Abstract
Purpose
This paper reviews chemical, physical, and biological problems of salt-affected soils and different reclamation methods applied to rehabilitate these soils.
Methods
Methods to increase C stocks in these lands are discussed with a focus on biochar application as a potential new approach to not only to increase the C content but also to improve soil properties. Gaps in research knowledge in this field are then identified.
Results
Given the concern on the continued worldwide expansion of salt-affected lands and the focus on C sequestration processes, this review has evaluated current knowledge on salt-affected soils and their remediation with organic materials and plants. The review of the published literature has highlighted important gaps in knowledge, which limit our current understanding of rehabilitation of salt-affected soils with organic amendments specially biochar and the associated carbon dynamic. Knowledge about application of biochar in salt-affected soils is scant, and to date, most studies have evaluated biochar use only in nonsalt-affected soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Globally, 75 countries have been recognized as having vast areas of salt-affected lands. Martinez-Beltran and Manzur (2005) estimated that nearly 831 million hectares of land are salt-affected worldwide. Salt-affected soils mostly exist in arid and semiarid regions of the world, and many salt-affected wastelands have been productive lands in the past (Qadir et al. 2000). Worldwide, about 95 million hectares of soils are under primary salinization (salt accumulation through natural processes in soils and water) whereas 77 million hectares suffer from secondary salinization (as a result of human activities and ever-rising groundwater) (Metternicht and Zink 2003). Of major concern is that 23 % of the arable lands in the world are affected by salinity and a further 10 % are saline sodic soils while 340 million hectares of lands suffer sodicity (NLWRA 2001; Szabolcs 1994). The high salt concentration negatively affects soil microbial activity as well as soil chemical and physical properties, thus causing a decline in soil productivity. Decline in vegetation growth due to salt toxicity and detrimental osmotic potential results in lower carbon (C) inputs into these soils and further deterioration of their physical and chemical properties (Wong et al. 2009). Therefore, over a long period of soil salinization, C storage decreases at a significant rate (Wong et al. 2010).
Application of organic matter (such as green manures, compost, and food processing wastes) can both ameliorate and increase the C stocks and fertility of saline soils. Biochar is a C-rich organic material produced during slow exothermic thermal decomposition of biomass at temperatures ≤700 °C under zero or low oxygen conditions (Lehman and Joseph 2009; Kwapinski et al. 2010). In recent years with increasing concern about climate change due to elevated anthropogenic CO2 emissions, research interest on biochar production and its application to soils has gained importance (Woolf et al. 2010). Apart from being a source of carbon, biochar as an organic soil amendment, has been shown to alter and improve physical, chemical, and biological properties of soils and as a result increase plant productivity (Rondon et al. 2007; Asai et al. 2009; Thies and Rilling 2009; Atkinson et al. 2010; Solaiman et al. 2010; Jones et al. 2012). Most biochar studies around the world have been carried out on nonsalt-affected soils (Cross and Sohi 2011; Jones et al. 2012; Herath et al. 2013; Xu et al. 2015). However, a small number of studies carried out on the application of biochar to salt-affected soils have indicated that biochar may also have positive effects of the chemical and biological properties of these problematic soils (Artiola et al. 2012; Lashari et al. 2013; Thomas et al. 2013; Akhtar et al. 2014; Lashari et al. 2014; Akhtar et al. 2015a, b, c; Chaganti et al. 2015a, Chaganti and Crohn 2015b; Hammera et al. 2015). Many review papers have already been published with topics of salt-affected soils, reclamation, and carbon dynamics (Rengasamy 2006, 2010; Wong et al. 2010); however, there is a lack of literature to review the research undertaken on biochar application in salt-affected soils. This article is the first review of exiting literature in this field. The review not only presents an overview of the extent and problems of salt-affected soils, their C content and dynamics, and reclamation through chemical and organic amendments but also specifically examines literature on the behavior of these soils following biochar application. The paper concludes by identifying research gaps in this field.
2 Definition and classification of salt-affected soils
Salt-affected soils are soils with high concentrations of dissolved mineral salts in their profiles such that these dissolved salts adversely affect crop production (Rengasamy 2006; Wong et al. 2010). The salts are primarily composed of carbonates, chlorides, sulfates, and bicarbonates of calcium (Ca2+), magnesium (Mg2+), and sodium (Na+) (Qadir et al. 2000; Manchanda and Garg 2008). Rain, weathering of rocks, application of soil amendments and soluble fertilizers, saline irrigation water, and capillary rise of saline ground water and seawater cause salt accumulation in the soil profile (Rengasamy 2010). Soils generally become salt-affected through primary and secondary salinization processes. Primary salinization includes soils with naturally high amounts of inherent salts from sources such as rock weathering (Rengasamy 2006). In contrast, secondary salinization is a consequence of human activities such as irrigation with saline water without sufficient leaching of salt, thus increasing salt concentration in the root zone (Ghasemi et al. 1995). Secondary salinization also occurs as a result of shallow groundwater tables together with poor drainage and high evaporation rate (Rhoades 1987; Smedema and Shiati 2002; Brinck and Frost 2009). Other contributors to soil salinity include excessive use of chemical fertilizers, overgrazing, and deforestation (Brenstein 1975; Lakhdar et al. 2009)
Salt-affected soils are generally classified on the basis of their electrical conductivity of the saturated extract (ECe), sodium adsorption ratio (SAR) and exchangeable sodium percentage (ESP), and pH (Richard 1954; Bohn et al. 2001; Rengasamy 2010). Based on these properties, salt-affected soils are thus classified as follows: (1)saline soils, containing high levels of soluble salts and characterized by having high ECe values (>4 dS m−1); (2) sodic soils, having high levels of exchangeable sodium with SAR >13 and/or ESP (>15); and (3) saline-sodic soils, in which both soluble salts and exchangeable sodium are high, i.e., ECe > 4 dS m−1, SAR > 13, and ESP > 15 (Ghasemi et al. 1995; Richard 1954). Due to the combined effects of salinity and sodicity on soil properties and plant growth in saline-sodic soils, these soils are considered to be the most degraded form of salt-affected soil (Rengasamy 2002) (Table 1).
3 The effects of salinity and sodicity on different properties of soils
3.1 Physical properties
Elevated levels of exchangeable Na+ cause structural deterioration of the affected soils resulting in low pore volume and poor soil-water and soil-air relations in salt-affected soils (Rengasamy and Olsson 1991). Sodicity affects soil hydraulic properties such as hydraulic conductivity and infiltration rate due to aggregate breakdown. Slaking, clay swelling, and dispersion are the main mechanisms involved in aggregate breakdown in sodic soils (Rengasamy and Sumner 1998). Slaking happens during soil wetting when the entrapped air expands and results in the breakup of individual low-strength aggregates. Furthermore, clay swelling shrinks pore sizes, as swelling exceeds the attractive forces of the clay particles. Consequently, they are dispersed into individual clay particles (Frenkel et al. 1978; Rengasamy and Sumner 1998). Dispersion is an irreversible process and can cause the translocation of individual soil particles and consequently the permanent blockage of water navigating pores although swelling is a reversible process (Sumner 1993). The mechanism can be explained by diffuse double layer (DDL) theory whereby intermolecular and electrostatistic forces result in attraction and repulsion between ions in soil solution and soil particles. Soil pore systems and structural stability are influenced by these forces (Quirk 1994; Rengasamy and Olsson 1991). When sodium increases on the exchange sites of the soil particles, the repulsive forces are increased which in turn enlarges the interparticulate distance in the DDL and brings about dispersion and breakdown of soil aggregates which negatively affects soil structure (Oster and Shainberg 2001). Soil hydraulic conductivity and infiltration also decrease due to swelling and dispersion in Na+-dominated soils.
Total electrolyte concentration (TEC) of irrigation water and soil solution also affects clay swelling and dispersion. Soil flocculation is favored by high electrolyte concentration of the soil solution; therefore, higher levels of salinity can improve permeability and positively affect soil structure on. Conversely, low salinity and high sodicity result in considerable reduction of infiltration and hydraulic conductivity by inducing swelling and dispersion (Quirk and Schofield 1955; McNeal et al. 1968; Frenkel et al. 1978; Shainberg and Lety 1984; Quirk 2001; Dikinya et al. 2006).
Following slaking and dispersion in the surface layer of sodic soils, a thin layer of high shear strength is formed after drying which is known as a “surface crust” (Agassi et al. 1981). This surface crust makes the surface soil susceptible to extreme erosion and also to waterlogged conditions (Moore and Singer 1990; Shainberg et al. 1992). “Hard setting” is another important characteristic of saline-sodic soils which functionally is very similar to surface crusting, but its formation processes take place in the lower depths of the soil profile rather than on the soil surface, thus creating an impermeable subsoil layer with high bulk density (Qadir and Schubert 2002). Loss of structure and reduction in transmitting capacity for air and water in soils with high sodium concentration will therefore result in the formation of massive structure, poorly aerated and waterlogged soils. Such conditions are unfavorable for the establishment and the growth of the plants (Nelson et al. 1998).
3.2 Chemical properties
Soils high in salinity and sodicity have high values of ECe, ESP, SAR, and pH (Table 1). Salt-affected soils generally suffer from deficiencies of nitrogen (N), phosphorus (P), and potassium (K). However, their high pH also adversely affects the availability of micronutrients such as Fe, Al, Zn, Mn, and Cu (Pessarakli and Szabolcs 1999; Lakhdar et al. 2009).
