Abstract
This study evaluated the effect of biochar on the soil nutrients, soil enzyme activity, and rice yield in a heavily saline-sodic paddy soil using a 2-year field experiment conducted in Jilin province in the northeastern part of China. The soil was amended with biochar at 0 biochar (B0), 33.75 t ha−1 (B1), 67.5 t ha−1 (B2), and 101.25 t ha−1 (B3). The field experiment was arranged in a randomized complete block design. Each treatment was replicated three times. The results show that the addition of biochar significantly increased the availability of soil total N, available P, and available K, while it remarkably reduced the content of the soil’s alkali-hydrolysable nitrogen, among which NH4-N and NO3-N were reduced significantly in 2 years. Biochar applications significantly increased the soil organic matter and soil C/N ratio. The soil Na+/K+ ratio was significantly reduced after biochar application in both 2 years. All of the biochar amendment applications improved the soil catalase activity, soil alkaline phosphatase activity, soil urease activity, and soil sucrose activity. The rice biomass, grain yield, and harvest index were significantly increased. Biochar applications can improve the soil nutrient status, decrease Na+/K+ concentration in soil, promote rice growth, and increase the rice yield in heavily saline-sodic paddy soils. It is anticipated that the study results will be useful for formulating novel management ways for improving crop production on saline-sodic soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Saline-sodic soils with a high electrical conductivity of soil saturation extract (ECe), pH, and sodium adsorption ratio (SAR) are an important type of salt-affected soils (Chi et al. 2012). This type of soil seriously inhibits plant growth and soil fertility in arid and semi-arid regions (Aikaraki 1997). Salt-affected soils are prone to nutrient deficiencies and ionic toxicity because of the degradation of the soil’s physicochemical and biological properties (Qadir and Schubert 2002). Munns and Tester (2008) reported that the essential nutrient availability and acquisition were inhibited in salt-affected soils due to the direct competition between the sodium ions and mineral nutrients; moreover, the mass flow of mineral nutrients to plant root system was greatly decreased due to the raise of solution osmotic pressure in salt-affected soils as a result of excessive Na+. Lakhdar et al. (2009) reported low levels of nitrogen, phosphorus, and potassium in the salt-affected soil because of the lower organic matter input from the plant biomass and higher losses of organic matter. Furthermore, the solubility of organic matter in saline-alkali soils increased due to the limiting of soil microorganism activities by high pH, and so the loss rate of mineral nutrients was also increased (Nelson and Oades 1998; Saifullah et al. 2018). Rengasamy (2010) reported that the soil microbial population growth and activity were adversely affected by salt-affected soils, which indirectly influenced the conversion of nutrients and their accessibility to crops (Fageria et al. 2011). The volatilization losses of NH3 and N2O in salt-affected soils were accelerated because of the high level of salinity and sodicity, thus causing an increase in the proportions of nitrogen fertilizer losses from these soils (Ghosh et al. 2017; Li et al. 2017; Chen et al. 2020). Therefore, increasing the soil fertility is essential for restoring and utilizing saline-sodic soils.
Soil enzyme activity is strongly connected with the soil’s physicochemical characteristics, pH, soil organic matter, and soil nutrient cycling (Gaskin et al. 2010; Song et al. 2012; Qi et al. 2016). Moreover, excessive salt accumulation in salt-affected soils adversely influences enzyme activities (Karlen et al. 2008; Shi et al. 2019). A thorough understanding of the variation of the soil enzyme activities is necessary for the exploitation and utilization of saline-alkali soils (Shi et al. 2019). However, there are no studies on the effect of biochar on the soil enzyme activity in heavily saline-sodic paddy soil.
Recent research shows that biochar applications could decrease the loss of nutrients in salt-affected soils by increasing the soil organic carbon content, nutrient availability, soil CEC, and soil surface area, as well as by stabilizing the soil structure (Chaganti and Crohn 2015; Esfandbod et al. 2017). Moreover, biochar could provide a habitat for soil microorganisms and enhance their activity, thus indirectly improving the nutrient status of salt-affected soils (Saifullah et al. 2018). Our previous studies showed that biochar could significantly lower the Na+ content of the rhizosphere soil, reduce the Na+ accumulation in crops, decrease the saline-alkali stress on crops, and improve crop growth (Jin et al. 2018; Ran et al. 2019).
Although many studies of biochar applications on salted soil have been reported, most of the studies have been conducted under laboratory or glasshouse conditions utilizing small pails; most studies focused on dryland crops. To date, there are few reports on the effect of biochar on soil nutrients, soil enzyme activities, and rice yield in a heavily saline-sodic paddy field. And the potential mechanism needs to be clarified urgently. In the present study, we hypothesized that biochar application may improve the nutrient statue of saline-sodic paddy field and alleviate the detrimental effects of saline-sodic on rice growth and soil enzyme activities. Therefore, our study aimed to evaluate the response of the soil nutrients and chemical properties, soil enzyme activities, and rice yield to biochar applications in a heavily saline-alkali paddy field and explore the underlying mechanisms of action. It is prognosticated that the research finding will be helpful to improve the health parameters of saline-sodic paddy field and promote the rice production in saline-sodic lands.
2 Materials and Methods
2.1 Study Site Description and Soil Sampling Analysis
The field experiments were conducted in 2017 and 2018 in Sheli Country, Baicheng City, Jilin Province, north-eastern China (45° 35′ N, 123° 50′ E), where the average annual air temperature, precipitation, and evaporation are 4.7 °C, 413.7 mm, and 1696.9 mm respectively. The main soil in the experiment is of the solonchak type according to the World Reference Base for Soil Resources (IUSS Working Group 2014). The experimental field was cultivated for 3 years before the test. The physicochemical characteristics of the trial soil were determined as shown in Table 1.
