Abstract
This paper generalizes previous work on physically based nonlinear orthotropic invariants for thermomechanical response of soft materials to include the inelastic process of homeostasis, which causes a biological tissue to approach its homeostatic state. This process of homeostasis can cause a homogeneous material (one with the same constitutive equations and material constants as each material point) to develop a nonuniform state. Within the context of biological tissues, this means that the tissue cannot be unloaded elastically to a zero-stress state. A simplified version of the theory is used to describe elastic response of an artery from its nonuniform homeostatic state using a Fung-type exponential orthotropic strain energy function with material constants determined for a human carotid artery. As discussed in Safadi and Rubin (Int. J. Eng. Sci. 118(40), 2017), the approach of assuming a homeostatic state at systolic pressure and limiting extrapolation of the constitutive response to the physiological pressure range reduces uncertainty in the stress distributions in the artery. The specific results here show that the circumferential stress in the physiological pressure range exhibits a strong sensitivity to residual stresses known to exist in the cut unloaded state. This approach suggests that detailed experimental data on the response of the artery in its physiological pressure range and more complete understanding of mechanobiological processes during homeostasis are essential for determining an accurate constitutive equation of an artery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Biological materials are heterogeneous mixtures of components that can flow relative to each other and can interact mechanically, chemically and electrically [2,3,4,5,6]. Within the context of continuum mechanics, it is natural to model the response of tissues using mixture theory [7,8,9] and a simplified mixture theory with only one velocity field was proposed in [2]. Continuum models for growth and remodeling can be found in [10,11,12,13,14,15,16,17,18,19]. Specifically, in [15, 17], a Lagrangian formulation was proposed with constitutive equations based on the elastic deformation tensor Fe, defined by
where F is the total deformation from a reference configuration and the growth tensor Fg is determined by integrating an evolution equation of the form
with the growth rate Λg specified by a constitutive equation. The theory of multiple natural configurations in [20] can also be expressed in a form characterized by Eqs. 1 and 2.
Eckart [21] developed an Eulerian formulation of a theory for inelastic response of solids based on an evolution equation for an elastic deformation measure. Leonov [22] proposed a similar theory for polymeric liquids. Both of the theories modeled elastically isotropic response. An Eulerian formulation for elastically anisotropic response was developed in [23] which proposed evolution equations for a triad of microstructural vectors that model elastic deformations and the changing orientations of anisotropy. Based on these ideas, an Eulerian formulation of growth was developed in [24]. Physical invariants for large deformation orthotropic elastic response were proposed in [25, 26] and recently [27] these invariants were generalized for orthotropic thermomechanical response of soft materials.
In contrast with Lagrangian formulations of inelastic response, the Eulerian formulation is insensitive to arbitrariness of specifications of a reference configuration, an intermediate configuration, a total deformation measure, and an inelastic deformation measure. This is particularly important for materials that grow and remodel due to homeostasis, which is the process of the material attempting to achieve a homeostatic state, because homeostasis occurs in the current state of the material and depends only on the current state variables.
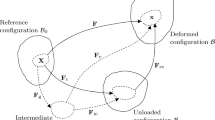
One objective of the present paper is to generalize the nonlinear orthotropic invariants developed in [27] for the themoelastic response of soft materials to include the inelastic effect of homeostasis. General forms of the evolution equations are proposed but specific forms for homeostasis still need to be discovered. Another objective is to examine the influence of homeostasis on the prediction of stress distributions in the passive behavior of arteries. The classical approach to modeling stresses in arteries [28, 29] is to model the artery as a homogeneous, uniform circular cylindrical tube made of a hyperelastic material. These works assume that the artery has zero-stress in its cut unloaded state (see Fig. 1) and that stresses in other intact states can be determined by considering the artery to be an incompressible material with applied internal pressure, zero external pressure, and applied uniform axial stretch relative to its cut unloaded state. Further axial cuts of the cut unloaded artery revealed that the curvature of the cut segments remains nearly uniform [29], which indicates that any residual stress in the cut unloaded artery is independent of circumferential coordinate but does not ensure that the stress field is independent of the radial coordinate.