Decline in vegetation growth due to salt toxicity, high osmotic suction, and degraded soil structure of salt-affected soils results in lower C inputs in these soils and further deterioration of their physical and chemical properties (Wong et al. 2009). Surface crusting and sealing, as discussed previously, also cause considerable erosive losses of organic matter so organic matter is low in these soils due to both low input and high losses (Qadir et al. 1997; Nelson and Oades 1998). Chander et al. (1994) showed that with increasing soil sodicity, due to irrigation with sodium-rich water, total N and organic C decreased. Adu and Oades (1978) also proposed that increased dispersion of aggregates in sodic soils exposes their locked up organic matter to rapid decomposition by soil microbes.
Gandhi and Paliwal (1976) reported that by increasing salinity, N mineralization decreased and gaseous NH3 losses increased. Pathak and Rao (1998) found that increasing salinity and sodicity of arid soils treated with organic amendments led to decreased C and N mineralization. Nelson et al. (1996) however reported an increase in C decomposition in salt-affected soils with increasing sodicity, due to solubilization of organic matter, whereas salinity decreased C mineralization. Frankenberger and Bingham (1982) found that the activity of soil enzymes with a role in C, N, P, and S cycles decreased with increasing salinity. High ion concentration, primarily Na+ and Cl−, reduces the ability of plants to take up water, which in turn affects the plant cells and growth (Muneer and Oades 1989a).
3.3 Biological properties
Soil microbial and biochemical processes, which are important for maintaining soil ecological functions, are negatively affected by changes in soil chemistry (Rietz and Haynes 2003). Microbial growth and activity are adversely impacted by increasing soil salinity, as high salt concentrations in soils causes’ osmotic stress and dehydration of microbial cells (Oren 1999; Wichern et al. 2006). Furthermore, in addition to salt stress, Na+ toxicity; nutritional deficiency such as Ca2+ deficiency; toxic levels of other ions such as carbonate, bicarbonate, and chloride; and loss of organic matter because of structural degradation all significantly contribute to decreasing microbial populations and activities in salt-affected soils (Zahran 1997; Nelson and Oades 1998). Garcia et al. (1994) studied the effects of salinity on the composition of microbial community in soils collected from arid regions of south east Spain and found increasing salinity had a negative effect on soil microbial communities. McClung and Frankenberger (1985) reported that enzyme activities and C and N mineralization rates decreased at high salinity levels. In their studies, nitrification rate increased up to 83 % with increasing salinity to 20 dS m−1, which stimulated ammonia losses through volatilization. Wichern et al. (2006) proposed that fungal communities are more exposed to increasing salt concentration than bacterial populations. Rietz and Haynes (2003) also reported that soil microbial and biochemical activities were negatively affected by irrigation-induced salinity and sodicity. They observed an exponential decrease in microbial biomass C with increasing soil ECe and a linear decrease in biomass C with increasing ESP and SAR. They also evaluated various biochemical enzyme activities and reported that enzyme activities linearly decreased with increasing ECe, ESP, and SAR. Several other studies have also reported that salinity and sodicity significantly decrease the soil microbial biomass and related enzyme activities (Garcia and Hernandez 1996; Tripathi et al. 2006, 2007). Rietz and Haynes (2003) and Wong et al. (2008) showed that with increasing salinity and sodicity, metabolic quotient (respiration per unit biomass) increases, indicating a more stressed microbial community. Gollarata and Raiesi (2007) also found an increase in metabolic quotient with increase in soil salinity. Similarly, Yuan et al. (2007) observed that with increasing salinity, a shift in soil microbial community takes place, with lower metabolisms, which can be an adaptive mechanism to reduce salt stress. Soil ecological functions are significantly affected by microbial and biochemical activities. These activities also play a central role in enhancing soil structure by increasing and improving formation and stabilization of soil aggregations (Six et al. 2004).
4 The effects of salinity and sodicity on soil carbon (C) dynamics
4.1 Soil organic C pools
The largest organic C pool of the continental biosphere is soil organic matter (SOM), with 1550 Pg accumulated in soil over thousands of years. It is about three times that of vegetation C (560–650 Pg) and higher than the atmospheric C pool (750 Pg) (Lal et al. 2003). The soil C pool comprises two parts: soil organic carbon (SOC) and soil inorganic carbon (SIC). The SOC is the most common form of C in humid regions while the SIC pool dominates in soils of arid and semi-arid regions. Based on the turnover time of C in soil, SOC can be partitioned into three main pools (Jenkinson and Raynor 1977; Parton et al. 1987):
-
1.
The active pool which is largely controlled by residue inputs and climate. This can be a nutrient source for plants. This pool is made up of readily oxidizable materials, and its turnover time is in the order of weeks (Schnurer et al. 1985; Wong et al. 2009).
-
2.
The slow pool with a turnover time that is in the order of decades. It contains particulate organic C and moderately decomposable material within aggregates (Parton et al. 1987; Wong et al. 2010).
-
3.
The passive pool with a turnover time in the order of millennia. Group 3 is also called the recalcitrant pool and contains stable C that is chemically resistant to further microbial degradation. Charcoal is one of the largest sources of this pool (Schimel et al. 1994; Skjemstad et al. 1996; Clough and Skjemstad 2000; Skjemstad et al. 2002; Lehmann et al. 2008).
The SIC pool includes carbonate minerals such as gypsum and calcite. This pool comprises two predominant components:
-
1.
Lithogenic or primary carbonates which are formed after weathering of parent material
-
2.
Pedogenic or secondary carbonates formed after dissolution of CO2 in soil water which produces carbonic acid and re-precipitation with Ca+2 or Mg+2 added to soil from different sources (Lal et al. 2006). In Australia, pedogenic carbonate often occurs in southern and inland regions and is estimated to occur in 50 % of the landscape. It is usually found in conjunction with sodic soils (Fitzpatrick and Merry 2000). The formation of pedogenic CaCO3 is linked to the development of sodicity in soils (Pal et al. 2000).
4.2 Carbon in salt-affected soils
It is generally accepted that once organic matter becomes an integral part of soil aggregates through the processes of aggregation, it is physically protected from rapid decomposition. Thus, any processes which contribute to the breakdown of soil aggregates also contribute to organic matter decomposition. For instance, tillage increases the disruption of aggregates and consequently raises the organic C mineralization rate (Wong et al. 2010). The loss of SOM is directly linked to the factors which affect its accessibility by the microbial population and enzymes (Dalal and Mayer 1986). In sodic soils, wetting and drying processes affect C accessibility in different ways. When sodic soils are wetted, dispersion of aggregates increases the availability of SOM and accelerates C loss, while on drying, SOM availability decreases due to an increase in soil bulk density and a decrease in water-holding capacity (Muneer and Oades 1989b). After solubilization of SOM, the initial increase in substrate availability can put some environmental stress on soil microbial communities and decrease the adverse effects of NaCl on microorganisms (McCormic and Wolf 1980; Pathak and Rao 1998). Wong et al. (2008) reported a higher soil microbial biomass (SMB) in soils with high salinity treatments. They related this increase in SMB to increased decomposability and accessibility of SOM which resulted in an increase in substrate. In another study, Wong et al. (2009) found that an initial increase in SMB and respiration rate happened after the addition of organic material to a highly saline-sodic soil. However, after the initial flush in available substrate and therefore increasing SMB, the salt and Na stress decrease substrate decomposition and availability. Consequently over time with microbial adaptation to the high saline environment, microbes continue to mineralize the remaining SOM (Poloneko et al. 1981; Zahran 1997). In another study, Setia et al. (2011) found EC to have a negative impact on CO2 emission in salt-affected landscapes, so in estimating CO2 release from these lands, this parameter needs to be taken into account.
In general, high salinity has a similar effect on both plant health and microbial activity through ion toxicity and osmotic effects (Batra and Manna 1997). Therefore, over a long period of time, where the salinization and sodicity processes are taking place, C storage decreases (Wong et al. 2010). In a study of arid saline soils in Spain (Garcia et al. 1994), low microbial activity was observed in a degraded land where high EC levels inhibited respiration. Similarly, they found that salinity decreased soil microbial biomass and concluded that this decline in soil microbial activity was due to a change in microbial community structure from fungi-dominated to bacteria-dominated. The latter are less diverse, competitive, and active (Garcia et al. 1994; Pankhurst et al. 2001; Sadinha et al. 2003). Chander et al. (1994) observed that SMB decreased with increasing sodicity, while the rate of organic matter mineralization increased. They concluded that increasing sodicity places stress on plants and decreases plant carbon inputs. In contrast, a slightly negative effect of sodicity on mineralization was reported by Nelson et al. (1997). This contradiction may be caused by the varying quantity and quality of added substrate in these two studies.
Soil enzyme activities have also been reported to decrease with increasing levels of salinity (Batra and Manna 1997). The inhibition of microbial and enzymatic activity is higher with NaCl than with Na2SO4 or CaCl2 (Frankenberger and Bingham 1982). Laura (1973) also found that increasing Na2CO3 concentration led to a decrease in total carbon (TC). Exchangeable Na increases due to an increase in Na2CO3 concentration which results in higher ESP values. On the other hand, precipitation of CaCO3 and MgCO3 increases pH values. These two processes (increased ESP and precipitation of CaCO3 and MgCO3) decrease SOC stocks in salt-affected soils. In contrast, Pathak and Rao (1998) found that even in highly saline and sodic conditions, biochemical mineralization can occur by soil enzymes. Wong et al. (2008) showed that SOC stocks in scalded and eroded soils were about a third of those in vegetated soils due to very low inputs of plant biomass carbon. Similarly, Pankhurst et al. (2001) attributed low SOC levels in saline soils to reduce presence of plant cover. Wong et al. (2008) reported that revegetation of scalded and eroded areas can increase SOC stocks to the similar levels of native pastures after about 10 years (Table 2). Leguminous trees can decrease ESP and pH and increase SMB of the salt-affected soils once they are established in such soils (Bhojvaid and Timmer 1998; Garg 1999; Mishra and Sharma 2003).
