Abstract
Green approach is one of eco-benign synthesis techniques and in the current investigation, manganese dioxide (MnO2) NPs are prepared using potato leaf extract and as a nanofertilizer, their effects were evaluated on photosynthetic pigments, growth, and antioxidant properties of cowpea (Vigna unguiculata) cultivar. The green-synthesized NPs were characterized for surface morphology and structure using SEM and XRD techniques. The functional groups were monitored by FTIR (Fourier transform infrared) spectroscopy analysis, which reveals that nanoparticles were of variable shapes, uniformly distributed with average particle size of 26 nm. The MnO2 NPs were applied as nanofertilizer to cowpea via soil and foliar applications. For both applications, five treatments were used, including control, salt, and three concentrations of MnO2 NPs (25, 50, and 75 mg/kg). The NPs after sonication were applied directly through soil and a spraying bottle was used for foliar application. A significant increase in plant growth by 67.1 and 45.3 (%), photosynthetic pigments by 52.8 and 39.2 (%), and non-enzymatic antioxidant activity by 56.25 and 49.6 (%) were observed for soil and foliar application, respectively. The effect of soil application was better versus foliar application to enhance the chlorophyll, growth characteristics, and non-antioxidant enzymes. Findings revealed that the MnO2 (nanofertilizer) increased the growth and yield of cowpea cultivars and has the potential to be used as a nanofertilizer for crops grown in Mn-deficient soils to increase crop productivity and yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
To date, the supplement of food to the world’s increasing population in a sustainable way is the major global issue [1, 2]. The recent global production of food is 7.8 billion, which is increasing day by day and will reach around 10 billion by 2050 [3]. It is described that 70 to 100 (%) additional food will be essential to feed the increasing population. Therefore, the use of fertilizers, genetically modified and disease-resistant diversities has become common in agriculture. Nutrient fertilization governs an important part in maintaining the soil quality and increasing crop yield and productivity. In fertilization practice, chemical fertilizers not only contain macronutrients, nitrogen, phosphorous, calcium, potassium, and magnesium, but also micronutrients that are also important to enhance growth and ultimately the field of the crops. It is observed that the 70% of crop production and yield depend on fertilizers and remaining parts depend on the forming practice [4, 5]. The fertilizer application as a nutrient directly to soil or foliar application increased the yield of crops [6]. The continuous conventional applications of synthetic fertilizers have caused a failure in soil expected fertility, biodiversity, economic losses, and environmental pollution. In this regard, the scientists/agriculturists are paying more attention for more effective fertilization of land for the betterment of agriculture [7,8,9,10].
Micronutrients, also known as trace elements, are crucial for plant growth. Their shortage can disrupt healthy vegetation and result in plant diseases and ultimately leads to low yield [9]. Micronutrient deficit is common in Asian countries owing to the argillaceous flora of topsoil, low organic matter percentage in soil, continuous drought, high pH, salt stress, bicarbonate content in water, and excessive application of different chemical fertilizers. Various effects of deficiency of micronutrients persuaded stress in different plants resulting in low yield and production, defective plants morphology, and extensive influx of different diseases [6]. In the case of argillaceous soils, the conformist conception is that micronutrients enhance crop yield by 15 to 30 (%), and in some cases, grain yields up to 50% and enhances micronutrient proficiency. By delivering plants with different micronutrients via foliar, soil supplement, and seed treatment, the yield and quality of crop can be enhanced. The Mn is an imperative micronutrient as it plays a crucial role in the metabolism of plants, which is delivered to plants in different forms including Mn (II, III, and IV). It controls the free radical effects which damage the cell [9]. It enhances the photosynthesis and respiration process in plants. Enzyme activated by Mn includes PEP, malic, PAL and isocitrate dehydrogenase. Different proteins are formed in the plants in the presence of Mn, which enhance the synthesis of flavonoids, amino acids, and lignin [11]. The Mn is an important part of the photosystem II (PSII) machinery and it provides the take part in the photosynthetic process in an electron transport chain. Mn also takes part in the enzyme activation and is a part of chlorophyll synthesis (cofactor), hormone acceleration, and amino acid formation (Ducic and Polle, 2005). To cope against environmental stresses, the Ms is also very imperative in plants, which induce a resistance in the plant during stress stages [12].
