 2.33 mg g−1, and 3.31 and 1.63 mg g−1 respectively). On the contrary, carotenoids accumulated in plants treated with pure Ti NPs and recorded the greatest value (1.4 mg g−1) followed by Cu-and Fe-doped NPs (1.2 and 1.1 mg g−1).
When cowpea plants were sprayed with nanoparticles, their metabolic process fluctuated and physiological process displayed total free amino acids, total soluble sugars, and total soluble protein contents that showed great variation in cowpea plants under different Ti NPs. Total soluble sugars increased in response to pure TiO2 NPs comparing with Cu-, Fe-, and N-doped TiO2 nanoparticles; however, TiO2 pure NPs showed no significant effect on amino acids and total soluble protein contents. On the other hand, Cu-doped TiO2 NP treatment showed the highest values for amino acids and protein contents and the less values for total soluble sugar content (Table 2).
Phytohormones are important growth-regulating compounds synthesized by plants. The cowpea plant growth regulator distribution showed fluctuations as affected with different treatments (Table 3). Growth-promoting in hormone gibberellic acid (GA3) and indole acetic acid (IAA) contents increased in plants that experienced Cu-, Fe-, and N-doped titanium oxide NPs. The greatest value was recorded in Cu-doped NPs (0.047 and 0.52 µg g-1) respectively. Pure TiO2 NPs do not affect the activating plant growth hormone contents significantly. On the other side, growth-inhibitory phytohormone abscisic acid (ABS) decreased in plants that received doped TiO2 NPs. The less values were given by Cu-doped TiO2 NPs (0.012 µg g-1) comparing with pure TiO2 NPs treatment and control groups (0.062 and 0.053 µg g-1) respectively. There are no significant changes detected in ABA content between the control group and the pure Ti NP-treated group.
Pure TiO2 treatment induced no significant changes in cowpea plant mineral uptake; however, Cu-coped TiO2 NPs showed significant increment in macroelements N, P, and K (3.2, 0.31, and 3.18 mg g−1) as compared with control plants (1.5, 0.21, and 2.47 mg g−1). Microelements Cu, Fe, and Ti contents fluctuated as shown in Table 4. The values 2.23, 0.98, and 0.074 mg g−1 were given due to Cu-doped NP treatment while, 1, 0.72, and 0.035 mg g−1 values were recorded in control plants.
Malondialdehyde (MDA) caused by overproduction of reactive oxygen species (ROS) is considered an indicator of membrane lipid peroxidation determined to evaluate TiO2NPs influence on cell membrane integrity. Table 5 demonstrates that lipid peroxidation, hydrogen peroxide, and membrane ion leakage decreased significantly in cowpea plants treated with either pure TiO2 NPs or Cu-, Fe-, and N-coped TiO2 NPs, which may lighten the TiO2 NPs’ ability in plant oxidative stress alleviation.
The lowest oxidative stress values were given in plants treated with Cu-doped TiO2 NPs.
4 Discussion
Our results showed a significant increment in plant growth in response to doped TiO2 NPs treatment as compared with untreated plants. Consistent with our results, Spinacia oleracea plants treated with TiO2 NPs recorded an enhancement in the root, shoot lengths, and biomass (Azmat et al., 2020). TiO2 NP application enhanced shoots and root lengths and wheat grain quality (Ullah et al., 2020). TiO2 application promotes the lengths and dry and fresh weights of roots and shoots in Artemisia absinthium plants (Bami et al., 2021). Titanium (Ti) has a biological effect on plant growth and performance; it was found that titanium increased the intensity of green color in plant leaves, activity of enzymes, enhancing growth hormone content, and increasing nutrients uptake (Lyu et al., 2017a, b). According to the present study, the greatest growth values were given by Cu compared with pure and Fe- and N-doped TiO2 NPs.
Our results (Fig. 6) revealed an increment in the number of pods and seeds per plant as well as the weight of 100 seeds in cowpea plants that received pure and doped TiO2 NPs. Previous studies supported our results. Onion plants treated with TiO2 NPs showed an increment in fruit production (Raskar and Laware 2013). The same results were recorded in coriander (Khater 2015), sunflower (Abdul Hafeez et al., 2015), and wheat (Kolenčík et al., 2020). Yield increment is combined with plant growth and photosynthesis enhancement. The application of titanium showed a positive impact on plant growth, productivity, and crop quality (Lyu et al., 2017a, b). Titanium NP treatment has a potential role in increasing plant tolerance to inadequate conditions such as drought, salinity, and cold stress by increasing enzymatic activities and plant performance. In addition, the accumulation of vitamin C, anthocyanin, and flavonoids in fruits was proved in many studies in response to TiO2 NP treatment (Skupien, and Oszmia ´ nski 2007).