Erosional processes are very common in salt-affected landscapes. During these processes, SOC can be removed from the surface layer. The particulate fraction of SOC is the most prone to being removed because of its low density (Lal 2001). In salt-affected scalded soils, the less fertile B horizon often gets exposed after removal of the A horizon by erosion (Murphy et al. 1998). The eroded materials either get deposited in the downslope section of the landscape or are transported to aquatic environments such as lakes and reservoirs causing water pollution. Such transported organic matter can be protected from decomposition in the water bodies, thus contributing to C sequestration (Izaurralde et al. 2001; McCarty and Ritchie 2002). Table 2 presents data on C content of salt-affected soil in some different studies on these soils. Based on these data, SOC has decreased with increasing EC and ESP/SAR.
5 Carbon dynamics and reclamation of salt-affected soils
Different reclamation processes are used for saline and sodic soils. Leaching of salts from saline soils is the most commonly used method of reclaiming these soils (Jury et al. 1979; Abrol et al. 1988; Tanton et al. 1988; Djedidi et al. 2005; Letey et al. 2011; Yan and Marschner 2013). This involves infiltration of good quality water through the soil profile, together with the provision of an adequate drainage system to remove the leached salts away from the reclaimed area.
On the other hand, maintaining sustainability of water resources is a worldwide priority for a growing population. Population growth and urbanization have imposed extreme pressures on water resources resulting in reduced availability of fresh water for irrigated agriculture (Levine and Asano 2004; Qadir and Oster 2004). Therefore, current and future generations need to use water resources very efficiently. However, maintaining or even increasing agricultural productivity is also very important to meet the food demands of the planet’s growing population. Therefore, reducing the acreage under irrigation is not an option as a considerable amount of land is already lost due to various land degradation problems. Due to this fact, alternatives to using potable water resources for irrigation have received increased attention. As a consequence, treated waste waters from municipal treatment plants (reclaimed water), agricultural drainage waters, and agricultural and urban runoff are often considered as viable options for irrigation waters or to meet other nonpotable water demands (Corwin and Bradford 2008; Oster 1994). These waters are generally referred to as “degraded waters” due to their deterioration in physical, chemical, and biological properties (O’Connor et al. 2008). Reuse of such waters helps to reduce or prevent their discharge into water bodies, thus reducing their degradation effects on receiving environments (Grattan et al. 2008; Toze 2006). In some countries such as the USA, there is high reuse of agricultural drainage and reclaimed waters to irrigate agricultural lands (Kinney et al. 2006; Wu et al. 2009). However, when such waters are diverted for agricultural irrigation the chemical constituents, most importantly salts, can accumulate in soils causing soil degradation such as the loss of soil structure and reduction in soil infiltration and permeability (Stevens et al. 2003). Constraints involved in using these low-quality waters are mostly with respect to their salinity and sodicity (SAR) (Suarez et al. 2006).
Application of waters with high salinity and sodicity and their consequent effects on soil properties have been long studied and are well documented (Chander et al. 1994; Beltran 1999; Grattan and Oster 2003; Rietz and Haynes 2003; Emdad et al. 2004; Suarez et al. 2006; Choudhary et al. 2011), but some research gaps still remain unsolved. For example, while many researchers have studied the effects of high SAR (>15) waters on soil properties, the use of relatively moderate SAR (<8) waters, such as reclaimed waters, has been less investigated especially for leaching salt-affected soils (Mace and Amrhein 2001; Mandal et al. 2008). Understanding the effect of using water with moderate SAR to leach a saline-sodic soils treated with organic amendments is important with respect to soil and water management and thus warrants further study.
5.1 Chemical reclamation
Reclamation of saline-sodic soils requires the replacement of Na+ on the soil exchange sites by divalent cations such as Ca2+, followed by a salt leaching process (Gupta and Abrol 1990). Na-organic compounds are very soluble in saline-sodic soils, resulting in dissolved Na-humates in percolating and runoff waters which increase dispersion, mobilization, and losses of clay particles (Sumner et al. 1998). In highly alkaline soils with low base saturation status and high Na2CO3contents, Na+ must first be replaced by Ca2+ to form a stable linkage between organic matter and Ca2+-saturated particles (Rengasamy and Olsson 1991). This is because organomineral interactions mainly depend on Ca2+ bridges rather than Na+, and sodic soils are not able to retain products of decomposition due to the higher proportion of Na+ on their exchange sites compared to Ca2+ (Naidu and Rengasamy 1993).
Gypsum is commonly used to supply Ca2+. The effect of gypsum on the reclamation of saline-sodic soils has been extensively studied (Armstrong and Tanton 1992; Ilyas et al. 1997; Oster et al. 1999; Ghafoor et al. 2001; Mace and Amrhein 2001; Qadir et al. 2001a; Lebron et al. 2002; Choudhary et al. 2004; Gharaibeh et al. 2009, 2010). Sulfur and sulfuric acid are the other commonly used inorganic amendments which upon application to calcareous soil increase soil Ca2+ levels by dissolving native calcium carbonate in these soils (Niazi et al. 2001; Amezketa et al. 2005; Sadiq et al. 2007; Vance et al. 2008).
5.2 Phytoreclamation
Phytoremediation or vegetative bioremediation of salt-affected soils was introduced as early as 1937 by Kelly (1937) when a sodic soil was ameliorated by bermuda grass. Since then, many researchers have studied the potential of crop and crop-based approaches in the reclamation of salt-affected soils (Robbins 1986; Qadir et al. 1996; Qadir et al. 2001b; Qadir and Schubert 2002; Ahmad et al. 2006; Ammari et al. 2008; Gharaibeh et al. 2011). Phytoremediation occurs through several mechanisms in salt-affected soils (Qadir et al. 2007) including the following:
-
1.
Plant roots increase the dissolution rate of calcite, resulting in enhanced levels of Ca2+ in soil solution and increased exchange with Na+. Many sodic and saline-sodic soils have an inherently good source of Ca2+, typically in the form of calcite (CaCO3), at varying depths within the profile. However, unlike other Ca2+ sources used in the amelioration of sodic and saline-sodic soils, calcite is not sufficiently soluble to effect the displacement of Na+ from the cation exchange complex, unless remediated by plants. Production of Ca2+ in the soil solution by formation of carbonic acid and solubilization of native calcite occurs due to an increase in partial pressure of CO2 (PCO2) in the root zone. Enhanced levels of PCO2 and H+ therefore assist in increasing the dissolution rate of calcite (Qadir et al. 2005).
-
2.
The generation of protons (H+) released by roots of certain plant species enhances Na+ uptake by plants and its subsequent removal from the field at harvest.
-
3.
Improving drainage and salt leaching through the pores formed by roots and root penetration. Ghaly (2002) found that after the second year of a field experiment the role of native grass species in reducing salt content was more effective than the gypsum only treatment. He attributed this to enhanced salt uptake by plant. This was evidenced firstly by higher carbon inputs to soil resulting in reclamation of these soils within 2 years and secondly by increased amount of Na+ uptake and accumulation in the grass shoots.
5.3 Reclamation by organic amendment
Organic matter application is another method used to ameliorate salt-affected soils as organic matter helps binding soil particles into aggregates (Nelson and Oades 1998). Deprotonation of humic and fluvic acids leads to formation of large organic polyanions. These can bind clay particles into microaggregates by forming [(Cl-P-OM)x]y complexes where Cl, P, and OM are clay particles, polyvalent cations, and organic matter, respectively (Edward and Bremner 1967; Tisdall and Oades 1982). Chemical, physical, and biological properties of soils can be affected by organic material addition. For instance, poultry manure and compost addition to a salt-affected soil can affect its chemical properties, enhancing both CEC and the soluble and exchangeable K+. Potassium competes with Na+ in terms of being adsorbed in sodic soils and will limit the entry of Na+ onto the exchange sites (Walker and Bernal 2008). Chorom and Rengasamy (1997) added green manure to an alkaline sodic soil and observed that decomposition and microbial respiration decreased pH and increased the solubility of CaCO3 due to increase in partial pressure of CO2. Similarly, Liang et al. (2003) reported significant stimulation of soil respiration, urease, and alkaline phosphatase activity after incorporation of organic manure to a soil derived from alluvial and marine deposits with 3.3 g kg−1 total salts. Also, Barzegar et al. (1997) observed that dispersible clay decreases after the addition of pea straw irrespective of SAR. They concluded that organic material addition to sodic soils can enhance structural stability even without initial reclamation of sodicity. Combined application of gypsum and organic matter has also been successful in decreasing ESP and reducing adverse effects of high Na+ ions. Vance et al. (1998) observed that combined application of organic matter and gypsum to the surface of saline-sodic soils reduced dispersion and EC more than gypsum alone.
Application of organic amendments is considered as an effective management strategy for both amelioration of salt-affected soils and improvement of plant growth. Therefore, organic amendments such as green manures, farmyard manures, and municipal solid wastes provide both soil nutrients and organic matter for lasting improvements in soil fertility. Soil salinity effects on organic matter turnover and the mineralization of C and N depend on the type of organic material incorporated (Walpola and Arunakumara 2010). For example, combined application of rice straw and pig manure affected enzymatic and microbial activity more significantly than applying these amendments alone (Liang et al. 2005). Application of organic material can therefore provide an effective approach to reducing toxic saline conditions. However, excessive use of some organic amendments, for instance chicken manure, can increase the risk of secondary salinization in regions with abundant rainfall. Therefore, important factors to be considered prior to using organic amendments are the following: the proper selection of organic amendment and appropriate timing and correct method of application to the soil (Diacono and Montemurro 2010).