It is observed that very minute amount of applied conventional fertilizer reaches the utility points plants without losing of dose, hydrolyses, drift, and photolytic degradation (Qureshi et al., 2018). To overcome this loss and increase the crop productivity, nanotechnology with potential applications as nanofertilizers and bio-fertilizers plays an important role, due to antimicrobial activity and nanomaterial coating [13]. The word “nanofertilizer” refers to a nanomaterial that is used to fertilize either in terms of macronutrients or micronutrients, or plant nutrients with small size (nm range). Due to less quantity used and easy reach to target site in minute quantity, nanofertilizer is favored versus chemical fertilizers, which enhanced nutrient uptake and increased plants growth, yield, and production without any negative impact on plants and soil. Nanofertilizer is a revolutionary field in agriculture that is effective and eco-friendly. Nanotechnology used in the field of agriculture has the capability to raise and implement the innovative tools, for nutrient precise forming, nutrient absorption, and disease treatment [5, 6, 12]. In the future, the nanostructure-based catalyst with low dose will enhance the efficiency of herbicides and pesticides. Accurate farming has increased the productivity by providing exact information about soil and weather conditions. Nanotechnology helps to detect plant diseases and to cure the disease using different materials [13, 14].
Nanoparticles are the particles having a size in 10 to 100 nm range, at least in one dimension not more than 100 nm. Different techniques of producing nanoparticles from bulk materials result in exclusively improved surface area, thermal, electrochemical, and optical characteristics [ 15,16,17]. These ideal assets of NPs are accountable for their use in every field of life, i.e., biomedical, catalysis, energy, industry, electronics, communications, engineering, microbiology, and agriculture [18,19,20,21]. Two main methods used for the nanoparticles (NPs) synthesis include top-down and bottom-up approaches. Top-down method is a destructive method of synthesis, in which nanoparticles are synthesized from bulk materials, and in bottom-up method, small atoms and molecules are aggregated for the preparation of NPs [ 22,23,24]. Nanofertilizers (NFs) can be established from synthetic materials, or green synthesized using different plant materials with different biological, mechanical, or chemical approaches using nanotechnology. Metal and their analogous oxides broadly transformed into nanoparticles by using physicochemical and biological methods. In chemical method of synthesis, different metal salts are used as precursor for the fabrication of NPs, and different reducing and capping agents are also used during the chemical reaction for stabilization of NPs [25, 26]. In biological method of synthesis, different plant materials are used as reducing agents with metal salts as precursor. The supremacy of green synthesis over chemical method is that the plant extract acts as both stabilizing and capping agents for the synthesis of nanoparticles. With increasing demand for green process and system for sustainable development, the green methods for the synthesis of nanomaterials via plants, microbes, and other biotical agents attracted much attention in recent decades [20, 27, 28]. Green fabrication of NPs has been a focus of recent studies by researchers using an eco-friendly technique. Much study has been conducted on the synthesis of plant extract–assisted NPs and their prospective uses in numerous fields due to their low cost, nontoxic approach, ease of availability, and environmental friendliness. The green routes for NP formation follow the principles of green chemistry for the synthesis of nanoparticle to use them as a nanofertilizer [22, 25].
The extracts of different plants, bacteria, fungi, algae, and yeast are used in green synthesis and bioactive agents in the extract act as a reducing agent. The potato is a tuberous crop noted for its high carbohydrate content. It has an abundance of starch molecules as well as benign, biodegradable chemicals and is water soluble; as a result, it may be efficiently used in the green synthesis of metal NPs that serve as reducing, capping, and stabilizing agent [19, 29]. Hydrolyzing the boiling fresh starch-rich potato yields massive amounts of glucose and starch. Furthermore, other organic biomolecules in potato extract (aldehyde, hydroxyl and carboxyl groups) contribute more functional moieties, which play role in the formation of NPs [30]. Following our prior investigations on NPs, the green route is simple, low cost, and efficient approach for NPs fabrication, i.e., magnetite NPs using starch-rich potato extract were prepared without using chemical additives. Before this study, green synthesis of pure magnetite NPs has also been undertaken using potato extract [31] and however, the synthesis of MnO2 has not been reported previously, which has been investigated in this study.