As reported in previous studies, rutile and anatase phases of titanium oxide NPs enhanced photosynthesis and plant pigment content in tomato, cucumber, and brad bean plants (Zhang et al., 2008, Qi et al., 2013 and Abdel Latef et al., 2017) while titanium nanoparticle application on Raphanus sativus plants showed no changes in photosynthetic pigment content comparing with control plants (Tighe-Neira et al., 2020). The same results were provided by Song et al. (2020) in cucumber plants. Cowpea photosynthetic pigment content enhancement in response to pure, and doped TiO2 NP treatment as compared with control plants was proved by the present study. Applying titanium NPs on plants induced immobilization of Ca+ ions in guard cells of leaf stomata leading to a delayed stoma closer (Tighe-Neira et al., 2020 and Wang et al., 2021). Khater (2015) claimed that enhancing pigments content in coriander plants in response to TiO2 NPs treatment may return to protecting thylakoid in the chloroplast, recovering chlorophyll molecular structure, and light-absorbing process. Nanoparticles affect photosynthesis efficiency by increasing carbon gaining and enhanced the activity of Ribulose1,5-bisphosphate carboxylase/oxygenase enzyme (Lyu et al., 2017a, b).
Data in Table 1 revealed the most enhancement in photosynthetic pigment content was recorded in cowpea plants treated with Cu-doped TiO2 NPs. This could be explained according to the results given in Fig. 4. The Cu-doped TiO2 NPs showed the minimum band gap compared with other TiO2 NPs (3.01) eV. Hence, it has the potential to be activated by visible light and improving photosensitivity (Isari et al., 2020). Copper ions contribute to the photosynthesis process and pigment formation in thylakoids and influence primary electron donors in photosystem I (PSI). Applying Cu treatment in low doses could enhance chlorophyll content and the photosynthesis process in citrus plants (Giannakoula et al., 2021).
Total soluble sugars reduced in cowpea plants after being treated with Cu-, Fe-, and N-doped TiO2 NPs while soluble proteins and amino acids increased. Soluble sugar reduction could be explained by Azmat et al. (2020) who claimed reduction in soluble sugar content in Spinacia oleracea plants in response to NP treatment may indicate the active transfer of these molecules into starch contents. The authors suggested that increasing decreasing soluble sugar content could be associated with increasing photosynthesis process and starch formation. Supporting our results, Zhang et al. (2020) revealed starch and amino acids accumulated in rice plants under TiO2 NP treatment. Titanium ions could bind to biologically active molecules like proteins and amino acids resulting in increasing molecule solubility in water as indicated by Zierden and Valentine (2016). A possible role of titanium in the living cell could be the maintenance of Fe ions which play the role as a co-factor for some enzymes. Also, many studies claimed that titanium affected gene expression for important enzymes (Lyu et al., 2017a, b). The increment of total amino acids supports the growth enhancement results; it could be associated with the effect of titanium to improve plant nitrogen status (Abdel latef et al., 20,017).
Copper-doped Ti NPs showed the less spherical size (5 nm) that could catalyze chemical reactions responsible for the synthesis of amino acids and soluble protein as compared with larger nanoparticles (pure, Fe-and N-doped TiO2). Small NPs were associated with high large surface area and showed a significant acceleration in chemical reactions (Abdal Dayem et al., 2017).
Plant hormones are naturally occurring organic compounds that regulate plant growth. IAA and GA3 regulate cell division, cell elongation, stem elongation, seed germination and dormancy, and presence of flowers and decrease senescence of leaves and fruit while ABA is involved in stomatal closure, cell division inhibition, and growth inhibition (Takatsuka and Umeda 2014). The present study revealed a reduction in ABA combined with estimation of IAA and GA3 synthesis in cowpea plants in response to pure and doped Ti NP treatment. In alignment with our results, Jiang et al. (2017) reported that both IAA and GA3 contents increased in wheat seedlings under titanium oxide NP application while ABA content decreased. The effect of TiO2 NPs in improving plant nitrogen, amino acid content, and enzyme activities was reported by Lyu et al. (2017a, b). This could be a probable reason for cowpea plant hormone alternation. According to Abdelaal et al. (20,121), the production of indole acetic acid, gibberellic acid, and abscisic acid in the plant was controlled by the content of N, P, amino acid, and enzyme activities.