2.2 Biochar Characteristics
The biochar used for this field experiment was provided by the Jihefu Agricultural Development Company, Liaoning, China. The feedstock of the biochar was peanut shells. The biochar was made from pyrolysis of peanut shells at a temperature of approximately 350–550 °C for 4 h. The biochar was ground and then filtered through a 2-mm sieve before use. The biochar characteristics and peanut shell properties are given in Table 2.
2.3 Experimental Setup
By adding 0 g, 15 g, 30 g, and 45 g per kilogram of soil in the plow layer (0–15 cm), the field experiment was set up at four biochar applied rates. Zero-biochar application served as the control (B0), 33.75 t ha−1 of biochar applied served as B1, 67.5 t ha−1 of biochar applied served as B2, and 101.25 t ha−1 of biochar applied served as B3. Biochar was applied on only one occasion in the spring of 2017. Each treatment was replicated three times. The experiment was conducted using 12 field plots, which were arranged in a randomized complete block design. Each plot in this experiment was 5 m × 6 m (30 m2). The individual plots were separated by buffer rows (0.6 m in width), and each plot had an irrigation and drainage outlet. In May 2017, biochar was thrown on the surface of the saline-sodic paddy soil, thoroughly incorporated into the soil by a wooden rake, and then plowed to a depth of over 15 cm. The rice variety Changbai 9 was cultivated for the field experiment. The rice seeds were sown in trays in a greenhouse for seeding on 10 April 2017 and 9 April 2018. Forty-day-old seedlings were transplanted into the field plots on 20 May 2017 and 19 May 2018, respectively. The spacings of the hills and rows were 30 cm and 16.5 cm, respectively, and three seedings were planted per hill. The rice was harvested at the mature stage (30 September 2017 and 1 October 2018). The base fertilizer before transplanting included 300 kg of (NH4)2SO4 per hectare, 150 kg of diammonium phosphate per hectare and 75 kg of K2SO4 per hectare. At the tilling stage, N fertilizer as urea was applied at 150 kg per hectare. Sixty kg ha−1 urea and 50 kg ha−1 K2SO4 were applied at the panicle stage. Other types of field management followed local practices.
2.4 Analysis of Soil Nutrients and Soil Enzyme Activity
After the rice harvest, the soil samples of each plot were gathered from the 0–20 cm stratum of the heavily saline-sodic paddy soil with an auger in five randomly selected places, and then the residues of the biomass and roots were removed. The soil samples were divided into two groups. In the first group, the soil sample was stored in a refrigerator at 4 °C at field moisture after being filtered through a 2-mm sieve. In another group, the soil sample was ground after being air-dried and then passed through a 2-mm sieve. The total organic carbon and nitrogen were measured by the dry combustion method (Jin et al. 2015) using an Elementar Vario Max CNS Analyser (Elementar Company, Germany), and we then calculated the C/N ratio. The alkali-hydrolysable nitrogen content was determined using the alkali-hydrolysed diffusion method (Liang et al. 1997). The contents of ammonium nitrogen (NH4-N) and nitrate-nitrogen (NO3-N) were measured by the method of Cabrera and Beare (1993) with a continuous flow analyzer (Auto Analyzer 3, Seal Analytical, Germany). The contents of available P and available K were determined by adopting the Egner-Riehm method (Egner et al. 1960). The flame meter method (M410 Sherwood England) was used to determine and quantify the ion contents of Na+ in the soil after nitric-perchloric acid (1%) digestion (Bastías et al. 2004). Catalase activity in the saline-sodic paddy soil was assessed by back titration residual H2O2 added to the soil with 0.1 M KMnO4, as described by Jin et al. (2009). Alkaline phosphatase activity was measured by spectrophotometry at 400 nm of the p-nitrophenol released from 1.0 g of soil after a 60-min incubation at 37 °C with a 0.025 M p-nitrophenyl phosphate substrate, in 4 ml of 0.17 M universal buffer at pH 11 (Tabatabai and Bremner 1969). Urease activity was determined by spectrophotometry at 578 nm as the NH4-N released from 1.0 g of soil after 24 h incubation at 37 °C with 10% (w/v) urea solution, in 20 ml of 1 M citrate buffer at pH 6.7 (Klose and Tabatabai 1999). The activity of sucrase in the saline-sodic paddy soil was assayed on the basis of the release and quantitative determination of products of glucose (Guan 1986). Soil samples were incubated for 24 h at 37 °C with 15 ml 8% sucrose solution, 5 ml phosphate buffer at pH 5.5, and 0.1 ml toluene, and spectrophotometric measurements were performed at 508 nm.
2.5 Analysis of the Rice Yield
All of the rice plants within 5 m2 from each trial plot were harvested at the mature stage. These rice plants were desiccated at 105 °C for 45 min and then dehydrated to a constant weight at 60 °C (Jin et al. 2018). The biomass yield was recorded. The grain yield was calculated by measuring the remaining plants from every plot after side rows were removed, and then it was converted into kilograms per hectare with a water content of 14%. The harvest index is equal to the ratio of the grain yield to the biomass yield.
2.6 Statistical Analysis
Descriptive statistics were used to test the mean and standard deviation of the measured parameters. The effects of biochar on the measured parameters were evaluated using one-way ANOVA (analysis of variance). The two-way ANOVA was employed to identify the interactive effects of biochar treatment and planting year on the measured parameters. Significant differences among means were detected using the least significant difference (LSD) test at P < 0.05. All of the statistical analyses were performed by SPSS 18.0 software.