Holzapfel et al. [30] described the artery as having three layers: the intima, media, and adventitia. Since the intima has little mechanical strength, the artery was modeled using two layers, each with orthotropic elastic response but it was also assumed that the cut unloaded state is at zero stress. Later [31] sectioned the cut unloaded state and determined that the residual stress distributions are three-dimensional and cannot be fully characterized by the geometry of the cut unloaded state. Also, constitutive equations based on structurally motivated orthotropic invariants are discussed in [32].
Using the formulation for growth of biological tissues developed in [24], Safadi and Rubin [1] studied the influence of homeostasis on stresses in arteries. Following this work, it is assumed that homeostasis is a slow process relative to normal deformations of the artery due to cyclic loading from the diastolic pressure Pd to the systolic pressure Ps and due to deformations caused by unloading and cutting the artery. This assumption suggests that the artery has had sufficient time to remodel to its homeostatic state (i.e., the homeostatic state is in mechanobiological equilibrium) and that deformations from this homeostatic state can be approximated as elastic with no influence of homeostasis.
Based on engineering considerations, different proposals for the mechanical response to homeostasis have been suggested. Much of the research on homeostasis, discussed in [1], suggests that homeostasis tends to cause the circumferential and axial stresses in the artery to become more uniform through thickness of the artery. These assumptions are used in the examples in this paper. In this regard, it is noted that [33] discusses the fundamental roles of axial stress, and flow-induced shear stress applied to the interior artery wall in the axial direction, which is not included in the present analysis.
Since the stresses in the artery are highest in its systolic state, Safadi and Rubin [1] specified this state as the homeostatic state of the artery with the homeostatic pressure Ph = Ps and predicted the stress distributions in the cut unloaded and intact unloaded states for a rabbit thoracic artery. Specifically, the circumferential and axial stresses in the homeostatic systolic state were assumed to be uniform, with these uniform values determined to match the measured cut unloaded geometry. This approach was also used to predict stress distributions due to homeostasis caused by hypertension.
In addition, in [1] estimates of the influence of residual stresses in a cut unloaded state were obtained by cutting the intact unloaded state into two layers, each of which produce different cut unloaded geometries with different internal and external radii and different opening angles. Furthermore, Safadi [34] examined the influence of residual stresses in the intact unloaded state on the stress distributions in the systolic state. Specifically, different homeostatic stress distributions were proposed for the intact unloaded state which are consistent with the measured geometry of the cut unloaded state. It was found that these different stress distributions have a significant influence on the predicted stresses in the systolic state.
Following with work in [1, 34], here, it is also assumed that the arterial tube is made of a homogeneous material (i.e., identical material properties at each point) but that homeostasis causes the arterial material to become nonuniform (i.e., the material cannot be fully unloaded elastically with a compatible deformation field to a zero-stress configuration). This is consistent with the experimental observations in [31] which showed that the geometry of the cut unloaded artery continues to change as more cuts are made that relieve residual stresses. Also, here, the loaded nonuniform homeostatic systolic state is used as a reference state for the determination of the stress-distributions in other states of the artery. Specifically, the systolic state has inner radius as, outer radius bs, and applied internal pressure Ps; the diastolic state has inner radius ad, outer radius bd, and applied internal pressure Pd; the intact unloaded state has inner radius au, outer radius bu, and zero applied internal pressure; and the cut unloaded state has inner radius ac, outer radius bc, and opening angle ωc (see Fig. 1). In this regard, it is noted that the opening angle of the cut unloaded state in Fig. 1 is identified as ωc/2 in [28, 29] and by ωc here and in [35]. The word intact is used to distinguish between an artery that is not cut and a cut artery. The equivalent uniform axial stress in the homeostatic state, which is taken to be the systolic state, is specified (see Eq. 86). Also, the equivalent uniform axial stress in the diastolic state is assumed to be reduced relative to that in the systolic state by the ratio of the applied pressures in the two states [see Eq. 84].