6 Biochar as an organic soil amendment
6.1 Biochar’s definition and characteristics
Biochar is a C-rich organic material produced during slow exothermic decomposition of biomass at temperatures ≤700 °C under zero oxygen or low oxygen conditions (Lehman and Joseph 2009; Kwapinski et al. 2010). In recent years, with increasing concern regarding climate change due to elevated anthropogenic CO2 emissions, research interest in biochar production and application has gained importance (Woolf et al. 2010). This product is highly recalcitrant due to its high aromaticity and can sequester C for very long periods (Fang et al. 2014; Kuzyakov et al. 2014; Lehmann et al. 2006). Therefore, biochar addition can be a potential pathway to improve and enhance native SOC and black C stabilization in cropping systems. For instance, Zhang et al. (2015) studied the effects of crushed corncob biochar application on aggregate-associated C concentrations in a 1-year field experiment. They observed higher C accumulation in large macroaggregates, suggesting that biochar-derived C can be physically protected within these aggregate sizes. Many studies have been undertaken on the effect of biochar application on SOC decomposition to find solutions for enhancing soil C sequestration (Wardle et al. 2008; Jones et al. 2011; Luo et al. 2011). However, previous studies have shown that biochar may both suppress and stimulate native SOC decomposition (Liang et al. 2010; Cross and Sohi 2011; Luo et al. 2011). Differences in the nature of soil and biochar, and incubation conditions used in different studies have led to these inconsistent results (Jones et al. 2011). Apart from being a C source, biochar as an organic soil amendment, has been shown to enhance plant growth either directly or indirectly. Biochar acts as a direct source of mineral nutrients such as Ca, Mg, K, and P, which promotes plant growth. Biochar also has a high adsorption capacity itself (determined by source feedstock and production conditions) and can be used in remediating contaminated soils (Uchimiya et al. 2011; Zhang et al. 2013; Paz-Ferreiro et al. 2014). However, biochar has indirect effects on mechanisms that alter physical, chemical, and biological properties of soils and consequently increase plant productivity (Rondon et al. 2007; Asai et al. 2009; Chan and Xu 2009; Spokas et al. 2009; Thies and Rilling 2009; Atkinson et al. 2010; Laird et al. 2010; Solaiman et al. 2010; Van Zwieten et al. 2010a; Lehman et al. 2011; Jones et al. 2012). Most biochar studies have been carried out on nonsalt-affected soils, where biochar application has resulted in the improvement of soil physical properties such as, infiltration, hydraulic conductivity, degree of aggregation, aggregate stability, bulk density, and water-holding capacity (Asai et al. 2009; Verheijen et al. 2010; Karhu et al. 2011; Uzoma et al. 2011; Liu et al. 2012; Jien and Wang 2013). For instance, Jien and Wang (2013) observed that application of 5 % biochar to a highly weathered soil increased macroaggregation of the soil and its saturated hydraulic conductivity (Ks). Similarly, Asai et al. (2009) observed a twofold increase in soil saturated hydraulic conductivity after the application of biochar at a rate of 16 t ha−1. Mukherjee and Lal (2013), Goerge et al. (2012), and Busscher et al. (2011) reported that soil aggregation increased after the addition of biochar. Piccolo et al. (1996) and (1997) showed that the primary binding agents responsible for enhancing soil aggregation are coal-derived humic substances. In their experiment, soil macroaggregate stability increased between 20 and 130 %, when coal-derived humic substances were added to four experimental soils from southern Nigeria.
Biochar influences different properties of soils under various mechanisms of fragmentation, oxidation, decay, and carboxylation (Kookana et al. 2011). For instance, biochar addition may decrease N availability by increasing N immobilization due to its effects on soil microbial activity, pH and CEC (Van Zwieten et al. 2010b; Reverchon et al. 2014, 2015). On the other hand, biochar may have no effect on soil N availability (DeLuca et al. 2006) or may even increase N availability (Hosseini Bai et al. 2015). The latter studied N cycling (using δ15N) and nutrient availability in soil and plant after 5 years of applying poultry litter and green waste biochar. A significant increase in both soil and foliar δ15N was observed with poultry litter biochar addition compared to green waste biochar and control treatments.
Biochar also has variable effects on phosphorus (P) availability. Slavich et al. (2013) observed an increase in available P after application of biochar derived from feedlot manure, while in another study, wood-based biochar addition noticeably reduced the availability of P (Van Zwieten et al. 2010b). This confirms that both soil properties and biochar feedstock are the important factors affecting biochar performance in soils (Macdonald et al. 2014).
6.2 Biochar and salt-affected soils
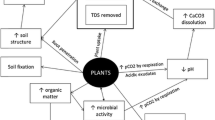
As previously stated, the addition of divalent cations such as Ca2+ and Mg2+ is critical in the reclamation of saline-sodic soils to offset excessive exchangeable Na+ and biochar may be able to play a positive role in this respect. In one study, Major et al. (2010) observed that the availability of Ca2+ and Mg2+ increased after the addition of biochar at a rate of 20 t ha−1 to a Columbian savanna oxisol. Likewise, Laird et al. (2010) observed that Ca2+ levels increased after the addition of oak-derived biochar to a Midwestern agricultural soil. Similarly, other researchers have also shown that divalent cation concentrations in soils increase after biochar addition (Chan et al. 2008; Gaskin et al. 2010; Novak et al. 2009). However, very few studies have investigated if biochar also has positive physical and chemical effects if applied to salt-affected soils.
Lashari et al. (2013) in a study on a salt-stressed cropland used biochar-manure compost in conjunction with pyroligneous solution. The results showed that soil pH and salt and sodium contents significantly decreased in amended treatments compared to the control. Also, they found significantly higher SOC and available P in amended soils. They concluded that biochar can be used as an ameliorant in salt-affected soils to reduce salinity (sodicity) stress by adsorption of Na+. Also, Lashari et al. (2014) in a similar study reported that a combined amelioration of manure compost and crop straw biochar plus pyroligneous solution could help reduce salinity stress to maize and improve productivity in salt-affected croplands. Furthermore, Akhtar et al. (2015a) studied the effects of biochar on growth, physiology, and yield of pot-grown wheat under salinity stress and also the underlying mechanism on reducing Na+ uptake. They reported positive effects of biochar on growth and yield of wheat. Also, biochar significantly affected the concentrations of Na+, K+, Ca2+, and Mg2+ in the leachate. The concentration of Na+ was not affected by biochar addition in nonsaline soils; however, in saline conditions, significant reduction of Na+ concentration was observed, indicating higher Na+ adsorption potential by biochar compared to control treatments. Also, increased amounts of Ca2+ and Mg2+ were reported in biochar-amended treatments. They concluded that the enrichment of exchangeable sites of soil profile with Ca2+ and Mg2+ can decrease the exchangeable Na+ concentration in these sites and improve soil physical properties in salt-affected soils which are relevant to reclamation of these soils. Similarly, another study by Akhtar et al. (2015b) reported that application of biochar to salt-affected soils can mitigate the salinity stress in potatoes due to biochar’s high Na+ adsorption potential. Thomas et al. (2013), in a glass house experiment, added salts on the surface of biochar (derived from lignocellulosic material) and concluded that biochar could ameliorate salt stress and mitigate its effect on plant performance through salt sorption. In this study, biochar material showed significantly increased electrical conductivity following salt addition. They also concluded that the observed increase in water-holding capacity of the biochar-amended treatments is another important factor in mitigating osmotic effects and ionic toxicity of excessive salts in soils. Hammera et al. (2015) reported similar results when they applied pellets of coniferous wood chips biochar along with arbuscular mycorrhizal (AM) fungi to salt-stressed soils in a greenhouse experiment with Lactuca sativa. They indicated that the biochar reduced salt stress by its ion sorption capacity, but additionally, it improved the nutritional condition of the soil which may be important variables in explaining the positive effects of biochar in salt-affected soils. They also noted the dependency of plants on symbiotic microorganisms to fully utilize the potential of biochar benefits in soils. The authors suggested a joint application of AM fungi and biochar in agricultural soils, after observing the positive effect of their combined use in increasing plant growth.
Biochar addition also promotes biological activity in soils. Although biochar is an organic amendment, it is more recalcitrant than any other organic substrate; thus, it less likely supports microbial growth. However, it has been shown that the release of temporary labile pyrolysis products can significantly promote soil biological activity after biochar addition to soils, although this effect could be temporary (Lehman et al. 2011). Biochar as a pure C source can also increase soil C stocks once added to soil. Providing a rich source of C for microbes is another possible reason for observed increases in biological activities in salt-affected soils after biochar addition. However, previous studies on the effects of biochar on microbial biomass carbon (MBC) are inconsistent. In some studies, there was no significant effect of biochar application on soil MBC (Castaldi et al. 2011; Zavalloni et al. 2011) while in others, biochar addition significantly decreased soil MBC (Dempster et al. 2012). In contrast, some other studies (Kolb et al. 2009; Liang et al. 2010; Lehman et al. 2011; Zhang et al. 2014) have found positive effects of biochar addition on microbial biomass. However, in general, MBC reflects any changes in SOC content and decomposition. Thus, any processes and materials which increase or decrease C content in soil can affect biomass and activity of microbial community. Therefore, biochar, as a pure C source, can provide more C for the microbial community and increase MBC.