3 Results
3.1 Effect of Biochar on Soil Nutrient and Chemical Properties
3.1.1 Soil Nitrogen Content
Biochar treatment significantly affected soil total nitrogen and alkali-hydrolysable nitrogen (Table 3). Compared with B0 (zero-biochar), the soil total nitrogen was significantly enhanced, while the soil alkali-hydrolysable nitrogen content was decreased significantly in both years. In 2017, the total nitrogen content was increased by 120% under B3, increased by 95% in B2, and increased by 50% under B1, compared with B0. In addition, a significant difference was found among the biochar treatments. Moreover, a significant interactive effect of biochar treatment and planting year on soil total nitrogen content was observed. The content of alkali-hydrolysable nitrogen was reduced by 8.72% in B1, 19.01% under B2, and 24.98% in B3, compared with that of B0, but there was no significant difference among the biochar treatments. Similar results were observed in 2018, in which the three biochar treatments B1, B2, and B3 exhibited increased total nitrogen contents by 33.33%, 66.67%, and 123.81%, respectively, while the reduced the alkali-hydrolysable nitrogen contents were reduced by 26.20%, 32.70%, and 43.28% compared with B0.
Table 3 shows that biochar treatment and planting year significantly decreased the soil NH4-N content in the saline-sodic paddy field. The effect of the biochar treatments showed the order of B3 < B2 < B1 < B0 in both years, and the difference among the treatments reached a significant level. For each biochar treatment, the soil NH4-N content was observed to decrease with planting year. Compared with B0, the content of NO3-N greatly decreased after the biochar was employed in both years (Table 3). The order was as follows: B3 < B2 < B1 < B0. The difference among the biochar treatments reached a significant level, while no significant difference between planting years was found. In addition, biochar treatment and planting year exhibited obvious interactive effects on soil NH4-N content and soil NO3-N content.
3.1.2 Soil Organic Matter and C/N Ratio
There was a positive effect of the biochar treatment and planting year on the soil organic matter content (SOM) and soil C/N ratio in the saline-sodic paddy field in 2017 and 2018, while no significant interaction between biochar treatment and planting year was detected (Table 3). SOM showed a trend of B3 > B2 > B1 > B0 in both years. In 2017, the SOM of B1, B2, and B3 was enhanced by 60.56%, 129.58%, and 277.46%, respectively, compared with B0. In the three biochar treatments, B1, B2, and B3, SOM increased by 32.56, 125.58, and 222.09%, respectively, compared with B0 in 2018. The differences among all treatments reached a significant level in both years. The SOM values were higher in 2018 than in 2017. Both biochar treatment and planting year significantly increased the soil C/N ratio (Table 3). A higher soil C/N ratio was found when biochar application was combined with planting year, and the effect increased with increasing biochar application rate and planting year. A significant difference was observed among all treatments in both years. The soil C/N ratios of the three biochar application treatments in 2018 were 17.09% higher than those in 2017 on average.
3.1.3 Soil Available P and Available K
Table 3 shows that the content of soil available P increased with increasing biochar application rates in the saline-sodic paddy fields in both years. On average, compared to the zero-biochar (B0), the addition of biochar at different rates (33.75, 67.50, and 101.25 t ha−1) increased soil available P by 36.21–192.59% in 2017 and by 95.38–269.23% in 2018. The content of soil available K was influenced by biochar treatment (P < 0.01) and planting year (P < 0.05). However, no significant interaction between biochar treatment and planting year was observed (Table 3). The content of soil available K significantly increased after biochar application in the saline-sodic paddy field in both years and increased with increasing biochar application rate. An obvious difference was found between the biochar treatments and B0, and the differences among the biochar treatments were also significant. In 2018, the soil available K was lower than that in 2017, while no interactive effect was observed.
3.1.4 Soil Na+/K+ Ratio
Biochar treatment and planting year significantly affected the soil Na+/K+ ratio (Table 3). The application of biochar significantly decreased the soil Na+/K+ ratio in the saline-sodic paddy soil (Table 3). The soil Na+/K+ ratio was reduced by 70.89% in B1, reduced by 80.41% under B2, and reduced by 85.30% in B3, compared with that of B0 in 2017. In addition, a significant difference was found among the biochar treatments. A similar result was observed in 2018, in which the three biochar treatments (33.75, 67.50, and 101.25 t ha−1) decreased the soil Na+/K+ ratio by 68.23%, 79.05%, and 86.52% compared with the zero-biochar treatment (B0), respectively. A significant interactive effect of biochar treatment and planting year on the soil Na+/K+ ratio was observed. Averaging over 2017 and 2018, the addition of biochar at different rates (33.75, 67.50, and 101.25 t ha−1) decreased the soil Na+/K+ ratio by 69.56%, 79.73%, and 85.91%, respectively. A decrease in soil Na+/K+ ratio is very helpful to reduce saline-sodic stress.