The purely mechanical form of the model developed here introduces a homeostatic state of the tissue with the elastic dilatation Je and the elastic distortional microstructural vectors \(\mathbf {m}_{i}^{\prime }\) given by
where the homeostatic values Jh,h1, and h2 are specified by constitutive equations and pi is a right-handed orthonormal triad that determines the directions of elastic orthotropy. Next, it is convenient to define the relative deformation gradient Fr from the homeostatic state to another deformed state and to define the relative dilatation Jr and relative unimodular deformation tensor \(\mathbf {F}_{r}^{\prime }\) [36], by
Now, confining attention to elastic deformations from the homeostatic state, it follows that the deformed elastic dilatation Je and elastic distortional deformation microstructural vectors \(\mathbf {m}_{i}^{\prime }\) in a general deformed state are given by
As is common, the elastic response from the homeostatic state is assumed to be incompressible. However, this does not prevent homeostasis of growth and remodeling from changing the volume of the artery when it is active.
The sensitivity of the material parameters and errors in measurements has been considered in [35, 37,38,39,40] for different material models of arterial tissue. Finite element solutions were also used in [41] to examine influences of different test protocols on the determination of stress in tube models of orthotropic materials.
Recently, Emuna et al. [35] determined the sensitivity of material model parameters in two constitutive equations to uncertainties in stress-free measurements. To determine the sensitivity of the Fung model to residual stresses in the cut unloaded state, a modified Fung model (MF-c) is developed here which uses scaled values of the residual stresses predicted for the cut unloaded state as the homeostatic state. Then, the stress distributions in the unloaded, diastolic, and systolic states are predicted by elastically deforming this cut unloaded homeostatic state. When the scale parameter α vanishes, these stress fields are the same as those predicted by the standard Fung model. However, when 0 < α ≤ 1, the predictions of the MF-c model suggest that the circumferential stress predicted by extrapolating from the cut unloaded state is particularly sensitive to the residual stress field.
An outline of this paper is as follows. Section 2 presents the basic equations and balance laws. Section 3 introduces the evolution equations for the elastic dilatation Je, the distortional microstructural vectors \(\mathbf {m}_{i}^{\prime }\) with the influence of homeostasis and the nonlinear orthotropic thermoelastic invariants. General constitutive equations are developed in Section 4 and modeling of the homeostatic state is discussed in Section 5. Section 6 discusses the application of a simplified version of the model to determining stresses in arteries and Section 7 presents results of specific examples. Conclusions are discussed in Section 8 and details of some of the mathematical expressions are recorded in Appendix A.
2 Basic equations
The location of a material point in the current configuration at time t is denoted by x and the velocity v, velocity gradient L, rate of deformation tensor D, and spin tensor W are defined by
where a superposed dot \(\dot {()}\) denotes material time differentiation.
Following the work in [24, 27], the local forms of the balance laws of mass, linear momentum and entropy are given by
where ρ is the current mass density, rm is the external rate of mass supply per unit mass, b is the external body force per unit mass, T is the Cauchy stress, η is the entropy per unit mass, s is the external rate of entropy supply per unit mass, ξ is the internal rate of entropy production per unit mass due to dissipative mechanisms, and p is the entropy flux per unit current area. In these equations, I is the second-order unity tensor and A ⋅B = tr(ABT) is the inner product between two second-order tensors A and B. Also, the balances of angular momentum and energy are given by
where ε is the internal energy per unit mass, 𝜃 is the absolute temperature, and b is the external rate of energy supply per unit mass due to mechanobiological processes. Using the result
the definition
and the balance of entropy, the balance of energy can be expressed in the form
Moreover, using the definition of the Helmholtz free energy
it follows that
3 Evolution equations
Following the work in [27], the elastic dilatation Je is determined by the equation
where ρ0 is the density of the material in a zero-stress state at reference temperature 𝜃0, Γv controls the rate of volumetric homeostasis and Jh controls the homeostatic value of elastic dilatation. Also, the elastic distortional deformations and the orthotropic orientations are determined by the right-handed triad of linearly independent distortional vectors \(\mathbf {m}_{i}^{\prime }\)
which satisfy the evolution equations
where Γ controls the rate of distortional homeostasis, \(\bar {\mathbf {L}}_{g}^{\prime \prime }\) controls its direction, and the deviatoric part of a tensor is denoted by \(()^{\prime \prime }\) so, for example
Also, the symmetric \(\bar {\mathbf {D}}_{g}^{\prime \prime }\) and skew-symmetric \(\bar {\mathbf {W}}_{g}\) parts of \(\bar {\mathbf {L}}_{g}^{\prime \prime }\) are defined by
In addition, the reciprocal vectors \(\mathbf {m}^{i\prime }\) are defined by
and the covariant metric \(m_{ij}^{\prime }\) and contravariant metric \(m^{ij \prime }\) satisfy the equations
where \({\delta _{i}^{j}}\) is the Kronecker delta and the usual summation convention for repeated indices is applied.