Studies showed that alter sites and interaction strength between NPs and their targets depend on the particle size and shape (Missaoui et al., 2017) in the light of previous finding of the changes between Cu-, Fe-, and N-doped, and pure TiO2 NPs effect on cowpea plants could be cleared.
Nanoparticles travel in plant tissues through the phloem; it was proven that foliar NPs translocated from the leaves to stem, roots, and grains (Uhram et al., 2013). Our results revealed titanium accumulation in treated plants as compared with untreated plants (Table 4). In accordance with our results, Ti mineral detected in carrot plants treated with surface-modified TiO2 NPs combined with an elevation in Fe and K uptake (Wang et al., 2021). A positive correlation between Fe and Ti contents was mentioned by Jacob et al. (2013). Many reports postulated the involvement of titanium in improving the Fe activity in plant tissues and participating in N fixation in legume root nodules (Lyu et al., 2017a, b). Other reports claimed that TiO2 NPs may contribute to K ion regulation and increasing plant uptake through regulating cytokinins (Wang et al., 2021). A significant increase in K cucumber grown in soil treated with 500 mg/kg TiO2 NPs was recorded by Servin et al. (2013). In addition, TiO2 NPs improved wheat phosphorous (P) content without applying P-containing fertilizer (Ullah et al., 2020). Song et al. (2020) reported foliar exposure to TiO2 NPs could elevate K accumulation in cucumber stem potentially.
TiO2 NPs showed a protective role in alleviating cowpea plant oxidative stress activity, supporting our results. Castiglione et al. (2016) claimed a reduction in H2O2 content in Ti NP-treated faba bean; also, low doses of TiO2 NPs induced a significant reduction in lipid peroxidation in cucumber seedlings compared with untreated seedlings (Song et al., 2020). In maize plants, reduction in H2O2 and lipid peroxidation in response to Ti NP treatment was recorded (Zhao et al., 2019). Such reduction in oxidative stress might be associated with increased antioxidant response and cell membrane defensive role (Posmyk et al., 2009). It was found that in spinach plants, treatment with TiO2 NPs showed a reduction in the accumulation of superoxide radicals, H2O2, and MDA content as well as elevation activities of antioxidant enzymes catalase (CAT), ascorbate peroxidase (APX), superoxide dismutase (SOD), and guaiacol peroxidase (Lei et al., 2008). According to Missaoui et al. (2017), 50 mg L−1 TiO2 NPs activated fenugreek ability to synthesis antioxidant enzymes and protective metabolites like flavonoids. Reactive oxygen species (ROS) generation in nano-treated plants increased by increasing particles sizes (Abdal Dayem et al., 2017) which explains the reduction in ROS in cowpea plants treated with Cu-doped TiO2 NPs as compared with other treatments.
5 Conclusion
Results confirmed the dispersion and replacement of Ti by copper Cu, iron Fe, and nitrogen N in the titanium dioxide nanoparticles (TiO2 NPs) structure. Differences in doped particle shapes and sizes were confirmed by transmission electron microscope (TEM) and X-ray diffraction analysis. Cu-doped NPs showed less size. The average crystallite size of pure TiO2 and Cu-, Fe-, and N-doped TiO2 nanoparticles was estimated, and it was found to be 30.20, 9.50, 15.50, and 23.59 nm respectively. The Ultraviolet-visible diffuse reflectance (UV-Vis DRS) analysis results obviously presented the shift of absorption band gap towards the visible region upon doping TiO2 with both Cu, Fe, and N, with the minimum value of 3.01 eV for Cu-doped TiO2. Pure and doped TiO2 NP treatments on cowpea plants showed enhancement in plant growth, productivity, photosynthetic pigment content, and growth hormones. Total soluble sugar content decreased combined with increment in amino acids, soluble protein, and mineral uptake. Cowpea plant showed decreasing in oxidative stress in response to treatment with pure and doped TiO2 NPs while the less values were recorded for Cu-doped NPs compared with pure TiO2 NP treatment and untreated plants. However, further studies are needed to clarify the factors affecting TiO2 NP utilization by plants.