3.2 Effect of Biochar on Soil Enzyme Activities
Biochar treatment and planting year significantly affected catalase activity and alkaline phosphatase activity (Table 4). The catalase activity and alkaline phosphatase activity increased with increasing biochar application rates. The order was B3 < B2 < B1 < B0 in both years. On average, compared to B0 (zero-biochar), the addition of biochar alone at different rates (33.75, 67.50, and 101.25 t ha−1) increased catalase activity by 20–54.76% and alkaline phosphatase activity by 17.54–21.13%. For the biochar treatment, the catalase activity and alkali-phosphatase activity were observed to increase with extended planting year. The results of two-way ANOVA showed that there were significant interactions between biochar treatments and planting years on catalase activity and alkaline phosphatase activity; namely, the effect of biochar application rate on catalase activity and alkali-phosphatase activity showed differences among different planting years. Biochar treatment and planting year had significant effects on urease activity, but showed no interactive effect on urease activity (Table 4). The urease activity of B2 was the highest, that of B3 was second highest, and that of B0 was the lowest in both years. A significant difference was found between the 0 biochar treatment. The addition of biochar at different rates (33.75, 67.50, and 101.25 t ha−1) increased urease activity by 40.00–53.33% in 2017 and by 47.06–58.82% in 2018. For the biochar treatment, the urease activity significantly increased with planting year. The sucrase activity was significantly improved by biochar application (Table 4); it showed a trend of B3 > B2 > B1 > B0. The values for B3, B2 and B1 were enhanced by 52.19%, 40.98%, and 14.75% in 2017, respectively, compared with B0. In 2018, the sucrase activity in the three biochar treatments B3, B2, and B1 increased by 110.34, 68.97, and 43.45%, respectively, compared with B0.
3.3 Effect of Biochar on Rice Yield
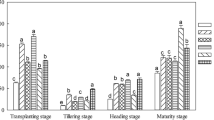
Table 5 shows the changes in the rice yield. The biomass yield, grain yield, and harvest index were increased by biochar addition in both years, and an obvious difference between the biochar treatments and B0 (0 biochar) was observed, while no significant difference was found among the biochar treatments. The biomass yield showed the trend of B3 > B2 > B1 > B0; the grain yield showed the trend of B2 > B3 > B1 > B0; and the harvest index showed the trend of B2 > B1 > B3 > B0. In addition, planting year significantly affected grain yield. On average, the grain yield of the biochar treatments in 2018 was 7.06% higher than that in 2017.
4 Discussion
4.1 Effect of Biochar on Soil Nutrient and Chemical Properties
Due to the degradation of soil’s physicochemical and biological characteristics, nitrogen availability and utilization in saline-sodic soils decrease significantly (Qadir and Schubert 2002). Biochar application can improve the nitrogen status of salt-affected soil directly by releasing nitrogen and can indirectly affect the abundance and activities of bacteria that can promote N transformations and enhance nitrogen status (Bhaduri et al. 2016; Ye et al. 2020). Consistent with this, a significant increase in the soil total nitrogen content was observed in the heavily saline-sodic paddy field over at least 2 years, and these increases were directly related to the amount of biochar added (Table 3). This result suggests that adding biochar will enhance the soil nutrient retention of saline-sodic paddy soil, especially at higher application rates. However, in contrast to the effect on the increased total N content, biochar application in the heavily saline-sodic paddy field significantly reduced the alkali-hydrolysable nitrogen content and the contents of NH4-N and NO3-N (Table 3). Similar results were also reported by Agbna et al. (2017), who found that over 25 t ha−1 wheat straw biochar application significantly reduced both the nitrate N and ammonium N contents. The underlying reason may be that (i) adding biochar can improve the rhizosphere environment, promote root growth (Bruun et al. 2014; Xiao et al. 2016), and increase crop yield (Table 5), thus improving the alkali-hydrolysable nitrogen acquisition capacity and uptake of crops, and (ii) biochar application significantly increased the soil C/N ratio (Table 3) and the abundance and activity of soil microbes (Bhaduri et al. 2016; Chen et al. 2020), thus accelerating the absorption of soil microorganisms for alkali-hydrolyzale nitrogen. Compared with 2017, soil alkali-hydrolysable nitrogen content increased in 2018 because the biochar contained available nitrogen, which was subsequently released to the soil more effectively in the second season (Hua et al. 2014; Agbna et al. 2017; Chen et al. 2020). Furthermore, biochar has been proven to be capable of changing the rate of nitrogen cycling and reducing nitrogen loss in soil (Bhaduri et al. 2016; Li et al. 2017).
Saline-sodic soils have little organic matter because of the degraded soil’s physicochemical and biological properties, high pH, and salt content. The decomposition of soil organic matter could accelerate the development of saline-sodic soil due to the swelling and dispersion of soil aggregates (Nelson and Oades 1998). A decrease in soil organic matter negatively affects soil nutrient availability and microbial activity (Jin et al. 2019). An emerging pool of knowledge shows that biochar can improve the soil structure and nutrient state, increase soil organic carbon, and maintain organic matter balance (Demisie et al. 2014; Liu et al. 2017; Bohara et al. 2018). In this study, biochar application greatly increased the soil organic matter content, which increased significantly with an increase of the amount in biochar application in both years (Table 3) and increased with the time of application. An increase in soil organic matter in heavily saline-sodic paddy soil helps to promote the increase in inorganic nutrients (Table 3) and provides a carbon source for soil enzyme and microorganism activities (Table 4). In experimental research carried out by Kim et al. (2016), biochar application significantly decreased the Na+ content in the root zone, improved soil aggregation, and increased the soil microbial population and soil organic matter. On the other hand, the increase in soil organic matter in the second planting year may indicate that biochar increases soil organic matter with time. This is supported by results from Hua et al. (2014), who reported that the potential of biochar to increase the organic carbon content is a function of incubation time.
Lakhdar et al. (2009) reported that the available P content was reduced in salt-affected soils due to their high pH value and low organic matter content. Many prior studies have indicated that biochar can increase the effectiveness and absorption of P in salt-affected soils directly by acting as a source of P and indirectly by improving the soil texture (Lashari et al. 2013; Drake et al. 2016; Saifullah et al. 2018). Consistently, a significant increase in available P content was found in the heavily saline-sodic paddy field in both years (Table 3). The availability of phosphorus is considered a key factor for inhibiting crop growth in saline-alkali soils (Munns and Tester 2008). This increase in the available P in heavily saline-sodic paddies is an important factor for improving rice yields and soil alkali-phosphatase activity. Liu et al. (2017) showed that biochar application could increase phosphate-solubilizing bacteria and phosphatase abundance and activity.