In these equations, \(\bar {\mathbf {D}}_{g}^{\prime \prime }\) controls the direction of the homeostasis distortional deformation rate and \(\bar {\mathbf {W}}_{g}\) controls the direction of inelastic spin. To motivate a form for \(\bar {\mathbf {D}}_{g}^{\prime \prime }\) it is recalled from [27] that the metrics \(m_{ij}^{\prime }\) and \(m^{ij \prime }\) in any hydrostatic state of stress (HSS) take the forms
where ηi are positive dependent constitutive functions of the elastic dilatation Je and temperature 𝜃 satisfying the restrictions
It also follows that for any HSS
where the right-handed orthonormal triad pi
characterizes the principal directions of orthotropy.
The six invariants βi introduced in [27] are defined by
Moreover, it can be shown that
where the scalar functions Ni and Ai and the deviatoric tensors \(\mathbf {B}_{i}^{\prime \prime }\) are defined in Appendix A.
4 Constitutive equations
The Helmholtz free energy per unit mass is specified in the form
and the stress and entropy are determined by
Now, using Eqs. 14, 26, and 28, the constitutive equation for \(\xi ^{\prime }\) in Eq. 13 is given by
Furthermore, the Helmholtz free energy is restricted by the conditions that
so that a zero-stress state is characterized by
5 Modeling a homeostatic state
A homeostatic state of the material is defined by Eq. 3, where Jh,h1,h2, and h3 characterize the homeostatic state. It then follows from Eq. 102 that for a homeostatic state
which indicates that the stress in a homeostatic state can be controlled by the values Jh and hi, and Pi are orthogonal second-order unit tensors.
From the evolution (14), it also follows that in the absence of deformation rate D = 0, the process of homeostasis causes Je to approach its homeostatic value Jh. To ensure that in the absence deformation rate L = 0, the evolution (16) causes \(\mathbf {m}_{i}^{\prime }\) to approach constant homeostatic values (3), the inelastic spin \(\bar {\mathbf {W}}_{g}\) is restricted to vanish in the homeostatic state. Also, \(\bar {\mathbf {W}}_{g}\) is restricted to vanish in a zero-stress state. Then, it is convenient to define the deviatoric tensors \(\bar {\mathbf {H}}_{i}^{\prime \prime }\) by
to express the tensor \(\bar {\mathbf {D}}_{g}^{\prime \prime }\) by
which vanishes for a homeostatic state given by Eq. 3. A more general form of \(\bar {\mathbf {D}}_{g}^{\prime \prime }\) with constitutive coefficients times each of the terms \(\bar {\mathbf {H}}_{i}^{\prime \prime }\) in the series to describe orthotropic homeostasis could be considered but is not pursued here.
Thus, with the help of Eqs. 14, 16, 22, 31, 33, and the restriction that \(\bar {\mathbf {W}}_{p}\) vanishes in a zero stress state, it follows that the zero-stress growth rate Dg, with constant \(\mathbf {m}_{i}^{\prime }\), is given by
6 Application to stresses in arteries
Motivated by the work in [1], use is made of the orthotropic model proposed in this paper to study stress distributions in a human carotid artery. All solutions are consistent with measured values of the cut unloaded state. The HS-Ps model described below specifies the systolic state as the homeostatic state and considers the influence of a nonuniform homeostatic circumferential stress on the stress distributions in other states. Also, the modified Fung model MF-c described below specifies the cut unloaded state as the homeostatic state and considers the influence of a family of homeostatic stress distributions on the stress distributions in other states. In this regard, it is recalled that [34] considered of the influence of different homeostatic stress fields specified for the intact unloaded state of a rabbit thoracic artery.