Change history
08 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42729-021-00682-y
References
Abdal Dayem A, Hossain M, LEE, Kim, SB, Saha K, Yang S, Choi GM, Cho H, (2017) The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci 18:120. https://doi.org/10.3390/ijms18010120
Abdelaal K, AlKahtani M, Attia K, Hafez Y, Király L, Künstler A (2021) The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 10:520. https://doi.org/10.3390/biology10060520
Abdel Latef AH, Srivastava AK, Abd El-sadek MS, Kordrostami M, Phan Tran LS (2017) Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad Dev 29:1065–1073. https://doi.org/10.1002/ldr.2780
Abdul Hafeez A, Mahmood T, Muhammad HJ (2015) Potential of copper nanoparticles to increase growth and yield of wheat. J Nanosci Adv Tech 1(1):6–11. https://doi.org/10.24218/jnat.2015.02
Ali T, Ahmed A, Alam U, Uddin I, Tripathi P, Muneer M (2018) Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater Chem Phys 212:325–335. https://doi.org/10.1016/j.matchemphys.2018.03.052
AOAC. (1995) In: “Official Methods of Analysis”. 16th ed. Association of Official Analytical Chemists, Arlington, Virginia.
Arunachalam A, Dhanapandian S, Manoharan C, Sivakumar G (2015) Physical properties of Zn doped TiO2 thin films with spray pyrolysis technique and its effects in antibacterial activity, Spectrochim. acta part A Mol. Biomol Spectrosc 138:105–112. https://doi.org/10.1016/j.saa.2014.11.016
Azmat R, Altaf I, Moin S, (2020) The reflection of the photocatalytic properties of TiO2 nanoparticles on photosynthetic activity of Spinacia oleracea plants. Pak. J. Bot., 52(4): https://doi.org/10.30848/PJB2020-4(2).
Bami SS, Khavari-Nejad RA, Ahadi AM et al (2021) TiO2 nanoparticles effects on morphology and physiology of Artemisia absinthium L. under salinity stress. Iran J Sci Technol Trans Sci 45:27–40. https://doi.org/10.1007/s40995-020-00999-w
Bo Y, Jin CY, Liu YM, Yu WJ, Kang HZ (2014) Metabolomic analysis on the toxicological effects of TiO2 nanoparticles in mouse fibroblast cells: from the perspective of perturbations in amino acid metabolism. Toxicol Mech Methods 24(7):461–469. https://doi.org/10.3109/15376516.2014.939321
Carofiglio M, Barui S, Cauda V, Laurenti M, (2020) Doped zinc oxide nanoparticles: synthesis, characterization and potential use in nanomedicine. Appl. Sci. (Basel, Switzerland), 10(15), 5194. https://doi.org/10.3390/app10155194.
Castiglione MR, Giorgetti L, Bellani L, Muccifora S, Bottega S, Spanò C (2016) Root responses to different types of TiO2 nanoparticles and bulk counterpart in plant. model system Vicia faba L. Environ Exp Bot 130:11–21. https://doi.org/10.1016/j.envexpbot.2016.05.002
Chapman HD, Pratt PE, (1978) Method of analysis for soil plant and water. University of California, Dep. of Agric. Sci. U.S.A.: 1–309. https://doi.org/10.1097/00010694-196201000-00015.