In view of promising efficiency of nanofertilizer, a lot of work has been done by the researchers and promising response has been reported for different metal oxide NPs for different cultivars, i.e., Azam et al. [6] documented the application of zinc nanofertilizer effect on zea mays plant. A promising acceleration in growth, photosynthetic pigments, and antioxidant moieties has been reported. Similarly, Salama et al. [32] investigated the effect of foliar MnO2 NPs on growth (vegetative), yield, and biochemical of the common dry bean and it was observed that Mn in nano form absorbed very well by the plants and a significant improving its development and productivity was observed. Also, Shebl et al. [33] documented similar observation that MN-Zn ferrite as a nanofertilizer has promising efficiency on the growth of squash plant. The use of ferrites as foliar nanofertilizer boosted squash plant development and yield across two seasons, with the maximum fruit output per hectare (54.8 and 55.2 t/ha).
Despite the fact that micronutrients are compulsory and nanofertilizer has a tremendous potential to overcome, these deficiencies of the soil and application of nanofertilizer is required at a moderate rate. In this context, the present research work was carried out to prepare the MnO2 and its subsequent application as a nanofertilizer was performed for Cowpea cultivar. Different physiological and biochemical parameters were observed at different concentration of NP doses applied as a nanofertilizer and response thus observed were compared with controls.
2 Material and method
2.1 Green synthesis of MnO2 NPs
For potato leaf extract, fresh plant leaves were obtained from the Botanical Garden, University of Agricultural (UAF), FSD, Paki, washed with distilled water thoroughly, chopped, and grinded using juice extractor. The extract was heated at 50 °C by adding 1:2 ratio of leaves and water for the extraction, filtered using Whatman filter paper, cooled, and stored at 4 °C till further use. A 0.1 M of MnCl2.4H2O solution was prepared in a 500-mL beaker and stirred using a magnetic stirrer at 50 °C. Freshly prepared 50 mL of extract was added drop-wise in the above solution using a burette. During stirring, a color change was seen from colorless to brown, which was an indication of reduction of ions to atoms. The pH of the solution was adjusted to basic using hydrazine. Stirring was continued for 3 h for complete reduction. The resultant solution was centrifuged for the removal of impurities using water and ethanol. The precipitates were dried at 60 °C, and after drying, the nanoparticles were calcined at 400 °C 3 h. The NPs thus collected were stored in air-tight bottles for future use.
2.2 Properties of the nanoparticles
The phase formation and structure (crystal) of the samples were studied using XRD analysis with a Cu-K X-ray source (1.5406) in the scanning angle range of 2θ = 20–80° (Philips X'Pert PRO, XRD). The SEM analysis has employed surfaces using Hitchi-S-3400 N SEM at 30 kV voltage. FTIR was performed to check the presence of functional groups. Zeta potential was measured as it is helpful in measuring surface charge of the NPs. It also provides the information of stability of nanoparticles, and magnitude of zeta potential represents the increase in stability due to electrostatic repulsion present in the nanoparticles.
2.3 Application as nanofertilizer
2.3.1 Growth conditions, treatments, and design
The current study was conducted at the PARS (Post Agriculture Research Station), UAF. The seeds of Vigna unguiculata (Cowpea) were procured from the AARI, Faisalabad, and planted at a depth of 2 cm in sand pots filled with 5.5 kg black sand. The treatment used a randomized fully block design (RCBD) with six replicates per treatment to eliminate any spatial impact. Five treatments were prepared for the soil application (application of nanofertilizer through roots) of nanofertilizer including (1) a pot of control, with no nanofertilizer, (2) a pot, which received Mn salt, and (3) 3 pots with 25, 50, and 75 mg/kg concentration of nanofertilizer were applied. Soil application (application of nanofertilizer through roots) was done by mixing nanofertilizer with water and sonicated for 1 h at 40 °C temperature to make the suspension each time. Each above-mentioned concentration was poured into each pot, respectively. For the foliar application of nanofertilizer, 3 treatments were designed at 25, 50, and 75 mg/L concentration along with a control pot. Foliar application was done using spraying machine. For both foliar and soil nanofertilizer application, 5 plants were grown in each pot. Hoagland solution was made in stock and applied to pots to provide other necessary nutrients to the plants [34]. The nanofertilizer solution is prepared distilled water and sonicated for 1 h at 40 °C to make the suspension each time. Foliar application was done at alternate days for 14 days starting after 4 weeks of germination using spraying machine. After 42 days (germination), the sample were collected, and shoots and roots are separated and cleaned for the removal of mud clumps in roots (0.2 mM CaCl2).