6.1 Constitutive equation of Fung
Within the context of the purely mechanical theory, the Fung model for biological tissues can be expressed in the form
where QF is a normalized strain energy function of the Lagrangian strain E defined in terms of the total deformation gradient F from a zero-stress reference configuration by
the scalar w controls the nonlinearity caused by the exponential function and c is a constant that has magnitude unity with the dimensions of stress. When this function is used for an incompressible material it describes distortional deformation only. Consequently, it is more convenient to write QF in terms of the distortional Lagrangian strain \(\mathbf {E}^{\prime }\) defined by
where J is the total dilatation and \(\mathbf {F}^{\prime }\) and \(\mathbf {C}^{\prime }\) are unimodular tensors with their determinants equal to unity.
To describe deformations of a cylindrical tube from a zero-stress reference configuration, use is made of the cylindriical polar base vectors
and the unit tensors
to write QF in the form
where cR,cΘ,cZ,cRΘ,cRZ, and cΘZ are material constants. In the limit of small strains, cQF characterizes the small deformation strain energy function for any positive value of w. Furthermore, it can be shown that
with similar expressions for the derivatives of \(\mathbf {E}^{\prime } \cdot \mathbf {N}_{{\varTheta }}\) and \(\mathbf {E}^{\prime } \cdot \mathbf {N}_{z}\). Then, the deviatoric stress \(\mathbf {T}^{\prime \prime }\) in Eq. 28 is given by
For an incompressible material, Je = J = 1 and the pressure p in Eq. 28 is an arbitrary function of position and time.
The material constants for a human carotid artery based on the experiments in [42], as reported in [35] for the Fung model, are recorded in Table 1. Due to the presence of w in the definition of ψ in Eq. 36, the values of the constants in Table 1 are those in [35] multiplied by the numerical value of c0 without units there so, for example, cR in Table 1 is equal to c0cR in [35]. Also the numerical value of w is given by w = 2/c0 without units for c0.
6.2 Purely mechanical response of an incompressible material
For the purely mechanical response of an incompressible material at constant reference temperature 𝜃0, the functions ηi = 1 in Eq. 22 and there is no advantage to using the formulation based on the invariants βi defined in Eq. 25. Moreover, for incompressible response, it is assumed that there is no volumetric growth with
However, distortional homeostasis still causes distortions of the material due to \(\mathbf {D}_{g}^{\prime \prime }\) in Eq. 18. Specifically, the Helmholtz free energy for distortional deformation is specified by
Then, with the help of the evolution (20) for \(m_{ij}^{\prime }\), the constitutive equation for the Cauchy stress is given by Eq. 28 with the deviatoric tensor \(\mathbf {T}^{\prime \prime }\) determined by
where due to the incompressibility constraint, the pressure p is an arbitrary function of position and time.
6.3 Specific constitutive equation
When the (i = 1,2,3) directions of \(\mathbf {m}_{i}^{\prime }\) are identified with the er,e𝜃, and ez material directions, respectively, the Helmholtz free energy
yields identical elastic response to the Fung model in Eqs. 36 and 41 with the material constants
specified in Table 1. Also, the associated deviatoric stress is given by
6.4 Homeostatic state
Using \(\mathbf {m}_{i}^{\prime }\) in Eq. 3, the homeostatic expression for \(\mathbf {T}^{\prime \prime }\) in Eq. 49 is given by
where \(m_{11}^{\prime }, m_{22}^{\prime }\) and \(m_{33}^{\prime }\) are specified by
with hi interpreted as elastic stretches.