In reclamation of salt-affected soils with biochar, it is critical to consider both the soil and biochar properties. Soil texture, the level of salinity (and sodicity), nutrient concentrations, and soil native C content are important soil properties which need to be considered before commencing reclamation processes. Also, the type of biochar (acidic or alkaline) and the source feedstock used for producing biochar are key factors determining the effectiveness of biochar as an organic amendment for reclamation of salt-affected soils. The current data on reclamation of salt-affected soils with biochar addition are inconsistent and it is difficult to compare across literature studies. This is probably because of the wide variety of biochar and soil types used in these studies. Moreover, there is a lack of relevant long-term field experiments to verify the mechanisms observed in existing pot or incubation studies. With such a dearth of information on the effects of biochar on saline and saline-sodic soils, further research on the suitability and the functioning mechanism of biochar in salt-affected soils is needed. Table 3 presents a summary of possible changes biochar may induce in salt-affected soils.
7 Conclusions
Given the concern on the continued worldwide expansion of salt-affected lands and the focus on C sequestration processes, this review has evaluated current knowledge on salt-affected soils and their remediation with organic materials and plants. The above review of the published literature has highlighted important gaps in knowledge, which limit our current understanding of rehabilitation of salt-affected soils with organic amendments and the associated carbon dynamic. These knowledge gaps include the following:
-
1.
Assessment of the effects of rehabilitation processes on C cycling and C stocks in salt-affected lands. Of particular importance is how to maximize the accumulation of C stocks in salt-affected lands, where SOC stocks are very small.
-
2.
More studies over longer periods of time are needed to fully understand the mechanism through which biochar affects the properties of saline soils.
-
3.
The benefits of biochar incorporation on reclamation of degraded lands especially salt-affected soils. Knowledge about this is scant, and to date, most studies have evaluated biochar use only in nonsalt-affected soils.
-
4.
The functioning of biochar in different saline soils and the responses of soil to different biochars need to be further investigated in the field.
-
5.
Further studies using long-term field and pot trials with different types of biochar made from various sources and pyrolysis temperatures and different soil types are required. This will enable determination of the ideal biochar to be applied to a specific soil type and the required length of time to effect changes. This will provide ideal information on how biochar can rehabilitate saline-sodic soils or increase SOC stocks permanently.
References
Abrol I, Yadav J, Massoud F (1988) Salt-affected soils and their managements. Soil Resources, Management and Conservation Service FAO Land and Water Development Division, Rome
Adu JK, Oades JM (1978) Physical factors influencing decomposition of organic material in soil aggregates. Soil Biol Biochem 10:109–115
Agassi M, Shainberg I, Morin J (1981) Effect of electrolyte concentration and soil sodicity on infiltration rate and crust formation. Soil Sci Soc Am J 45:848–851
Ahmad S, Ghafoor A, Qadir M, Aziz MA (2006) Amelioration of a calcareous saline-sodic soil by gypsum application and different crop rotations. Int J Agr Biol 8:142–146
Akhtar SS, Li G, Andersen MN, Liu F (2014) Biochar enhances yield and quality of tomato under reduced irrigation. Agr Water Manage 138:37–44
Akhtar SS, Andersen MN, Liu F (2015a) Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agr Water Manage 158:61–68
Akhtar SS, Andersen MN, Liu F (2015b) Biochar mitigates salinity stress in potato. J Agron Crop Sci 201:368–378
Akhtar SS, Andersen MN, Naveed M, Zahir ZA, Liu F (2015c) Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Funct Plant Biol 42:770–781
Amezketa E, Aragues R, Gazol R (2005) Efficiency of sulfuric acid, mined gypsum and two gypsum by products in soil crusting prevention and sodic soil reclamation. Agron J 97:983–989
Ammari TG, Tahboub AB, Saoub HM, Hattar BI, Al-Zubi YA (2008) Salt removal efficiency as influenced by phyto-amelioration of salt-affected soils. J Food Agric Environ 6:456–460
Armstrong A, Tanton T (1992) Gypsum application to aggregated saline-sodic clay topsoils. Eur J Soil Sci 43:249–260
Artiola JF, Rasmussen C, Freitas R (2012) Effects of a biochar-amended alkaline soil on the growth of romaine lettuce and bermudagrass. Soil Sci 177:561–570
Asai H, Samson BK, Stephan HM, Songykhanguthor K, Homma K, Kiyono Y, Horie T (2009) Biochar amendment techniques for upland rice production in Northern Laos: 1. Soil physical properties, leaf SPAD and grain yield. Field Crop Res 111:81–84
Atkinson C, Fitzgerald J, Hipps N (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Barzegar AR, Nelson PN, Oades JM, Rengasamy P (1997) Organic matter, sodicity and clay type: influence on soil aggregation. Soil Sci Soc Am J 61:1131–1137
Batra L, Manna MC (1997) Dehydrogenase activity and microbial biomass carbon in salt-affected soils of semiarid and arid regions. Arid Soil Res Rehab 11:295–303
Beltran JMN (1999) Irrigation with saline water: benefits and environmental impact. Agr Water Manage 40:183–194
Bhojvaid PP, Timmer VR (1998) Soil dynamics in an age sequence of Prosopis Juliflora planted for sodic soil restoration in India. Forest Ecol Manage 106:181–193
Bohn HL, McNeal BL, O’Connor GA (2001) Soil chemistry. Wiley, New York
Brenstein L (1975) Effects of salinity and sodicity on plant growth. Ann Rev Phytopathol 13(1):295–312
Brinck E, Frost C (2009) Evaluation of amendments used to prevent sodification of irrigated fields. Appl Geochem 24:2113–2122
Busscher WJ, Novak JM, Ahmedna M (2011) Physical effects of organic matter amendment of southeastern US coastal loamy sand. Soil Sci 176:661–667
Castaldi S, Riondino M, Baronti S, Esposito, Marzaioli R, Rutigliano FA, Vaccari FP, Miglietta F (2011) Impact of biochar application to a Mediterranean wheat crop on soil microbial activity and greenhouse gas fluxes. Chemosphere 85:1464–1471
Chaganti VN, Crohn DM (2015) Evaluating the relative contribution of physiochemical and biological factors in ameliorating a saline–sodic soil amended with composts and biochar and leached with reclaimed water. Geoderma 259–260:45–55
Chaganti VN, Crohn DM, Simunek J (2015) Leaching and reclamation of a biochar and compost amended saline–sodic soil with moderate SAR reclaimed water. Agr Water Manage 158:255–265
Chan KY, Xu ZH (2009) Biochar: nutrient properties and their enhancement. Biochar Environ Management: Sci Technol, pp 67–84
Chan KY, Van Zwieten L, Meszaros I, Downi A, Joseph S (2008) Agronomic values of greenwaste biochar as a soil amendment. Soil Res 45:629–634
Chander K, Goyal S, Kapoor KK (1994) Effect of sodic water irrigation and farmyard manure application on soil microbial biomass and microbial activity. Appl Soil Ecol 1:139–144
Chorom M, Rengasamy P (1997) Carbonate chemistry, pH and physical properties of an alkaline sodic soil as affected by various amendments. Aust J Soil Res 35:149–161
Choudhary OP, Josan AS, Bajwa MS, Kapur ML (2004) Effect of sustained sodic and saline-sodic irrigation and application of gypsum and farmyard manure on yield and quality of sugarcane under semi-arid conditions. Field Crop Res 87:103–116
Choudhary OP, Ghuman BS, Bijay S, Thuy N, Buresh RJ (2011) Effects of long term use of sodic water irrigation, amendments and crop residues on soil properties and crop yields in rice-wheat cropping system in a calcareous soil. Field Crop Res 121:363–372
Clough A, Skjemstad JO (2000) Physical and chemical protection of soil organic carbon in three agricultural soils with different contents of calcium carbonate. Aust J Soil Res 38:1005–1016
Corwin DL, Bradford SA (2008) Environmental impact and sustainability of degraded water reuse. J Environ Qual 37:1–7
Cross A, Sohi SP (2011) The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol Biochem 43:2127–2134
Dalal RC, Mayer RJ (1986) Long term trends in fertility of soils under continuous cultivation and cereal cropping in Southern Queensland. II. Total organic carbon and its rate of loss from the soil profile. Aust J Soil Res 24:280–292
DeLuca TH, McKenzie MD, Gundale MJ, Holben WE (2006) Wildfire produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci Soc Am J 70:448–453
Dempster DN, Gleeson DB, Solaiman ZM, Jones DL, Murphy DV (2012) Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 354:311–324
Diacono M, Montemurro F (2010) Long-term effects of organic amendments on soil fertility: a review. Agron Sustain Dev 30:401–422
Dikinya O, Hinz C, Aylmore G (2006) Dispersion and re-deposition of fine particles and their effects on saturated hydraulic conductivity. Soil Res 44:47–56