2.3.2 Response measurements
Various characteristics were investigated to determine the impact of the nanofertilizer. The plant physical metrics, such as weight, length, and leaf area, were measured. Just after harvest, the fresh weight of the plants was measured. The dry weight of the plants was determined after drying them in an oven at 60 °C for 24 h. The chlorophyll a, chlorophyll b, and carotenoids contents were measured [35]. Plant material (0.1 g) was ground in a mortar and pestle acetone (2 mL, 80%) and then 8 mL of 80% acetone was added. After 12 h, absorbance was recorded at 645, 663, and 480 (nm) for chlorophyll b, a, and total carotenoids, respectively (CE Cecil 7200, UK).
The TFC was monitored using Folin-Ciocalteu reagent [36]. A 100 μL of extract was mixed in 0.2 mL FC reagent, left in dark for 10 min at 25 °C, and then, 0.6 mL of 0.2 mM of Na2CO3 is added and kept for 2 h and at 765 nm absorbance was recorded and TPC was expressed as mg GAE/g dry weight of the sample. The TFC was estimated using Alara et al. [37]. A 2 mL of methanolic extract is mixed with AlCl3 (2 mL, 2%) in ethanol and kept at 25 °C for 2 h and at 420 nm absorbance was monitored and TFC was expressed mg quercetin equivalent/g of sample. Anthocyanin was measured following Wahid [38]. For this, extraction of leaves is performed in acidified methanol (1% HCl) and filtered and absorbance was measured at 535 nm. Ascorbic acid was determined following Mukherjee and Choudhuri method [39]. The dinitrophenyl hydrazine 2% (2 mL) was mixed with extract (4 mL) fowling thiourea addition (1 drop of 10% in 70% ethanol), boiled for 15 min, and cooled down and H2SO4 (5 ml of 80%, v/v) was added at 0 °C and finally, absorbance was noted at 530 nm and concentration was measured using vitamin C curve.
The root and shoot (dry) were ground to fine powder and mixed with 100 mL of methanol (250 mL flask), centrifuged for 8 h at 150 rpm, filtered and concentrated using a rotary evaporator to dryness, and weighed. The yield (%) was estimated using relation shown in Eq. 1 [40].
2.4 Statistical analysis
The ANOVA (analysis of variance) is undertaken to find the mean and standard deviation of triplicate sample and significance level among the treatments. The Tuckey test is employed for the comparison of effect of nanofertilizer for all the treatments using Minitab software.
3 Results and discussion
3.1 Characteristics of MnO2 NPs
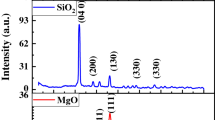
The morphological and surface analysis was done by scanning electron microscope (SEM) and responses thus observed are shown in Fig. 1. The average diameter of the nanoparticles calculated was 26 nm. These results expressed that nanoparticles are in nano-size range and uniformly distributed. The particle at nano-size can act as efficient fertilizer due to their fast absorption by the plant tissues. One of the major advantages of green-synthesized nanoparticles is their safety to use them as a nanofertilizer. Nanoparticles were of variable shapes, uniformly distributed, and in agglomerates form, which is due to the interaction among the biomolecules on the surface of NPs from the extracts. Figure 2 shows the XRD pattern of the MnO2 structure was tetragonal and crystalline (JCPDS #44–0141). Diffraction peaks at 2θ: 32.4°, 35.6°, 39°, 48.9°, and 58.8° can be indexed to the planes (200), (310), (101), (211), and (521), respectively. The average crystal size determined from XRD data using the Scherrer relation was around 26 nm (Fig. 3). FTIR with wavelength range of 650–4000 cm−1 measured MnO2 NPs shown in Fig. 4. The broad peak is observed at 3267 cm−1 which is due to –OH bond on the surface of nanoparticles. The peak observed at 2924 cm−1 indicates the -CH bond. The band observed at 1701 cm−1 is due to C = C. Sharp peak appeared at 1602 cm−1 is due to stretching of C = O bond. The peak at 1026 cm−1 represents the metal–oxygen bond (M–O) and it shows the presence of MnO2 NPs and also these observations are in line with the previous reports [41].