Chen H (2018) Metal based nanoparticles in agricultural system: behavior, transport, and interaction with plants. Chem Spec Bioavailab 30(1):123–134. https://doi.org/10.1080/09542299.2018.1520050
Chen X, Lu DF, Zhang SH, Huang BZ (2012a) Preparation and properties of SiO2 supported nitrogen-doped visible-light response TiO2-xNy/SiO2 photocatalysts. Chin J Inorg Chem 28:307–313. https://doi.org/10.1016/S1872-2067(06)60037-5
Chen XY, Lu DF, Lin SF (2012b) Preparation and properties of sulfur-doped visible light response S-TiO2/SiO2 photocatalyst. Chin J Catal 33:993–999. https://doi.org/10.3724/SP.J.1088.2012.11127
da Silva EB, Mussoline WA, Wilkie AC, Ma LQ (2019) Anaerobic digestion to reduce biomass and remove arsenic from as-hyperaccumulator. Pteris Vittate Environ Pollut 250:23–28. https://doi.org/10.1016/j.envpol.2019.03.117
Duan Y, Fu N, Liu Q, Fang Y, Zhou X, Zhang J, Lin Y (2012) Sn-doped TiO2 photoanode for dye-sensitized solar cells. J Phys Chem C 116:8888–8893. https://doi.org/10.1021/jp212517k
Elghniji K, Atyaoui A, Livraghi S, Bousselmi L, Giamello E, Ksibi M (2012) Synthesis and characterization of Fe3+ doped TiO2 nanoparticles and films and their performance for photocurrent response under UV illuation. J Alloys Compd 541:421–427. https://doi.org/10.1016/j.jallcom.2012.07.010
Gahlawat G, Choudhury AR (2019) A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv 9:12944–12967. https://doi.org/10.1039/C8RA10483B
Gallo V, Zappettini A, Villani M, Marmiroli N, Marmiroli M (2021) Comparative analysis of proteins regulated during cadmium sulfide quantum dots response in Arabidopsis thaliana wild type and tolerant mutants. Nanomaterials 11:615. https://doi.org/10.3390/nano11030615
Ghanbari Niaki AH, Bakhshayesh AM, Mohammadi MR (2014) Double-layer dyesensitized solar cells based on Zn-doped TiO2 transparent and light scattering layers: improving electron injection and light scattering effect. J Sol Energy 103:210–222. https://doi.org/10.1016/j.solener.2014.01.041
Giannakoula A., Therios I., Chatzissavvidis, C, (2021) Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. The role of antioxidants in oxidative damage as a response to heavy metal stress. Plants 10, 155. 10.3390/ plants10010155
Gohari G, Mohammadi A, Akbari A et al (2020) Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci Rep 10:912. https://doi.org/10.1038/s41598-020-57794-1
Gul I, Manzoor M, Kallerhoff J, Arshad M, (2020) Enhanced phytoremediation of lead by soil applied organic and inorganic amendments: Pb phytoavailability, accumulation and metal recovery. Chemosphere, 258, Article 127405. https://doi.org/10.1016/j.chemosphere.2020.127405.
Hamd Alla WA, Shalaby EM, Dawood RA, Zohry AA, (2014) Effect of cowpea (Vigna sinensis L.) with maize (Zea mays L.) intercropping on yield and its components. Int J Agric Biol Eng 8(11): 1200-1206. 10.5281/zenodo.1326836.
Hendren CO, Mesnard X, Droge J, Wiesner MR, (2011) Estimating production data € for five engineered nanomaterials as a basis for exposure assessment, Environ Sci Technol. 45 2562e2569. https://doi.org/10.1021/es103300g.
Homme PM, Gonzalez B, Billard J (1992) Carbohydrate content, frutane and sucrose enzyme activities in roots, stubble and leaves of rye grass (Lolium perenne L.) as affected by sources link modification after cutting. J Plant Physiol 140:282–291. https://doi.org/10.1016/S0176-1617(11)81080-1
Hu J, Wu X, Wu F, Chen W, White JC, Yang Y, Wang X, (2020) Potential application of titanium dioxide nanoparticles to improve the nutritional quality of coriander (Coriandrum sativum L.) J. Hazard. Mater., 389, Article 121837. https://doi.org/10.1016/j.jhazmat.2019.121837.
Ieggli, CV, Bohrer D, do Nascimento PC, de Carvalho LM, Garcia SC, (2010) Determination of sodium, potassium, calcium, magnesium, zinc, and iron in emulsified egg samples by flame atomic absorption spectrometry. Talanta, 80, 1282–1286. https://doi.org/10.1016/j.talanta.2009.09.024.
Irshad M A, Nawaz R, Rehman MZ, Adrees M, Rizwan M, Ali A, Ahmad S, Tasleem S, (2021) Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: a review, Ecotox. Environ. Safe., Volume 212,2021, 111978. https://doi.org/10.1016/j.ecoenv.2021.111978.
Isari AA, Hayati F, Kakavandi B, Rostami M, Motevassel M, Dehghanifard E (2020) N, Cu co-doped TiO2@functionalized SWCNT photocatalyst coupled with ultrasound and visible-light: an effective sono-photocatalysis process for pharmaceutical wastewaters treatment. Chem Eng J 392:123685. https://doi.org/10.1016/j.cej.2019.123685
Jacob DL, Borchardt JD, Navaratnam L, Otte ML, Bezbaruah AN (2013) Uptake and translocation of Ti from nanoparticles in crops and wetland plants. Int J Phytoremediat 15:142–153. https://doi.org/10.1080/15226514.2012.683209
Jahan S, Alias YB, Bakar AFBA, Yusoff IB (2018) Toxicity evaluation of ZnO and TiO2 anomaterials in hydroponic red bean (Vigna angularis) plant: physiology, biochemistry and kinetic transport. J Environ Sci 72:140–152. https://doi.org/10.1016/j.jes.2017.12.022
Jiang MY, Zhang JH (2002) Role of abscisic acid in water stress induced antioxidant defense in leaves of maize seedlings. Free Radic Res 36:1001–1015. https://doi.org/10.1080/1071576021000006563
Jiang F, Shen Y, Ma C, Zhang X, Cao W, et al., (2017) Effects of TiO2 nanoparticles on wheat (Triticum aestivum L.) seedlings cultivated under super-elevated and normal CO2 conditions. PLOS ONE 12(5): e0178088. https://doi.org/10.1371/journal.pone.0178088.