Excessive accumulation of Na and the impairment of K in plants are the principal limiting factors for plant growth in salt-affected soil (Akhtar et al. 2015; Chakraborty et al. 2016). Our previous research found that improving the K+/Na+ ratio in different rice organs by using biochar to enhance K availability was a useful way to improve growth in saline-alkali paddy soil (Ran et al. 2019). Lashari et al. (2013) found that increased soil K+ content was considered a key mechanism for offsetting salinization or/and alkalinization stress and encouraging crop growth. Consistent with this, the available K content (Table 3) significantly increased after biochar application in the heavily saline-sodic paddy field. In addition, we found a higher K+ concentration and lower Na+/K+ ratio in the soils with biochar treatments compared to that of zero-biochar control; such conditions can the improve soil water status and reduce osmotic stress (Table 3). Therefore, it can be concluded that the soil available potassium content effectively increased, while the Na+/K+ ratio significantly decreased after biochar addition in the heavily saline-sodic paddy field, which indicates that biochar addition not only helped reduce Na+ stress and improve cellular function and structural integrity (Ran et al. 2019) but also offset the problem of potassium deficiency and Na+ toxicity caused by saline-alkali stress. On the other hand, the peanut shell biochar contained certain contents of Ca2+ and Mg2+ (Table 2), which directly reduced the soil SAR and increased the soil cation exchange capacity, thus decreasing the Na+ content in the soil solution (Akhtar et al. 2015).
4.2 Effect of Biochar on Soil Enzyme Activities
Soil enzyme activities play an important role in the decomposition of soil organic matter and nutrient cycling (Song et al. 2019), and they are considered important potential indexes of soil quality (Liu et al. 2017). However, few studies are available on the effect of biochar on enzyme activity in heavily saline-sodic paddy soils. Liang et al. (2005) reported that salinity significantly decreased enzyme activities in the rhizosphere. In this field experiment, our results demonstrated that adding biochar had a positive effect on the soil enzyme activity in a heavily saline-sodic paddy field in both years (Table 4). Catalase is considered an indicator of aerobic microorganisms and reflects the redox ability in soils, and it is closely related to soil fertilizer and aerobic microorganism abundance (Liu et al. 2017). In this study, we found that soil catalase greatly increased after biochar was applied in the heavily saline-sodic paddy field (Table 4) and increased significantly with increasing biochar application amount, which is in agreement with the results of Masto et al. (2013). Many prior studies have shown that the addition of biochar can accelerate soil nitrogen and carbon cycling, improve soil quality, and then promote the activity of catalase (Amini et al. 2016; Yang et al. 2016). Similarly, here biochar application in heavily saline-sodic paddy field significantly increased the soil organic matter content and C/N ratio (Table 3). Soil phosphatase can convert organic phosphorus into inorganic phosphorus because it is involved in P cycling, which provides available P for plants and microorganisms (Bhaduri et al. 2016). The amount of soil alkali-phosphatase significantly increased with the amount of applied biochar in the heavily saline-sodic paddy field (Table 4); this may be one reason for the biochar increasing the available P (Table 3) in the saline-sodic paddy field. Similar to our results, existing studies have indicated that biochar application enhances the soil phosphatase activity (Liu et al. 2017; Ye et al. 2020). Soil urease participates in the hydrolysis of nitrogen-containing organic matter and increases the soil available N content, and it is considered an indicator of soil nitrogen content in soils (Demisie et al. 2014; Liu et al. 2017). Moreover, high salinity or sodicity has a significant influence on urease activity. In this study, the urease activity in heavily saline-sodic paddy field was greatly improved after biochar application (Table 4). Soil sucrose provides energy for soil organisms, which can hydrolyze sucrose, and its activity is considered an indicator of the utilization of soluble substances and soil organic matter. In our study, the soil sucrase activity in the heavily saline-sodic paddy field significantly increased with the addition of biochar and increased with increasing biochar application amount (Table 4), which was consistent with the results of Xu et al.’s (2017) research on paddy soil. In general, biochar had a positive effect on the soil enzyme activities in the heavily saline-sodic paddy fields, and biochar may become more effective in soils in the second planting year. These effects could promote the formation and degradation of soil organic matter and nutrient recycling and transformation. This trend might be explained by the improvements in the soil organic matter, C/N ratio, soil nutrient status (Table 3 and Bhaduri et al. 2016), microorganism diversity, and community structure (Drake et al. 2016; Li et al. 2018; Saifullah et al. 2018).
4.3 Effect of Biochar on Rice Yield
Nutrient deficiencies, ionic toxicity, and osmotic stress due to saline-sodic stress could be the major causes reduced rice yield (Qadir and Schubert 2002; Chi et al. 2012). Biochar is known to modify soil physical and chemical parameters (Huang et al. 2019), optimize root morphology and physiological traits, and then increase crop yield (Bruun et al. 2014; Xiao et al. 2016). The results of this study indicated that biochar application significantly increased the biomass yield, grain yield, and harvest index of rice in the heavily saline-sodic paddy field (Table 5). The underlying mechanism can be attributed to the following three aspects. First, biochar application to saline-sodic paddy soil to a large extent alleviates saline-sodic stress and promotes rice yield by the release of vital nutrients including K, P, Ca, and Mg (Tables 2 and 3), which could help reduce the proportion of Na+ in the exchange complexes (Table 3) and counteract the adverse effects of saline-sodic stress. In addition, biochar can effectively increase the absorption capacity of soil for NH4+, P+, and K+ and prevent loss from leaching or volatilization, thus facilitating nutrient absorption, slowing nutrient release, and improving nutrient utilization efficiency and crops yield (Saifullah et al. 2018). Second, the soil organic matter and C/N ratio significantly increased with biochar amendment (Table 3); such effects can improve soil physiochemical properties (Huang et al. 2019), promote the carbon-nitrogen cycle (Hua et al. 2014), and enhance soil enzyme activities (Table 4). Finally, our prior studies showed that biochar application in the saline-sodic soil can reduce the Na+ content, increase the K+/Na+ ratio, decrease leaf relative electrical leakage and increase leaf water status, which can offset saline-sodic stress, improve plant growth, and increase rice yield (Ran et al. 2019).