3.2 Effect of nanofertilizer on plant growth
The results of the trials were evaluated to determine the influence of soil and foliar applications of MnO2 nanofertilizer on plants’ various properties. Taking into account both treatments and their different concentrations, a one-way ANOVA was employed to examine the sources separately as well as their type of influence on the various plant attributes. Each approach, soil (Fig. 5) and foliar (Fig. 6) showed an accelerating effect on the growth characteristics of the plants. Varied amounts of MnO2 NPs elicited different responses from Vigna unguiculata in terms of physical and biochemical features. The nanofertilizer had a substantial effect on all of the plant parameters evaluated versus control plant under similar conditions. The data in Tables 1 and 2 revealed that all the three levels of MnO2 nanofertilizer treatments (soil and foliar), respectively, showed a significant impact (P < 0.05) on weight (dry and fresh) of root and shoot versus controls. In the case of foliar treatment, yield (%) obtained at FC2 (50 mg/L) was improved significantly versus FC1 (25 mg/L), FC3 (75 mg/L), and control. Similarly, the yield (%) for SC2 (50 mg/kg) was significantly higher versus controls, Mn salt treatment, SC1 (25 mg/kg), SC3 (75 mg/kg), and control in MnO2 soil treatment. The positive effect of Mn nanofertilizer was observed in all the aspects of plant growth characteristics. Also, previous findings support these observations that nanofertilizer has potential to improve the growth, biochemicals, and physiological attributes, i.e., the effect of MnO2 was studied in Phaseolus vulgaris plant and the NPs affected the growth and yield significantly versus control [32]. Similarly, Shebl et al. [33] also investigated the effect of nano MnO2 ferrites on the squash plant in different seasons, and resultantly, the growth and yield of plant were enhanced significantly. The negative effect of SC3 on the plant is due to the toxic effects of higher concentration of nanofertilizer. As a result, when applying nanofertilizer to plants, a moderate dose is crucial. In soil containing some micronutrients, even in trace amounts, the nanofertilizer concentration should be applied moderately to avoid the negative impact on plants as well as soil.
3.3 Photosynthetic pigments
The results revealed that applying nanofertilizer to plants resulted in a considerable improvement in the photosynthetic pigments of the cowpea plant. Table 3 displays all foliar and soil application levels’ effect on chlorophyll contents. The concentrations of chlorophyll (a, b) and carotenoids demonstrated a highly significant influence of nanofertilizer in both soil and foliar treatments. The photosynthetic pigment contents in cowpea plant treated with nanofertilizer is much higher than in control and Mn salt. The Mn is an important micronutrient that acts as a cofactor for multiple enzymes take part in various physiological processes and metabolic pathways, i.e., photosynthesis, ATP formation, chlorophyll, proteins, and fatty acids as well as secondary metabolites. The previous studies also support these findings, i.e., de França Bettencourt et al. [21] inspected the effect of nanofertilizer of Mn and resultantly, the photosynthetic pigments accelerated considerably in plants treated with nanofertilizer versus control. Similarly, Murgueitio-Herrera et al. [42] reported a 7.1% increase in chlorophyll contents in response of nanofertilizer application to the plants.