Djedidi Z, Drogui P, Ben Cheikh R, Mercier G, Blais J (2005) Laboratory study of successive soil saline leaching and electrochemical lead recovery. J Environ Eng 131:305–314
Edward AP, Bremner JM (1967) Microaggregates in soils. Eur J Soil Sci 18:64–73
Emdad M, Raine S, Smith R, Fardad H (2004) Effect of water quality on soil structure and infiltration under furrow irrigation. Irrigation Sci 23:55–60
Fang Y, Singh B, Singh BP, Krull E (2014) Biochar carbon stability in four contrasting soils. Eur J Soil Sci 65:60–71
Fitzpatrick RW, Merry RH (2000) Pedogenic carbonate pools and climate change in Australia. In: Lal R, Kimble JM, Eswran H, Stewart BA (eds) Global climate change and pedogenic carbonates. Lewis Publishers, Boca Raton, pp 105–120
Frankenberger WT, Bingham FT (1982) Influence of salinity on soil enzyme activities. Soil Sci Soc Am J 46:1173–1177
Frenkel H, Goertzen JO, Rhoades JD (1978) Effects of clay type and content, exchangeable sodium percentage, and electrolyte concentration on clay dispersion and soil hydraulic conductivity. Soil Sci Soc Am J 42:32–39
Gandhi AP, Paliwal KV (1976) Mineralization and gaseous losses of nitrogen from urea and ammonium sulphate in salt-affected soils. Plant Soil 45:247–255
Garcia C, Hernandez T (1996) Influence of salinity on the biological and biochemical activity of a calcirothird soil. Plant Soil 178:255–263
Garcia C, Hernandez T, Costa F (1994) Microbial activity in soils under Mediterranean environmental conditions. Soil Biol Biochem 26:1185–1191
Garg VK (1999) Leguminous trees for the rehabilitation of sodic wasteland in Northern India. Restor Ecol 7:281–287
Gaskin JW, Speir RA, Harris K, Das K, Lee RD, Morris LA, Fisher DS (2010) Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status and yield. Agron J 102:623–633
Ghafoor A, Gill M, Hassan A, Murtaza G, Qadir M (2001) Gypsum: an economical amendment for amelioration of saline-sodic waters and soils and for improving crop yields. Int J Agr Biol 3:266–275
Ghaly FM (2002) Role of natural vegetation in improving salt-affected soil in northern Egypt. Soil Till Res 64:173–178
Gharaibeh M, Eltaif N, Shunnar OF (2009) Leaching and reclamation of calcareous saline-sodic soil by moderately saline and moderate-SAR water using gypsum and calcium chloride. J Plant Nutr Soil Sci 172:713–719
Gharaibeh M, Eltaif N, Shra’ah S (2010) Reclamation of a calcareous saline sodic soil using phosphoric acid and by product gypsum. Soil Use Manage 26:141–148
Gharaibeh M, Eltaif N, Albalasmeh A (2011) Reclamation of highly calcareous saline sodic soil using Atriplex Halimus and by-product gypsum. Int J Phytoremediation 13:873–883
Ghasemi F, Jakeman AJ, Nix HA (1995) Salinisation of land and water resources: human causes, extent, management, and case studies. NSW University Press, Sydney
Goerge C, Wagner M, Kucke M, Rilling MC (2012) Divergent consequences of hydrochar in the plant-soil system: Arbuscular mycorrhiza, nodulation, plant growth and soil aggregation effects. Appl Soil Ecol 59:68–72
Gollarata M, Raiesi F (2007) The adverse effects of soil salinization on the growth of Trifolium alexandrinum L. and associated microbial and biochemical properties in a soil from Iran. Soil Biol Biochem 39:1699–1702
Grattan SR, Oster JD (2003) Use and reuse of saline-sodic waters for irrigation of crops. J Crop Prod 7:131–162
Grattan S, Benes S, Peters D, Diaz F (2008) Feasibility of irrigation Pickleweed (Torr) with hyper-saline drainage water. J Environ Qual 37:149–156
Gupta RK, Abrol I (1990) Salt-affected soils: their reclamation and management for crop production. Adv Soil Sci 11:223–288
Hammera E, Forstreutera M, Rilliga MC, Kohlera J (2015) Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl Soil Ecol 96:114–121
Herath HMSK, Camps-Arbestain M, Hedley M (2013) Effect of biochar on soil physical properties in two contrasting soils: an Alfi sol and an Andisol. Geoderma 209–210:188–197
Hosseini Bai S, Xu CY, Xu Z, Blumfield TJ, Zhao H, Wallace H, Reverchon F, Van Zwieten L (2015) Soil and foliar nutrient and nitrogen isotope composition (δ15N) at 5 years after poultry litter and green waste biochar amendment in a macadamia orchard. Environ Sci Pollut Res 22:3803–3809
Ilyas M, Qureshi RH, Qadir MA (1997) Chemical changes in a saline-sodic soil after gypsum application and cropping. Soil Technol 10:247–260
Izaurralde RC, Rosenberg NJ, Lal R (2001) Mitigation of climate change by soil carbon sequestration: issues of science, monitoring and degraded lands. Adv Agron 70:1–75
Jenkinson DJ, Raynor JH (1977) The turnover of soil organic matter in some of the Rothamsted classical experiments. Soil Sci 123:298–305
Jien SH, Wang CS (2013) Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena 110:225–233
Jones DL, Murphy DV, Khalid M, Ahmad W, Edwards-Jones G, DeLuca TH (2011) Short-term biochar-induced increase in soil CO2 release is both biotically and abiotically mediated. Soil Biol Biochem 43:1723–1731
Jones DL, Rousk J, Edwards-Jones G, Deluca TH, Murphy DV (2012) Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol Biochem 45:113–124
Jury WA, Jarrell WM, Devitt D (1979) Reclamation of saline-sodic soils by leaching. Soil Sci Soc Am J 43:1100–1106
Karhu K, Mattila T, Bergstrom I, Regina K (2011) Biochar addition to agricultural soil increased CH4 uptake and water holding capacity- Results from a short-term pilot field study. Agr Ecosyst Environ 140:309–313
Kelly W (1937) The reclamation of alkali soils. California Agric Exp Station Bull 617:1–40
Kinney CA, Furlong ET, Werner SL, Cahill JD (2006) Presence and distribution of wastewater-derived pharmaceuticals in soil irrigated with reclaimed water. Environ Toxicol Chem 25:317–326
Kolb SE, Fermanich KJ, Dornbush ME (2009) Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci Soc Am J 73:1173–1181
Kookana R, Sarmah A, Van Zwieten L, Krull E, Singh B (2011) Biochar application to soil: agronomic and environmental benefits and unintended C consequences. Adv Agron 112:103–143
Kuzyakov Y, Bogomolova I, Glaser B (2014) Biochar stability in soil: decomposition during eight years and transformation as assessed by compound-specific 14C analysis. Soil Biol Biochem 70:229–236
Kwapinski W, Byrne CM, Kryachko E, Wolfram P, Adley C, Leahy J, Hayes M (2010) Biochar from biomass and waste. Waste Biomass Valorization 1:177–189
Laird DA, Fleming P, Davis DD, Horton R, Wang B, Karlen DL (2010) Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 158:443–449
Lakhdar A, Rabhi M, Ghnaya T, Montemurro F, Jedidi N, Abdelly C (2009) Effectiveness of compost use in salt-affected soil. J Hazard Mater 171:29–37
Lal R (2001) Fate of eroded soil organic carbon: emission or sequestration. In Lal R (ed) Soil carbon sequestration and greenhouse effect. Soil Sci Soc Am J, USA, pp 173–182
Lal R, Follett RF, Kimble JM (2003) Achieving soil carbon sequestration in the United States: a challenge to the policy makers. Soil Sci 168:827–845
Lal R, Kimble J, Follett RF, Cole CV (2006) Enhancing crop yields through restoration of soil organic carbon pool in agricultural lands. Land Degrad Dev 17:197–206
Lashari MS, Liu Y, Li L, Pan W, Fu J, Pan G, Yu X (2013) Effects of amendment of biochar-manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from Central China Great Plain. Field Crop Res 144:113–118
Lashari MS, Ye Y, Ji H, Li L, Kibue GW, Lu H, Zheng J, Pan G (2014) Biochar–manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: a 2-year field experiment. J Sci Food Agr 95:1321–1327
Laura RD (1973) Effects of sodium carbonate on carbon and nitrogen mineralization of organic matter added to soil. Geoderma 9:15–26
Lebron I, Suarez DL, Yoshida T (2002) Gypsum effect on the aggregate size and geometry of three sodic soils under reclamation. Soil Sci Soc Am J 66:92–98
Lehman J, Joseph S (2009) Biochar for environmental management. Science and Technology. Earth Scan, pp 1–12
Lehman J, Rilling MC, Thies JE, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems – a review. Mitig Adapt Strategies Glob Chang 11:395–419
Lehmann J, Skjemstad JO, Sohi S, Carter J, Barson M, Fallon P, Krull E (2008) Australian climate-carbon cycle feedback reduced by soil black carbon. Nat Geosci 1:832–835
Letey J, Hoffman GJ, Hopmans JW, Grattan Suarez D, Corwin DL, Amrhein C (2011) Evaluation of soil salinity leaching requirement guidelines. Agr Water Manage 98:502–506
Levine AD, Asano T (2004) Peer reviewed: recovering sustainable water from wastewater. Environ Sci Technol 38:201–208
Liang YC, Yang YF, Yang CG, Shen QQ, Zhou JM, Yang LZ (2003) Soil enzymatic activity and growth of rice and barley as influenced by organic matter in an anthropogenic soil. Geoderma 115:149–160
Liang Y, Nikolic M, Peng Y, Chen W, Jiang Y (2005) Organic manure stimulates biological activity and barley growth in soil subject to secondary salinization. Soil Biol Biochem 37:1185–1195
Liang B, Lehmann J, Sohi SP, Thies JE, O’Neill B, Trujillo L, Gaunt J, Solomon D, Grossman J, Neves EG, Luizao FJ (2010) Black carbon affects the cycling of non-black carbon in soil. Org Geochem 41:206–213