On the other hand, the average grain yield with biochar treatment in 2018 was 7.06% higher than that in 2017. The yield increase under 101.25 t ha−1 biochar amendment was lower than that under 67.50 t ha−1, mainly due to the immobilization of alkali-hydrolysable nitrogen (Table 3). During the two planting years, the difference in biochar application rates (33.75, 67.50, 101.25 t ha−1) were not evident. Considering economic issues, 67.50 t ha−1 could be an appropriate biochar application rate for rice production in heavily saline-sodic paddy soil. Nonetheless, the high cost of biochar is still the major obstacle for profits from biochar application (Jin et al. 2019). Thus, developing low-cost techniques for biochar production from agricultural waste materials seems to be essential to facilitate the widespread use of biochar in poor soil, such as saline-sodic paddy soil.
5 Conclusion
The results of the current study showed that biochar could provide beneficial effects on soil total nitrogen, available phosphorus, available potassium, soil organic matter, C/N ratio, catalase activity, alkali-phosphatase activity, urease activity, and sucrase activity, which is of great benefit to low-fertility soils such as saline-sodic paddy soils. In addition, biochar significantly reduced the soil Na+/K+ ratio by transient Na+ binding due to its high adsorption capacity and by releasing mineral nutrients into the soil solution, and a significant interaction effect of biochar treatment and planting year on the soil Na+/K+ ratio was also observed. Furthermore, the greatest positive effect on rice yield performance was observed in the treatment with a biochar application rate of 67.5 t ha−1 in both planting years. This clearly showed the potential of biochar to be used to support rice productivity in saline-sodic paddy soil. However, further investigation is recommended to identify the long-term effects of biochar applications on soil nutrients, soil physicochemical properties, and rice yield in heavily saline-sodic paddy fields.
References
Agbna GHD, Dongli S, Zhipeng L, Elshaikh NA, Timm LC (2017) Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Sci Hortic 222:90–101. https://doi.org/10.1016/j.scienta.2017.05.004
Aikaraki GN (1997) Barley response to salt stress at varied levels of phosphorus. J Plant Nutr 20(11):1635–1643. https://doi.org/10.1080/01904169709365362
Akhtar SS, Andersen MN, Liu FL (2015) Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric Water Manag 158:61–68. https://doi.org/10.1016/j.agwat.2015.04.010
Amini S, Ghadiri H, Chen CR, Marschner P (2016) Salt-affected soils, reclamation, carbon dynamics, and biochar: a review. J Soils Sediments 16:939–953. https://doi.org/10.1007/s11368-015-1293-1
Bastías EI, González-Moro MB, González-Murua C (2004) Zea mays L. amylacea from the Lluta Valley (Arica-Chile) tolerates salinity stress when high levels of boron are available. Plant Soil 267:73–84. https://doi.org/10.1016/j.chemosphere.2015.12.130
Bhaduri D, Saha A, Desai D, Meena HN (2016) Restoration of carbon and microbial activity in salt-induced soil by application of peanut shell biochar during short-term incubation study. Chemosphere 148:86–98. https://doi.org/10.1016/j.chemosphere.2015.12.130
Bohara H, Dodla S, Wang JJ, Darapuneni M, Kongchum M, Fromme DD, Harrell D (2018) Impacts of N-stabilizers and biochar on nitrogen losses, nitrogen phytoavailability, and cotton yield in poultry litter-fertilized soils. Agron J 110:1–9. https://doi.org/10.2134/agronj2018.01.0007
Bruun EW, Petersen CT, Hansen E, Holm JK, Hauggaard-Nielsen H (2014) Biochar amendment to coarse sandy subsoil improves root growth and increases water retention. Soil Use Manag 30:109–118. https://doi.org/10.1111/sum.12102
Cabrera ML, Beare MH (1993) Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci Soc Am J 57:1007–1012. https://doi.org/10.2136/sssaj1993.03615995005700040021x
Chaganti VN, Crohn DM (2015) Evaluating the relative contribution of physiochemical and biological factors in ameliorating a saline-sodic soil amended with composts and biochar and leached with reclaimed water. Geoderma 259-260:45–55. https://doi.org/10.1016/j.geoderma.2015.05.005
Chakraborty K, Bhaduri D, Meena HN, Kalariya K (2016) External potassium (K+) application improves salinity tolerance by promoting Na+-exclusion, K+-accumulation and osmotic adjustment in contrasting peanut cultivars. Plant Physiol Biochem 103:143–153. https://doi.org/10.1016/j.plaphy.2016.02.039
Chen L, Liu MJ, Ali A, Zhou QC, Zhan SW, Chen YC, Pan XH, Zeng YJ (2020) Effects of biochar on paddy soil fertility under different water management modes. J Soil Sci Plant Nutr 20:1810–1818. https://doi.org/10.1007/s42729-020-00252-8