3.4 Antioxidant activity
The nanofertilizer application exerted a momentous effect on the antioxidant (non-enzymatic) system of cowpea plant. Tables 4 and 5 show control, salt, and all three levels of soil and foliar treatment data, respectively. Plants treated with nanofertilizer showed higher antioxidant concentrations, such as TPC, ascorbic acid, TFC, and anthocyanin as compared to chemical Mn fertilizer and the control. These findings were also supported by previous studies, i.e., Shebl et al. [33] studied the effect of Mn-Zn ferrites nanofertilizer in Cucurbita pepo L and found significant positive effect on the growth, biochemicals, and yield of plant. Also, Sedefoglu et al. [43] prepared ZnO nanoparticles via a green route using Ganoderma lucidum, which was applied as nanofertilizer. The ZnO NPs significantly enhanced the crop yield along with nutrient uptake in cherry tomato plants. In a more recent study, Azam et al. [6] prepared ZnO and used as a nanofertilizer for maize cultivar and it was observed that the growth, antioxidant moieties, and photosynthetic pigments were enhanced significantly. Based on the findings, the use of nanofertilizer application was suggested to enhance the plant growth and yield. Similarly, Abdulhameed et al. [10] reported the cabbage growth and yield in response of nanofertilizer and nanoparticles. The effect on the growth parameters of cabbage showed dependence on the type of nanofertilizer and nanofertilizer also enhanced the NPK uptake in cabbage plant and resultantly, the growth and yield were enhanced significantly. The nanofertilizer is more efficient versus conventional fertilizers because a different mechanism is involved for the working of NPs as a nanofertilizer, i.e., absorption of nanofertilizer is taken place via various modes like binds with carrier proteins, enter ion channels, or endocytosis or through aquaporins, binds with organic moieties. Hence, the nanofertilizer has absorbed the plant tissues more efficiently, which play an imperative role to avoid the loss of fertilizer in the environment. The use of nanofertilizer gained much attention in recent time and various types of nanofertilizer have been prepared and their effect on plant growth have also been trialed [6, 10]. However, still researcher attention is required to deduce optimal amounts of different nanofertilizer for their efficient use without any loss in the soil and environment. Moreover, nowadays, the environmental pollution is one of major issues due to anthropogenic activities [44,45,46,47]; therefore, there is need to develop and adopt the new methods for the synthesis of materials. In this regard, the green route for the synthesis of NPs is one of eco-benign, facile, cost-effective, and efficient methods, which could be preferred over of the conventional synthesis techniques [15, 17, 25, 26, 28, 29]. Also, the NPs prepared via green route are safer [23, 24] and more effective as a nanofertilizer versus chemical synthesized fertilizer.
4 Conclusion
The MnO2 was prepared via a green route and their efficiency as nanofertilizer was evaluated, which increase the growth, photosynthetic pigments, biochemicals, and antioxidant property of yield and nutritious values of cowpea plant. MnO2 nanofertilizer (soil and foliar) application showed a significant impact on the growth of the cowpea plants versus controls. The best yield and quality of plants were observed when MnO2 was applied @50 mg/kg of soil as a nanofertilizer. The MnO2 nanofertilizer treatments exerted a substantial influence on plant metrics such as plant height, plant yield, growth, photosynthetic pigment contents, and antioxidant (non-enzymatic) system. These observations revealed that the MnO2 synthesized via green route as highly efficient supplement for the cowpea crop and in comparison, to the chemical/synthetic fertilizer, the nanofertilizer prepared via green route is safer, which could be employed as a substitute to the conventional fertilizers to manage crops in a sustainable way.
Data availability
Not applicable.
References
Bhatia L, Bachheti RK, Garlapati VK, Chandel AK (2022) Third-generation biorefineries: a sustainable platform for food, clean energy, and nutraceuticals production. Biomass Convers Biorefin 12:4215–4230
Sene L, Pintro TC, Leonel LV, Bender S, da Cunha MAA (2022) Rice bran extract as an alternative nutritional supplement for Kluyveromyces marxianus. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-03252-z