Khater M.S, (2015) Effect of Titanium Nanoparticles (TiO2) on Growth, Yield and Chemical Constituents of Coriander Plants. Arab J Nucl Sci Appl, 48(4), (187–194).
Kolenčík M, Ernst D, Urík M et al (2020) Foliar application of low concentrations of titanium dioxide and zinc oxide nanoparticles to the common sunflower under field conditions. Nanomaterials 10:1619. https://doi.org/10.3390/nano10081619
Kouhi SMM, Lahouti M, Ganjeali A, Entezari MH (2014) Comparative phytotoxicity of ZnO nanoparticles, ZnO microparticles, and Zn2+ on rapeseed (Brassica napus L.): investigating a wide range of concentrations. Toxicol Environ Chem 96:861–868. https://doi.org/10.1080/02772248.2014.994517
Lateef A, Nazir R, Jamil N, Alam S, Shah R, Khan MN, Saleem M (2016) Synthesis and characterization of zeolite based nano–composite: an environment friendly slow release fertilizer. Microporous Microporous Mater 232:174–183. https://doi.org/10.1016/j.micromeso.2016.06.020
Lei Z, Mingyu S, Xiao W, Chao L, Chunxiang Q, Liang C, Hao H, Xiao- qing L, Fashui H, (2008) Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol Trace Elem Res 121:69–79. https://doi.org/10.1007/s12011-007-8028-0
Lian W, Yang L, Joseph S, Shi W, Bian R, Zheng J, Pan G, (2020) Utilization of biochar produced from invasive plant species to efficiently adsorb Cd (II) and Pb (II) Bioresour. Technol., 317, Article 124011. https://doi.org/10.1016/j.biortech.2020.124011.
Lichtenthaler HK, Buschmann C, (2001) Chlorophylls and Carotenoids: Measurement and Characterization by UV‐VIS Spectroscopy. Curr. Protoc. Food Anal. Chem., 1, F4.3.1‐F4.3.8, https://doi.org/10.1002/0471142913.faf0403s01.
Liu R, Lal R (2015) (2015) Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci Total Environ 514:131–139. https://doi.org/10.1016/j.scitotenv.2015.01.104
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Lutt S, Kinet JM, Bouharmont J (1996) NaCl induced senescence in leaves of rice cultivar differing in salinity resistance. Annal of Bot 78:389–398. https://doi.org/10.1006/anbo.1996.0134
Lyu S, Wei X, Chen J, Wang C, Wang X, Pan D (2017a) Titanium as a beneficial element for crop production. Front Plant Sci 8:597. https://doi.org/10.3389/fpls.2017.00597
Lyu S, Wei X, Chen J, Wang C, Wang X, Pan D, (2017) Titanium as a beneficial element for crop production. Front. Plant Sci., 8, Article 597. https://doi.org/10.3389/fpls.2017.00597.