Chi CM, Zhao CW, Sun XJ, Wang ZC (2012) Reclamation of saline sodic soil properties and improvement of rice (Oriza sativa L.) growth and yield using desulfurized gypsum in the west of Songnen Plain, northeast China. Geoderma 187-188:24–30. https://doi.org/10.1016/j.geoderma.2012.04.005
Demisie W, Liu ZY, Zhang MK (2014) Effect of biochar on carbon fractions and enzyme activity of red soil. Catena 121:214–221. https://doi.org/10.1016/j.catena.2014.05.020
Drake JA, Cavagnar TR, Cunningham SC, Jackson WR, Patti AF (2016) Does biochar improve establishment of tree seedlings in saline sodic soils? Land Degrad Dev 27:52–59. https://doi.org/10.1002/ldr.2374
Egner H, Riehm H, Domingo WR (1960) Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung de Nährstoffzustandes der Böden II. Chemische Extraktionsmethoden zur Phosphor- und Kaliumbetimmung Annaler 26:199–215
Esfandbod M, Phillips IR, Miller B, Rashti MR, Lan ZM, Srivastava P, Singh B, Chen CR (2017) Aged acidic biochar increases nitrogen retention and decreases ammonia volatilization in alkaline bauxite residue sand. Ecol Eng 98:157–165. https://doi.org/10.1016/j.ecoleng.2016.10.077
Fageria NK, Gheyi HR, Moreira A (2011) Nutrient bioavailability in salt affected soils. J Plant Nutr 34:945–962. https://doi.org/10.1080/01904167.2011.555578
Gaskin WJ, Speir A, Harris K, Das KC, Dewey LR, Morris AL, Fisher SD (2010) Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron J 102:623–633. https://doi.org/10.2134/agronj2009.0083
Ghosh U, Thapa R, Desutter T, Yangbo HE, Chatterjee A (2017) Saline–sodic soils: potential sources of nitrous oxide and carbon dioxide emissions? Pedosphere 27:65–75. https://doi.org/10.1016/S1002-0160(17)60296-0
Guan S (1986) Enzyme in soils and their study methods. China Agriculture Press, Beijing, pp 260–313 (in Chinese)
Hua L, Lu ZQ, Ma HR, Jin SS (2014) Effect of biochar on carbon dioxide release, organic carbon accumulation, and aggregation of soil. Environ Prog Sustain Energy 33:941–946. https://doi.org/10.1002/ep.11867
Huang MG, Zhang ZY, Zhai YM, Lu PR, Zhu CL (2019) Effect of straw biochar on soil properties and wheat production under saline water irrigation. Agronomy 457(9):1–15. https://doi.org/10.3390/agronomy9080457
IUSS Working Group WRB (2014) World Reference Base for soil resources 2014: international soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports 106; FAO: Roma, Italy
Jin K, Sleutel S, Buchan D, Neve SD, Cai DX, Gabriels D, Jin JY (2009) Changes of soil enzyme activities under different tillage practices in the Chinese Loess Plateau. Soil Tillage Res 104:115–120. https://doi.org/10.1016/j.still.2009.02.004
Jin VL, Potter KN, Johnson MVV, Harmel RD, Arnold JG (2015) Surface-applied biosolids enhance soil organic carbon and nitrogen stocks but have contrasting effects on soil physical quality. Appl Environ Soil Sci 2015:9–11. https://doi.org/10.1155/2015/715916
Jin F, Ran C, Anwari Q, Geng YQ, Guo LY, Li JB, Han D, Zhang XQ, Liu X, Shao XW (2018) Effects of biochar on sodium ion accumulation, yield and quality of rice in saline-sodic soil of the west of Songnen plain, northeast China. Plant Soil Environ 64:612–618. https://doi.org/10.17221/359/2018-PSE
Jin ZW, Chen C, Chen XM, Jiang F, Hopkins I, Zhang XL, Han ZQ, Billy G, Benavides J (2019) Soil acidity, available phosphorus content, and optimal biochar and nitrogen fertilizer application rates: a five-year field trial in upland red soil, China. Field Crop Res 232:77–87. https://doi.org/10.1016/j.fcr.2018.12.013
Karlen DL, Tomer MD, Neppel J, Cambardella A (2008) A preliminary watershed scale soil quality assessment in North Central Iowa, USA. Soil Tillage Res 99:291–299. https://doi.org/10.1016/j.still.2008.03.002
Kim HS, Kim KR, Yang JE, Ok YS, Owens G, Nehls T, Wessolek G, Kim KH (2016) Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 142:153–159. https://doi.org/10.1016/j.chemosphere.2015.06.041
Klose S, Tabatabai MA (1999) Urease activity of microbial biomass in soils. Soil Biol Biochem 31:205–211. https://doi.org/10.1016/S0038-0717(98)00090-X
Lakhdar A, Rabhi M, Ghnaya T, Montemurro F, Jedidi N, Abdelly C (2009) Effectiveness of compost use in salt-affected soil. J Hazard Mater 171:29–37. https://doi.org/10.1016/j.jhazmat.2009.05.132
Lashari MS, Liu Y, Li L, Pan W, Fu J, Pan G, Zheng J, Zheng J, Zhang X, Yu X (2013) Effects of amendment of biochar-manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from Central China great plain. Field Crop Res 144:113–118. https://doi.org/10.1016/j.fcr.2012.11.015
Li YY, Huang LH, Zhang H, Wang M, Liang ZW (2017) Assessment of ammonia volatilization losses and nitrogen utilization during the rice growing season in alkaline salt affected soils. Sustainability 132(9):1–15. https://doi.org/10.3390/su9010132
Li YC, Li ZW, Lin WW, Jiang YH, Weng BQ, Lin WX (2018) Effects of biochar and sheep manure on rhizospheric soil microbial community in continuous ratooning tea orchards. J Appl Ecol 29:1273–1282. https://doi.org/10.13287/j.1001-9332.201804.036