Elemike EE, Uzoh IM, Onwudiwe DC, Babalola OO (2019) The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl Sci 9:499
Siddique A, Hassan A, Khan SR, Inayat A, Nazir A, Iqbal M (2018) Appraisal of heavy metals and nutrients from phosphate rocks, Khyber Pakhtunkhwa, Pakistan. Chem Int 4:1–6
Golbashy M, Sabahi H, Allahdadi I, Nazokdast H, Hosseini M (2017) Synthesis of highly intercalated urea-clay nanocomposite via domestic montmorillonite as eco-friendly slow-release fertilizer. Arch Agron Soil Sci 63:84–95
Azam M, Bhatti HN, Khan A, Zafar L, Iqbal M (2022) Zinc oxide nano-fertilizer application (foliar and soil) effect on the growth, photosynthetic pigments and antioxidant system of maize cultivar. Biocatal Agric Biotechnol 42:102343
Majolagbe AO, Adeyi AA, Osibanjo O, Adams AO, Ojuri OO (2017) Pollution vulnerability and health risk assessment of groundwater around an engineering Landfill in Lagos, Nigeria. Chem Int 3:58–68
Majolagbe AO, Adeyi AA, Osibanjo O (2016) Vulnerability assessment of groundwater pollution in the vicinity of an active dumpsite (Olusosun), Lagos, Nigeria. Chem Int 2:232–241
Barker AV, Pilbeam DJ (2015) Handbook of plant nutrition. CRC Press
Abdulhameed MF, Taha AA, Ismail RA (2021) Improvement of cabbage growth and yield by nanofertilizers and nanoparticles. Environ Nanotechnol Monit Manag 15:100437
Hebbern CA, Laursen KH, Ladegaard AH, Schmidt SB, Pedas P, Bruhn D, Schjoerring JK, Wulfsohn D, Husted S (2009) Latent manganese deficiency increases transpiration in barley (Hordeum vulgare). Physiol Plant 135:307–316
Millaleo R, Reyes-Díaz M, Ivanov A, Mora M, Alberdi M (2010) Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. J Soil Sci Plant Nutr 10:470–481
Mikula K, Izydorczyk G, Skrzypczak D, Mironiuk M, Moustakas K, Witek-Krowiak A, Chojnacka K (2020) Controlled release micronutrient fertilizers for precision agriculture–a review. Sci Total Environ 712:136365
Waghchaure RH, Adole VA, Jagdale BS, Koli PB (2022) Fe3+ modified zinc oxide nanomaterial as an efficient, multifaceted material for photocatalytic degradation of MB dye and ethanol gas sensor as part of environmental rectification. Inorg Chem Commun 140:109450
Salem NM, Awwad AM (2022) Green synthesis and characterization of ZnO nanoparticles using Solanum rantonnetii leaves aqueous extract and antifungal activity evaluation. Chem Int 8:12–17
Shammout MW, Awwad AM (2021) A novel route for the synthesis of copper oxide nanoparticles using Bougainvillea plant flowers extract and antifungal activity evaluation. Chem Int 7:71–78
Al-Zahrani FAM, Al-Zahrani NA, Al-Ghamdi SN, Lin L, Salem SS, El-Shishtawy RM (2022) Correction to: Synthesis of Ag/Fe2O3 nanocomposite from essential oil of ginger via green method and its bactericidal activity. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-03353-9
AL-Dharob MH, Mouhamad RS, Al Khafaji KA, Al-Abodi EE (2022) Antibacterial efficacy of cotton nanofiber soaked in Ag, ZnO and TiO2 nanoparticles. Chem Int 8:58–67
Igwe OU, Nwamezie F (2018) Green synthesis of iron nanoparticles using flower extract of Piliostigma thonningii and antibacterial activity evaluation. Chem Int 4:60–66
Remya V, Abitha V, Rajput P, Rane A, Dutta A (2017) Silver nanoparticles green synthesis: a mini review. Chem Int 3:165–171
de França Bettencourt GM, Degenhardt J, Zevallos Torres LA, de Andrade Tanobe VO, Soccol CR (2020) Green biosynthesis of single and bimetallic nanoparticles of iron and manganese using bacterial auxin complex to act as plant bio-fertilizer. Biocatal Agric Biotechnol 30:101822
Ali F, Hamza M, Iqbal M, Basha B, Alwadai N, Nazir A (2022) State-of-art of silver and gold nanoparticles synthesis routes, characterization and applications: a review. Z Phys Chem 236:291–326
Konappa N, Joshi SM, Dhamodaran N, Krishnamurthy S, Basavaraju S, Chowdappa S, Jogaiah S (2022) Green synthesis of Callicarpa tomentosa routed zinc oxide nanoparticles and their bactericidal action against diverse phytopathogens. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-13022-03438-13395
Tabrizi Hafez Moghaddas SS, SamarehMoosavi S, KazemiOskuee R (2022) Green synthesis of calcium oxide nanoparticles in Linum usitatissimum extract and investigation of their photocatalytic and cytotoxicity effects. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-13022-02643-13396
Naseer A, Iqbal M, Ali S, Nazir A, Abbas M, Ahmad N (2022) Green synthesis of silver nanoparticles using Allium cepa extract and their antimicrobial activity evaluation. Chem Int 8:89–94