Liu XH, Han PF, Zhang XC (2012) Effect of biochar on soil aggregates in the Loess Plateau: results from incubation experiments. Int J Agr Biol 14:975–979
Luo Y, Durenkamp M, De Nobili M, Lin Q, Brookes PC (2011) Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol Biochem 43:2304–2314
Macdonald L, Farrell M, van Zwieten L, Krull E (2014) Plant growth responses to biochar addition: an Australian soils perspective. Biol Fertil Soil 50:1035–1045
Mace J, Amrhein C (2001) Leaching and reclamation of a soil irrigated with moderate SAR waters. Soil Sci Soc Am J 65:199
Major J, Rondon M, Molina D, Riha SJ, Lehmann J (2010) Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 333:117–128
Manchanda G, Garg N (2008) Salinity and its effects on the functional biology of legumes. Acta Physiol Plant 30:595–618
Mandal UK, Bhardwaj AK, Warrington DN, Goldstein D, Bar Tal A, Levy GJ (2008) Changes in soil hydraulic conductivity, runoff and soil loss due to irrigation with different types of saline-sodic water. Geoderma 144:509–516
Martinez-Beltran J, Manzur CL (2005) Overview of salinity problems in the world and FAO strategies to address the problem. Paper presented at the International salinity forum, Riverside
McCarty GW, Ritchie JC (2002) Impact of soil movement on carbon sequestration in agricultural ecosystems. Environ Pollut 116:423–430
McClung G, Frankenberger WT (1985) Soil nitrogen transformation as affected by salinity. Soil Sci 139:405–411
McCormic RW, Wolf DC (1980) Effect of sodium chloride on CO2 evolution, ammonification and nitrification in a sassafras sandy loam. Soil Biol Biochem 12:153–157
McNeal BL, Layfield DA, Norvell WA, Rhoades JD (1968) Factors influencing hydraulic conductivity of soils in the presence of mixed -salt solutions. Soil Sci Soc Am J 32:187–190
Metternicht G, Zink J (2003) Remote sensing of soil salinity: potentials and constraints. Remote Sens Environ 85:1–20
Mishra A, Sharma SD (2003) Leguminous trees for the restoration of degraded sodic wasteland in Eastern Uttar Pradesh, India. Land Degrad Dev 14:245–261
Moore DC, Singer MJ (1990) Crust formation effects on soil erosion processes. Soil Sci Soc Am J 54:1117–1123
Mukherjee A, Lal R (2013) Biochar impacts on soil physical properties and greenhouse gas emissions. Agron J 3:313–339
Muneer M, Oades JM (1989a) The role of Ca-organic interactions in soil aggregate stability. II. Field studies with 14C-labelled straw, CaCO3 and CaSO4.2H2O. Aust J Soil Res 27:401–409
Muneer M, Oades JM (1989b) The role of Ca-organic interactions in soil aggregates stability I. Laboratory studies with 14C-glucose, CaCO3 and CaSO4.2H2O. Aust J Soil Res 27:389–399
Murphy DV, Sparling GP, Fillery IRP (1998) Stratification of microbial biomass C and N and gross N mineralization with soil depth in two contrasting Western Australian agricultural soils. Aust J Soil Res 36:45–55
Naidu R, Rengasamy P (1993) Ion interactions and constraints to plant nutrition in Australian sodic soils. Aust J Soil Res 31:801–819
Nelson PN, Oades JM (1998) Organic matter, sodicity and soil structure. In: Sumner ME, Naidu R (eds) Sodic soils: distribution, properties, management and environmental consequences. Oxford University Press, New York
Nelson PN, Ladd JN, Oades JM (1996) Decomposition of 14C-Labelled plant material in a salt-affected soil. Soil Biol Biochem 24:433–441
Nelson PN, Barzegar AR, Oades JM (1997) Sodicity and clay type: influence on decomposition of added organic matter. Soil Sci Soc Am J 61:1052–1057
Nelson PN, Baldock JA, Oades JM (1998) Changes in dispersible clay content, organic carbon content and electrolyte composition following incubation of sodic soil. Soil Res 36:883–898
Niazi B, Ahmed M, Hussain N, Salim M (2001) Comparison of sand, gypsum and sulphuric acid to reclaim a dense saline sodic soil. Int J Agr Biol 3:316–318
NLWRA (2001) National dryland salinity assessment. National land and water resources audit
Novak JM, Busscher WJ, Laird DA, AHmedna M, Watts DW, Niandou MA (2009) Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci 174:105–112
O’Connor GA, Elliott HA, Bastian RK (2008) Degraded water reuse: an overview. J Environ Qual 37:157–168
Oren A (1999) Bioenergetic aspects of halophilism. Microbiol Mol Biol Rev 63:334–348
Oster JD (1994) Irrigation with poor quality water. Agr Water Manage 25:271–279
Oster JD, Shainberg I (2001) Soil responses to sodicity and salinity: challenges and opportunities. Soil Res 39:1219–1224
Oster JD, Shainberg I, Abrol I (1999) Reclamation of salt-affected soil. Agricultural Drainage. Agronomy Monograph 38:315–346
Pal DK, Dasong GS, Vadivelu S, Ahuja RL, Bhattacharyya T (2000) Secondary calcium carbonate in soils of arid and semiarid regions on India. In: Lal R, Kimble JM, Eswran H, Stewart BA (eds) Global climate change and pedogenic. Lewis Publishers, Boca, Raton, pp 149–186
Pankhurst CE, Yu S, Hawke BG, Harch BD (2001) Capacity of fatty acid profiles and substrate utilization patterns to describe differences in soil microbial communities associated with increased salinity or alkalinity at three locations in South Australia. Biol Fert Soil 33:204–217
Parton WJ, Schimel DS, Cole CV (1987) Analysis of factors controlling soil organic matter levels in great plains grasslands. Soil Sci Soc Am J 51:1173–1179
Pathak H, Rao DLN (1998) Carbon and nitrogen mineralization from added organic matter in saline and alkali soils. Soil Biol Biochem 30:695–702
Paz-Ferreiro J, Lu H, Fu S, Méndez A, Gascó G (2014) Use of phytoremediation and biochar to remediate heavy metal polluted soils: a review. Solid Earth 5:65–75
Pessarakli M, Szabolcs I (1999) Soil salinity and sodicity as particular plant-crop stress factors. Handbook of plant and crop stress. 2nd edition, revised and expanded. Marcel Dekker, Inc., New York, p 1254
Piccolo A, Pietramellara G, Mbagwu JSC (1996) Effects of coal derived humic substances on water retention and structural stability of Mediterranean soils. Soil Use Manage 12:209–213
Piccolo A, Pietramellara G, Mbagwu JSC (1997) Use of humic substances as soil conditioners to increase aggregate stability. Geoderma 75:267–277
Poloneko D, Mayfield C, Dumbroff E (1981) Microbial responses to salt-induced osmotic stress. Plant Soil 59:269–285
Qadir M, Oster JD (2004) Crop and irrigation management strategies for saline-sodic soils and waters aimed at environmentally sustainable agriculture. Sci Total Environ 323:1–19
Qadir M, Schubert S (2002) Degradation processes and nutrient constraints in sodic soils. Land Degrad Dev 13:275–294
Qadir M, Qureshi RH, Ahmad N (1996) Reclamation of a saline-sodic soil by gypsum and Leptochloa fusca. Geoderma 74:207–217
Qadir M, Qureshi RH, Ahmad N (1997) Nutrient availability in a calcareous saline-sodic soil during vegetative bioremediation. Arid Land Res Manag 11:343–352
Qadir M, Ghafoor A, Murtaza G (2000) Amelioration strategies for saline soils: a review. Land Degrad Dev 11:501–521
Qadir M, Ghafoor A, Murtaza G (2001a) Use of saline–sodic waters through phytoremediation of calcareous saline–sodic soils. Agr Water Manage 50:197–210
Qadir M, Schubert S, Ghafoor A, Murtaza G (2001b) Amelioration strategies for sodic soils: a review. Land Degrad Dev 13:275–294
Qadir M, Noble AD, Oster JD, Schubert S, Ghafoor A (2005) Driving forces for sodium removal during phytoremediation of calcareous sodic and saline-sodic soils: a review. Soil Use and Management 21: 173–180
Qadir M, Oster JD, Schubert S, Noble AD, Sahrawat KL (2007) Phytoremediation of sodic and saline‐sodic soils. In Donald LS (ed). Adv Agron 96:197–247
Quirk J (1994) Interparticle forces: a basis for the interpretation of soil physical behavior. Advances in Agronomy, Academic Press, Orlando, pp 121–183
Quirk J (2001) The significance of the threshold and turbidity concentrations in relation to sodicity and microstructure. Aust J Soil Res 39:1185–1218
Quirk J, Schofield RK (1955) The effect of electrolyte concentration on soil permeability. Eur J Soil Sci 6:163–178
Rengasamy P (2002) Transient salinity and subsoil constraints to dryland farming in Australian sodic soils: an overview. Aust J Exp Agr 42:351–362
Rengasamy P (2006) World salinization with emphasis on Australia. J Exp Bot 57:1017–1023
Rengasamy P (2010) Soil processes affecting crop production in salt-affected soils. Funct Plant Biol 37:613–620
Rengasamy P, Olsson KA (1991) Sodicity and soil structure. Aust J Soil Res 29:935–952
Rengasamy P, Sumner ME (1998) Processes involved in sodic behaviour. In: Sumner ME, Naidu R (eds) Sodic soils: distribution, properties, management and environmental consequences. Oxford University Press, New York, pp 35–50
Reverchon F, Flicker RC, Yang H, Yan G, Xu Z, Chen C, Bai SH, Zhang D (2014) Changes in δ15N in a soil–plant system under different biochar feedstocks and application rates. Biol Fertil Soils 50:275–283
Reverchon F, Yang H, Ho TY, Yan G, Wang J, Xu Z, Chen C, Zhang D (2015) A preliminary assessment of the potential of using an acacia—biochar system for spent mine site rehabilitation. Env Sci Pollut Res 22:2138–2144