Liang BC, Mackenzie AF, Schnitzer M (1997) Management induced change in labile soil organic matter under continuous corn in eastern Canadian soils. Biol Fertil Soils 26:88–94. https://doi.org/10.1007/s003740050348
Liang YC, Si J, Nikolic M, Peng Y, Chen W, Jiang Y (2005) Organic manure stimulates biological activity and barley growth in soil subject to secondary salinization. Soil Biol Biochem 37:1185–1195. https://doi.org/10.1016/j.soilbio.2004.11.017
Liu SN, Meng J, Jiang LL, Yang X, Lan Y, Cheng XY, Chen WF (2017) Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristics in three different soil types. Appl Soil Ecol 116:12–22. https://doi.org/10.1016/j.apsoil.2017.03.020
Masto RE, Kumar S, Rout TK, Sarkar P, George J, Ram LC (2013) Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. Catena 111:64–71. https://doi.org/10.1016/j.catena.2013.06.025
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Nelson PN, Oades JM (1998) Organic matter, sodicity and soil structure. In: Sumner ME, Naidu R (eds) Sodic soils: distribution, properties, management and environmental consequences. Oxford University Press, Oxford, pp 51–75
Qadir M, Schubert S (2002) Degradation processes and nutrient constraints in sodic soils. Land Degrad Dev 13:275–294. https://doi.org/10.1002/ldr.504
Qi RM, Li J, Lin ZA, Li ZJ, Li YT, Yang XD, Zhang JJ, Zhao BQ (2016) Temperature effects on soil organic carbon, soil labile organic carbon fractions, and soil enzyme activities under long-term fertilization regimes. Appl Soil Ecol 102:36–45. https://doi.org/10.1016/j.apsoil.2016.02.004
Ran C, Gulaqa A, Zhu J, Wang WW, Zhang SQ, Geng YQ, Guo LY, Jin F, Shao XW (2019) Benefits of biochar for improving ion contents, cell membrane permeability, leaf water status and yield of rice under saline-sodic paddy field condition. J Plant Growth Regul 39:370–377. https://doi.org/10.1007/s00344-019-09988-9
Rengasamy P (2010) Soil processes affecting crop production in salt-affected soils. Funct Plant Biol 37:613–620. https://doi.org/10.1071/FP09249
Saifullah, Dahlawi S, Naeem A, Rengel Z, Naidu R (2018) Biochar application for the remediation of salt-affected soils: challenges and opportunities. Sci Total Environ:320–335. https://doi.org/10.1016/j.scitotenv.2017.12.257
Shi SH, Tian L, Nasir F, Bahadur A, Batool A, Luo SS, Yang F, Wang ZC, Tian CJ (2019) Response of microbial communities and enzyme activities to amendments in saline-alkaline soils. Appl Soil Ecol 135:16–24. https://doi.org/10.1016/j.apsoil.2018.11.003
Song YY, Song CC, Yang GS, Miao YQ, Wang JY, Guo YD (2012) Changes in labile organic carbon fractions and soil enzyme activities after marshland reclamation and restoration in the Sanjiang Plain in Northeast China. Environ Manag 50:418–426. https://doi.org/10.1007/s00267-012-9890-x
Song YY, Song CC, Shi FX, Wang MQ, Ren JS, Wang XW, Jiang L (2019) Linking plant community composition with the soil C pool, N availability and enzyme activity in boreal peatlands of Northeast China. Appl Soil Ecol 140:144–154. https://doi.org/10.1016/j.apsoil.2019.04.019
Tabatabai MA, Bremner JA (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307. https://doi.org/10.1016/0038-0717(69)90012-1
Xiao Q, Zhu LX, Zhang HP, Li XY, Shen YF, Li SQ (2016) Soil amendment with biochar increases maize yields in a semi-arid region by improving soil quality and root growth. Crop Pasture Sci 67:495–507. https://doi.org/10.1071/CP15351
Xu M, Xia HX, Wu J, Yang G, Zhang XH, Peng H, Yu XY, Li L, Xiao H, Qi H (2017) Shifts in the relative abundance of bacteria after wine-lees-derived biochar intervention in multi metal-contaminated paddy soil. Sci Total Environ 599-600:1297–1307. https://doi.org/10.1016/j.scitotenv.2017.05.086
Yang X, Liu JJ, McGrouther K, Huang HG, Lu KP, Guo X, He LZ, Lin XM, Che L, Ye ZQ, Wang HL (2016) Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ Sci Pollut Res 23:974–984. https://doi.org/10.1007/s11356-015-4233-0
Ye LL, Campsarbestai M, Shen QH, Lehmann J, Singh B, Sabir M (2020) Biochar effects on crop yields with and without fertilizer: a meta-analysis of field studies using separate controls. Soil Use Manag 36(1):2–18. https://doi.org/10.1111/sum.12546
Funding
This study was funded by the National Natural Science Foundation of China (No.32071951) and Jilin Province Education Department Planning Project (No.JJKH20200340KJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yao, T., Zhang, W., Gulaqa, A. et al. Effects of Peanut Shell Biochar on Soil Nutrients, Soil Enzyme Activity, and Rice Yield in Heavily Saline-Sodic Paddy Field. J Soil Sci Plant Nutr 21, 655–664 (2021). https://doi.org/10.1007/s42729-020-00390-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-020-00390-z