Amer MW, Awwad AM (2021) Green synthesis of copper nanoparticles by Citrus limon fruits extract, characterization and antibacterial activity. Chem Int 7:1–8
Al-Fa’ouri AM, Abu-Kharma MH, Awwad AM (2021) Green synthesis of copper oxide nanoparticles using Bougainvillea leaves aqueous extract and antibacterial activity evaluation. Chem Int 7:155–162
Awwad AM, Amer MW, Salem NM, Abdeen AO (2020) Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Ailanthus altissima fruit extracts and antibacterial activity. Chem Int 6:151–159
Awwad AM, Salem NM, Aqarbeh MM, Abdulaziz FM (2020) Green synthesis, characterization of silver sulfide nanoparticles and antibacterial activity evaluation. Chem Int 6:42–48
Pirathiba S, Dayananda BS (2021) Potato peel waste as reductant for the biogenesis of gold and silver ultrafine particles. Mater Today Proc 42:1084–1090
Sharma R, Yadav S, Gupta R, Arora G (2019) Synthesis of magnetic nanoparticles using potato extract for dye degradation: a green chemistry experiment. J Chem Educ 96:3038–3044
Salama DM, Abd El-Aziz M, Osman SA, AbdElwahed MS, Shaaban E (2022) Foliar spraying of MnO2-NPs and its effect on vegetative growth, production, genomic stability, and chemical quality of the common dry bean. Arab J Basic Appl Sci 29:26–39
Shebl A, Hassan AA, Salama DM, Abd El-Aziz ME, AbdElwahed MSA (2020) Template-free microwave-assisted hydrothermal synthesis of manganese zinc ferrite as a nanofertilizer for squash plant (Cucurbita pepo L). Heliyon 6:e03596
Hothem SD, Marley KA, Larson RA (2003) Photochemistry in Hoagland’s nutrient solution. J Plant Nutr 26:845–854
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Portland Press Ltd.
Alara OR, Abdurahman NH, Olalere OA (2018) Ethanolic extraction of bioactive compounds from Vernonia amygdalina leaf using response surface methodology as an optimization tool. J Food Meas Charact 12:1107–1122
Alara OR, Abdurahman NH, Olalere OA (2018) Optimization of microwave-assisted extraction of flavonoids and antioxidants from Vernonia amygdalina leaf using response surface methodology. Food Bioprod Process 107:36–48
Wahid A (2007) Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J Plant Res 120:219–228
Mukherjee S, Choudhuri M (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170
Alara OR, Abdurahman NH, Ukaegbu CI (2018) Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J Appl Res Med Aromat Plants 11:12–17
Kumar H, Rani R (2013) Structural and optical characterization of ZnO nanoparticles synthesized by microemulsion route. Int Lett Chem Phys Astron 14:26–36
Murgueitio-Herrera E, Falconí CE, Cumbal L, Gómez J, Yanchatipán K, Tapia A, Martínez K, Sinde-Gonzalez I, Toulkeridis T (2022) Synthesis of iron, zinc, and manganese nanofertilizers, using Andean blueberry extract, and their effect in the growth of cabbage and lupin plants. Nanomaterials 12:1921
Sedefoglu N, Zalaoglu Y, Bozok F (2022) Green synthesized ZnO nanoparticles using Ganoderma lucidum: characterization and In Vitro Nanofertilizer effects. J Alloy Compd 918:165695
Iqbal M, Abbas M, Nazir A, Qamar AZ (2019) Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: a review. Chem Int 5:1–80
Ukpaka CP, Ugiri AC (2022) Biodegradation kinetics of petroleum hydrocarbon in soil environment using Mangnifera indica seed biomass: a mathematical approach. Chem Int 8:77–88
Jalal G, Abbas N, Deeba F, Butt T, Jilal S, Sarfraz S (2021) Efficient removal of dyes in textile effluents using aluminum-based coagulants. Chem Int 7:197–207
Chokor AA (2021) Total petroleum and aliphatic hydrocarbons profile of the River Niger surface water at Okpu and Iyiowa-Odekpe regions in South-Eastern, Nigeria. Chem Int 7:188–196
Acknowledgements
The authors extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R165), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R165), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
S. Samsoon: investigation, writing—original draft. M. Azam: methodology. A. Khan: data curation. M. Ashraf: software, resources. H. N. Bhatti: supervision, project administration. Samar Z. Alshawwa: resources, funding acquisition. M.N. Akhtar: data curation, writing—review and editing, M. Iqbal: validation, visualization, writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Samsoon, S., Azam, M., Khan, A. et al. Green-synthesized MnO2 nanofertilizer impact on growth, photosynthetic pigment, and non-enzymatic antioxidant of Vigna unguiculata cultivar. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03686-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03686-5