Missaoui T, Smiri M, Chmingui H, Hafiane A, (2017) Effects of nanosized titanium dioxide on the photosynthetic metabolism of fenugreek (Trigonella foenum-graecum L.). C. R. Biol. Volume 340, Issues 11–12, Pages 499–511, ISSN 1631–0691, https://doi.org/10.1016/j.crvi.2017.09.004
Mohammadi H, Esmailpour M, Gheranpaye A, (2016) Effects of TiO2 nanoparticles and water-deficit stress on morpho-physiological characteristics of dragonhead (Dracocephalum moldavica L.) plants. Acta Agric. Slov., 107 (2), pp. 385–396. https://doi.org/10.14720/aas.2016.107.2.11
Nah YC, Paramasivam I, Schmuki P (2010) Doped TiO2 and TiO2 nanotubes: synthesis and applications. ChemPhysChem 11:2698–2713. https://doi.org/10.1002/cphc.201000276
Odedeji JO, Oyeleke WA (2011) Comparative studies on functional properties of whole and dehulled cowpea seed flour (Vigna unguiculata). Pak J Nutr 10(9):899–902. https://doi.org/10.3923/pjn.2011.899.902
Picado A, Paixao SM, Moita L, Silva L, Diniz M, Lourenco J, Peres I, Castro L, Correia JB, Pereira J, Ferreira I, Matos APA, Barquinha P, Mendonca E (2015) A multi-integrated approach on toxicity effects of engineered TiO2 nanoparticles. Front Environ Sci Eng 9(5):793–803. https://doi.org/10.1007/s11783-015-0775-0
Posmyk M, Kontek R, Janas K (2009) Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicol Environ Saf 72:596–602. https://doi.org/10.1016/j.ecoenv.2008.04.024
Qi MF, Liu YF, Li TL (2013) Nano-TiO2 improve the photosynthesis of tomato leaves under mild heat stress. Biol Trace Elem Res 156(1–3):323–328. https://doi.org/10.1007/s12011-013-9833-2
Raliya R, Nair R, Chavalmane S, Wang WN, Biswas P (2015) Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 7:1584–1594. https://doi.org/10.1039/C5MT00168D
Rao S, Shekhawat GS, (2016) Phytotoxicity and oxidative stress perspective of two selected nanoparticles in Brassica juncea. 3 Biotech, 6(2): 244. 10. 1007/s13205–016–0550–3.
Raskar S, Laware SL (2013) Effect of titanium dioxide nano particles on seed germination and germination indices in onion. Plant Sci Feed 3(9):103–107
Reszczynska J, Grzyb T, Sobczak JW, Lisowski W, Gazda M, Ohtani B, Zaleska A (2014) Lanthanide co-doped TiO2: the effect of metal type and amount on surface properties and photocatalytic activity. Appl Surf Sci 307:333–345. https://doi.org/10.1016/j.apsusc.2014.03.199
Santos LM, Machado WA, França MD, Borges KA, Paniago RM, Patrocinio AOT, Machado AEH (2015) Structural characterization of Ag-doped TiO2 with enhanced photocatalytic activity. RSC Adv 5(125):103752–103759. https://doi.org/10.1039/C5RA22647C
Sean P, Tanmay Banerjee H, Dilbeck T, Hanson K (2015) Photon upconversion and photocurrent generation via self-assembly at organic–inorganic interfaces. J Phys Chem Lett 6(22):4510–4517. https://doi.org/10.1021/acs.jpclett.5b02120
Servin AD, Morales MI, Castillo-Michel H, Hernandez-Viezcas JA, Munoz B, Zhao L, Nunez JE, Peralta-Videa JR, Gardea-Torresdey JL (2013) Synchrotron verification of TiO2 accumulation in cucumber fruit: a possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environ Sci Technol 47:1159211598. https://doi.org/10.1021/es403368j
Singh BB, Mohan-Raj DR, Dashiel KE, Jackai LEN (1997) Advances in cowpea research- post harvest storage of cowpea in sub-Saharan Africa. I.I.T.A./JIRCA Publication, Ibadan, Nigeria, pp 302–312
Skupien K, Oszmia´ nski J, (2007) Influence of titanium treatment on ´ antioxidants content and antioxidant activity of strawberries. Acta Sci Pol Technol Aliment. 6, 83–93.