6.5 Specific expressions for the homeostatic stress
Figure 1 shows an artery modeled as a circular cylindrical tube in its homeostatic state with inner radius ah outer radius bh and internal pressure Ph. For the deformations of the artery considered in the following examples, the stress T and pressure are given by
where Trr,T𝜃𝜃, and Tzz are the cylindrical polar components of stress, which satisfy the equilibrium equation
and the functional forms of Nr,N𝜃, and Nz are defined in Eq. 40. Moreover, the homeostatic state is characterized by Eqs. 50 and 51.
To study the influence of the stress field in the homeostatic state, it is convenient to consider the following homeostatic stress fields, which satisfy the equilibrium (53)
and the boundary conditions
In these expressions, c1 and c2 are constants to be determined. Also, the pressure p and deviatoric components of stress are given by
The force f𝜃 per unit axial length applied in the e𝜃 direction and the moment mz per unit axial length about the middle surface of artery applied in the ez direction, both on the cross-section with outward normal e𝜃, and the equivalent uniform axial stress σz applied in the ez direction on the cross-section with outward normal ez over the region a ≤ r ≤ b are defined by
The homeostatic stress field (54) is defined so that
when the region of interest is the homeostatic state with a = ah and b = bh. These expressions show that c1 controls the magnitude of the moment mzh and c2 controls the equivalent uniform axial stress σzh.
6.6 Determination of the homeostatic values h 1,h 2, and p h
Given values of the material constants (48) and the constants ci in Eq. 54, the values of h1(r) and h2(r) are determined by solving the equations
where \(T_{rr}^{\prime \prime }\) and \( T_{\theta \theta }^{\prime \prime }(r)\) are specified by Eq. 56 and \(\mathbf {T}^{\prime \prime }(r)\) is specified by Eq. 52. Also, ph is specified by .
with p given by Eq. 56.
6.7 Deformations from a homeostatic state
For the analysis in this paper, it is assumed that deformations from the homeostatic state are elastic with no influence of homeostasis (Γm = Γ = 0). This means that the evolution (16) for elastic changes in \(\mathbf {m}_{i}^{\prime }\) are given by
Next, it is noted that the unimodular relative distortional deformation gradient \(\mathbf {F}_{r}^{\prime }\) in Eq. 4 from the homeostatic state of the artery satisfies the evolution equation and initial condition
It follows that the solution of Eq. 61 is given by
with hi specified by Eq. 3. To be specific, the homeostatic state is specified by the systolic state of the intact artery with applied systolic pressure Ps.
6.7.1 Deformation to the cut unloaded state
The radial r, circumferential 𝜃 and axial z coordinates in the homeostatic state are deformed to \(\tilde {r}\), \(\tilde {\theta }\), and \(\tilde {z}\), respectively, in the cut unloaded state such that
where \(\tilde {\lambda }_{c}\) is axial stretch from the homeostatic systolic state. It therefore follows that the radii of the cut unloaded state satisfy the equation
This assumed deformation field satisfies the incompressibility condition with \(\mathbf {F}_{r}^{\prime }\) given by
which determines the value of \(\mathbf {T}^{\prime \prime }\) by Eq. 50 with 𝜃 replaced by \(\tilde {\theta }\), \(m_{11}^{\prime }, m_{22}^{\prime }\), and \(m_{33}^{\prime }\) given by
and hi determined by the homeostatic systolic state.
6.7.2 Deformation to another intact state
The functional forms for \(\tilde {r}\), \(\tilde {\theta }\) and \(\tilde {z}\) in another intact state are given by
where \(\tilde {\lambda }\) is the axial stretch relative to the homeostatic systolic state. It therefore follows that the inner and outer radii (au,bu) of the intact unloaded state and (ad,bd) of the diastolic state are related by the equations
where \(\tilde {\lambda }_{u}\) and \(\tilde {\lambda }_{d}\) are, respectively, the axial stretches in the intact unloaded and diastolic states relative to the homeostatic systolic state.