Rhoades JD (1987) Use of saline water for irrigation. Water Quality Bull 12:14–20
Richard LA (1954) Diagnosis and improvement of saline and alkali soils. Soil Sci 78:154
Rietz DN, Haynes RJ (2003) Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol Biochem 35:845–854
Robbins C (1986) Sodic calcareous soil reclamation as affected by different amendments and crops. Agron J 78:916–920
Rondon M, Lehman J, Ramirez J, Hurtado M (2007) Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol Fert Soils 43:699–708
Sadinha M, Muller T, Schmeisky H, Joergensen RG (2003) Microbial performances in soils along a salinity gradient under acidic conditions. Appl Soil Ecol 23:237–244
Sadiq M, Hassan G, Mehdi S, Hussain N, Jamil M (2007) Amelioration of saline-sodic soils with tillage implements and sulfuric acid application. Pedosphere 17:182–190
Schimel DS, Braswell BH, Holland EA, McKeown R, Ojima DS, Painter TH, Townsend AR (1994) Climatic, edaphic and biotic controls over storage and turnover of carbon in soils. Global Biogeochem Cy 8:279–293
Schnurer JM, Clarholm M, Roswell T (1985) Microbial biomass and activity in an agricultural soil with different organic matter contents. Soil Biol Biochem 17:611–618
Setia R, Marschner P, Baldock J, Chittleborough D, Verma V (2011) Relationships between carbon dioxide emission and soil properties in salt-affected landscapes. Soil Biol Biochem 43:667–674
Shainberg I, Lety J (1984) Response of soils to sodic and saline conditions. Hilgardia 52:1–57
Shainberg I, Levy G, Rengasamy P, Frenkel H (1992) Aggregate stability and seal formation as affected by drops’ impact energy and soil amendments. Soil Sci 154:113–119
Six J, Bossuyt H, Degryze S, Denef K (2004) A history of research on the link between (micro) aggregates, soil biota and soil organic matter dynamics. Soil Till Res 79:7–31
Skjemstad JO, Clarke P, Taylor JA, Oades JM, McClure SG (1996) The chemistry and nature of protected carbon in soil. Aust J Soil Res 34:251–271
Skjemstad JO, Reicosky DC, Wilts AR, McGowan JA (2002) Charcoal carbon in US agricultural soils. Soil Sci Soc Am J 66:1249–1255
Slavich PG, Sinclair K, Morris SH, Kimber SWL, Downie A, Van Zwieten L (2013) Contrasting effects of manure and green waste biochars on the properties of an acidic ferralsol and productivity of a subtropical pasture. Plant Soil 366:213–227
Smedema L, Shiati K (2002) Irrigation and salinity: a perspective review of the salinity hazards of irrigation development in the arid zone. Irrig Drain 16:161–174
Solaiman ZM, Blackwell P, Abbott LK, Storer P (2010) Direct and residual effect of biochar application on mycorrhizal root colonisation, growth and nutrition of wheat. Soil Res 48:546–554
Spokas KA, Koskinen WC, Baker JM, Reicosky DC (2009) Impact of woodchip biochar addition on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 77:574–581
Stevens DP, McLaughlin MJ, Smart MK (2003) Effects of long term irrigation with reclaimed water on soils of the Northern Adelaide Plains, South Australia. Soil Res 41:933–948
Suarez D, Wood J, Lesch S (2006) Effect of SAR on water infiltration under a sequential rain-irrigation management system. Agr Water Manage 86:150–164
Sumner M (1993) Sodic soils—new perspectives. Soil Res 31:683–750
Sumner ME, Miller WP, Kookana RS, Hazelton P (1998) Sodicity, dispersion and environmental consequences. In: Sumner ME, Naidu R (eds) Sodic soils: distribution, properties, management and environmental consequences. Department of Crop and Soil Sciences, University of Georgia, Athens
Szabolcs I (1994) Soils and salinization. Hand book of plant and crop stress. Marcel Dekker, New York, pp 3–11
Tanton TW, Armstrong ASB, Rycroft DW (1988) The leaching of salts from restructured saline clay soils. Soil Use Manage 4:139–143
Tejada M, Garcia C, Gonzalez JL, Hernandez MT (2006) Use of organic amendment as a strategy for saline soil remediation: influence on the physical, chemical and biological properties of soil. Soil Biol Biochem 38:1413–1421
Thies JE, Rilling MC (2009) Characteristics of biochar: biological properties. Biochar Environ Management: Sci Technol, pp 85–105
Thomas SC, Frye S, Gale N, Garmon M, Launchbury R, Machado N, Melamed S, Murray J, Petroff A, Winsborough C (2013) Biochar mitigates negative effect s of salt additions on two herbaceous plant species. J Environ Manage 129:62–68
Tisdall JM, Oades JM (1982) Organic matter and water-stable aggregates in soils. Eur J Soil Sci 33:141–163
Toze S (2006) Reuse of effluent water-benefits and risks. Agr Water Manage 80:147–159
Tripathi S, Kumari S, Chakraborti A, Gupta A, Chakrabarti K, Bandyapadhyay BK (2006) Microbial biomass and its activities in salt-affected coastal soils. Biol Fert Soil 42:273–277
Tripathi S, Chakraborti A, Chakrabarti K, Bandyapadhyay BK (2007) Enzyme activities and microbial biomass in coastal soils of India. Soil Biol Biochem 39:2840–2848
Uchimiya M, Wartelle LH, Klasson KT, Fortier CA, Lima IM (2011) Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J Agric Food Chem 59:2501–2510
Uzoma KC, Inoue M, Andry H, Fujimaki H, Zahoor A, Nishihara E (2011) Effects of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manage 27:205–212
Van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Cowie A (2010a) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246
Van Zwieten L, Kimber S, Downie A, Morris S, Rust J, Chan KY (2010b) A glasshouse study on the interaction of low mineral ash biochar with nitrogen in a sandy soil. Aust J Soil Res 48:569–576
Vance WH, Tisdall JM, McKenzie BM (1998) Residual effects of surface application of organic matter and calcium salts on the sub-soil of a red-brown earth. Aust J Exp Agr 38:595–600
Vance GF, King LA, Ganjegunte GK (2008) Soil and plant responses from land application of saline-sodic waters: implications of management. J Environ Qual 37:139–148
Verheijen F, Jeffery S, Bastos A, Van Der Velde M, Diafas I (2010) Biochar application to soils: a critical scientific review of effects on soil properties, processes and functions. Joint Research Centre, Institute for Environment and Sustainability, Ispra
Walker DJ, Bernal PM (2008) The effects of olive mill waste compost and poultry manure on the availability and plant uptake of nutrients in a highly saline soil. Bioresour Technol 99:396–403
Walpola BC, Arunakumara KKIU (2010) Effect of salt stress on decomposition of organic matter and nitrogen mineralization in animal manure amended soils. J Agr Sci 5:9–18
Wardle DA, Nilsson MC, Zackrisson O (2008) Fire-derived charcoal causes loss of forest humus. Science 320:629
Wichern J, Wichern F, Joergensen RG (2006) Impact of salinity on soil microbial communities and decomposition of maize in acidic soils. Geoderma 137:100–108
Wong VNL, Dalal RC, Greene RSB (2008) Salinity and sodicity effects on respiration and microbial biomass of soil. Biol Fert Soil 44:943–953
Wong VNL, Dalal RC, Greene RSB (2009) Carbon dynamics of sodic and saline soils following gypsum and organic material additions: a laboratory incubation. Appl Soil Ecol 41:29–40
Wong VNL, Greene RSB, DALAL RC, Murphy BW (2010) Soil carbon dynamics in saline and sodic soils: a review. Soil Use Manage 26:2–11
Woolf D, Amonette JE, Street-Perrott FA, Lehman J, Joseph S (2010) Sustainable biochar on mitigate global climate change. Nat Commun 1:56
Wu L, Chen W, French C, Chang AC (2009) Safe application of reclaimed water reuse in the southwestern United States. ANR Publications, pp 1–21
Xu CY, Hosseini-Bai S, Hao Y, Rachaputi RCN, Wang H, Xu Z, Wallace H (2015) Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ Sci Pollut Res 22:6112–6125
Yan N, Marschner P (2013) Microbial activity and biomass recover rapidly after leaching of saline soils. Biol Fert Soil 49:367–371
Yuan BC, Li ZZ, Liu H, Gao M, Zhang YY (2007) Microbial biomass and activity in salt-affected soils under arid conditions. Appl Soil Ecol 35:319–328
Zahran HH (1997) Diversity, adaptation and activity of the bacterial flora in saline environments. Biol Fert Soil 25:211–223
Zavalloni C, Alberti G, Biasiol S, Vedove GD, Fornasier F, Liu J, Peressotti A (2011) Microbial mineralization of biochar and wheat straw mixture in soil: a short-term study. Appl Soil Ecol 50:45–51
Zhang X, Wang H, He L, Lu K, Sarmah A, Li J, Bolan NS, Pei J, Huang H (2013) Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ Sci Pollut Res 20:8472–8483
Zhang QZ, Dijkstra FA, Liu XR, Wang YD, Huang J, Lu N (2014) Effects of biochar on soil microbial biomass after four years of consecutive application in the north China plain. PLoS One 9:1–8
Zhang QZ, Du ZL, Lou Y, He X (2015) A one-year short-term biochar application improved carbon accumulation in large macroaggregate fractions. Catena 127:26–31
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yong Sik Ok
Rights and permissions
About this article
Cite this article
Amini, S., Ghadiri, H., Chen, C. et al. Salt-affected soils, reclamation, carbon dynamics, and biochar: a review. J Soils Sediments 16, 939–953 (2016). https://doi.org/10.1007/s11368-015-1293-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1293-1