Song C, Huang M, Jason C, Xiaofeng Zhang W, Wang W, Sarpong CK, Jamali HZ, Zhang H, Zhao L, Wang Y (2020) Metabolic profile and physiological response of cucumber foliar exposed to engineered MoS2 and TiO2 nanoparticles, NanoImpact, Volume 20. ISSN 100271:2452–748. https://doi.org/10.1016/j.impact.2020.100271
Sugano N, Tanaka T, Yamamoto E, Nishi A (1975) Behavior of phenylalanine ammonialyase in carrot cells in suspension cultures. Phytochem 14:2435–2440. https://doi.org/10.1016/0031-9422(75)80359-1
Syu YY, Hung JH, Chen JC, Chuang HW (2014) Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem 83:57–64. https://doi.org/10.1016/j.plaphy.2014.07.010
Takatsuka H, Umeda M (2014) Hormonal control of cell division and elongation along differentiation trajectories in roots. J Exp Bot 65(10):2633–2643. https://doi.org/10.1093/jxb/ert485
Tighe-Neira R, Adriano Nunes-Nesi M, Recio G, Carmonah E, Corgne A, Rengel Z, Inostroza-Blancheteau C (2020) Titanium dioxide nanoparticles provoke transient increase in photosynthetic performance and differential response in antioxidant system in Raphanus sativus L. Sci Hort 269:109418. https://doi.org/10.1016/j.scienta.2020.109418
Uhram S, Minjoo S, Gisuk L, Jinkyu R, Younghun K, Eun JL (2013) Functional analysis of TiO2 nanoparticle toxicity in three plant species. Biol Trace Elem Res 155(1):93–103. https://doi.org/10.1007/s12011-013-9765-x
Ullah S, Adeel M, Zain V, Rizwan M, Irshad MK, Jilani G, (2020) Physiological and biochemical response of wheat (Triticum aestivum) to TiO2 nanoparticles in phosphorous amended soil: a full life cycle study J. Environ. Manag., 263, Article 110365. https://doi.org/10.1016/j.jenvman.2020.110365.
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Vogel AT (1975) A Textbook of Practical Organic Chemistry, 3rd edn. Book Society and Longmans Group Ltd, London, pp 483–485
Wang Y, Deng C, Cota-Ruiz K, Tan W, Reyes A, Jose R, Peralta-Videa Jose A, Hernandez-Viezcas Chunqiang Li, Jorge L G, (2021) Effects of different surface-coated nTiO2 on full-grown carrot plants: Impacts on root splitting, essential elements, and Ti uptake. J Hazard Mater. 402, 2021, 123768. https://doi.org/10.1016/j.jhazmat.2020.123768.
Xie Y, Huang N, You S, Liu Y, Sebo B, Liang L, Fang X, Liu W, Guo S, Zhao X (2013) Improved performance of dye-sensitized solar cells by trace amount Cr-doped TiO2 photoelectrodes. J Power Sources 224:168–173. https://doi.org/10.1016/j.jpowsour.2012.09.078
Yemm EW, Cocking EC (1955) The determination of amino acids with ninhydrin. Analyst 80:209–213. https://doi.org/10.1039/AN95580FX009
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57(3):508–514. https://doi.org/10.1042/bj0570508
Zhang J, Fu D, Wang S, Hao R, Xi Y (2019) Photocatalytic removal of chromium (VI) and sulfite using transition metal (Cu, Fe, Zn) doped TiO2 driven by visible light: feasibility, mechanism and kinetics. J Ind Eng Chem 80:23–32. https://doi.org/10.1016/j.jiec.2019.07.027
Zhang J, Zhao Z, Wang X, Yu T, Guan J, Yu Z, Li Z, Zou Z (2010) Increasing the oxygen vacancy density on the TiO2 surface by La-doping for dye-sensitized solar cells. J Phys Chem C 114:18396–18400. https://doi.org/10.1021/jp106648
Zhang P, Cui HX, Zhang ZJ, Zhong RG (2008) Effects of nano-TiO2 photo semiconductor on photosynthesis of cucumber plants. Chi Agric Sci Bull 24:230–233. https://doi.org/10.1186/s11671-016-1721-1
Zhang Y, Liu N, Wang W, Sun J, Zhu L, (2020) Photosynthesis and related metabolic mechanism of promoted rice (Oryza sativa L.) growth by TiO2 nanoparticles. Front Environ Sci Eng. 14(6):103. https://doi.org/10.1007/s11783-020-1282-5.
Zhao L, Zhang H, White JC, Chen X, Li H, Qu X, Ji R (2019) Metabolomics reveals that engineered nanomaterial exposure in soil alters both soil rhizosphere metabolite profiles and maize metabolic pathways. Environ Sci Nano 6:1716–1727. https://doi.org/10.1039/C9EN00137A
Zierden MR, Valentine AM (2016) Contemplating a role for titanium in organisms. Metallomics 8:9–16. https://doi.org/10.1039/C5MT00231A
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The given name of the first author of this article has been changed and is now a first initial.
Rights and permissions
About this article
Cite this article
Kamal, R., Mogazy, A.M. Effect of Doping on TiO2 Nanoparticles Characteristics: Studying of Fertilizing Effect on Cowpea Plant Growth and Yield. J Soil Sci Plant Nutr 23, 325–337 (2023). https://doi.org/10.1007/s42729-021-00648-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-021-00648-0