Also, this assumed deformation field satisfies the incompressibility condition with \(\mathbf {F}_{r}^{\prime }\) given by
which determines the value of \(\mathbf {T}^{\prime \prime }\) by the expression (50) with \(m_{11}^{\prime }, m_{22}^{\prime }\), and \(m_{33}^{\prime }\) given by
In Eq. 68\((\tilde {a}, \tilde {b}, \tilde {\lambda })\), respectively, take the values \((a_{u}, b_{u}, \tilde {\lambda }_{u})\) for the intact unloaded state and \((a_{d}, b_{d}, \tilde {\lambda }_{d})\) for the diastolic state.
6.8 Equilibrium and boundary conditions
All of the solutions satisfy the equilibrium equation, which can be written in the equivalent forms
and the boundary conditions
where P is the applied internal pressure. Also, the pressure \(p(\tilde {r})\) is determined by integrating (73) to obtain
with x being a dummy variable of integration.
6.9 Boundary conditions for the cut unloaded state
For the cut unloaded state, the internal pressure P = 0 vanishes and integration of Eq. 73 requires
Moreover, using Eq. 57 with P = 0, integration of Eq. 74 automatically yields the result
which indicates that the circumferential force applied to the cut artery vanishes. In addition, the equivalent uniform axial stress in the cut unloaded state must vanish which, with the help of Eq. 57, requires
6.10 Boundary conditions for other intact states
For all intact states, the boundary condition at the inner surface is satisfied by specifying the appropriate value of the pressure P, and the boundary condition (75)2 at the outer boundary imposes the restrictions
for the intact unloaded, diastolic and systolic states, respectively. This boundary condition for the systolic state is automatically satisfied by this solution since the systolic state is assumed to be the homeostatic state specified by the stress field (54), which satisfies the boundary conditions (55).
In addition, it is necessary to specify a condition on the axial load for each state. For the intact unloaded state, the equivalent uniform axial stress vanishes
Motivated by the results in Figs. 9.4-9.6 in [28], the equivalent uniform axial stress in the diastolic state is assumed to be proportional to applied internal pressure so that
where the equivalent uniform axial stress σs in the systolic state is determined by
For this solution, the systolic state is specified by the homeostatic state so that σs is given by Eq. 58
6.11 A modified Fung model
The cut unloaded state is not a physiological state and therefore most likely is also not a homeostatic state, which is specified by values of Jh and hi. However, within the context of the proposed theory, it is possible to study the influence of different residual stress distributions in the cut unloaded state by specifying Jh and hi to be consistent with these residual stresses.
To this end, the modified Fung model, which uses the cut unloaded state as a homeostatic state, is defined by
with the stress field given by Eq. 50 with 𝜃 replaced by \(\tilde {\theta }\) and using the specifications (51). A deformed intact configuration is determined by
It then follows that the inner and outer intact radii (au,bu) of the intact unloaded state, (ad,bd) of the diastolic state and (as,bs) of the systolic state are related by the equations
where λu,λd, and λs are axial stretches from the cut unloaded state. Furthermore, the relative deformation gradient \(\mathbf {F}_{r}^{\prime }\) from the cut unloaded state and the elastic deformation measures are given by
where hi are determined by the homeostatic cut unloaded state and λ is the axial stretch from the cut unloaded state to the intact state in consideration. Also, the stresses are obtained by substituting these values of \(m_{11}^{\prime }, m_{22}^{\prime }\), and \(m_{33}^{\prime }\) into Eq. 50 with 𝜃 replaced by \(\tilde {\theta }\).
6.12 Solution procedure
The examples in this paper discuss solutions based on two models. Both models use the same constitutive (50) with 𝜃 replaced by \(\tilde {\theta }\) and the material constants for a human carotid artery recorded in Table 1. For clarity, the proposed model, which takes the systolic state to be the homeostatic state of the artery, is referred to as the HS-Ps model. The second model is the modified Fung model, which takes the homeostatic state to be the cut unloaded state predicted by the HS-Ps model, and is referred to as the MF-c model.
The solutions of both models satisfy the geometry of the cut unloaded state determined by [35]
Both the HS-Ps and MF-c models are parameterized by two constants
with λs determining the axial stretch of the systolic state relative to the cut unloaded state. For the examples considered in this paper, λs is specified by
and the influence of c1 in Eq. 54 on the homeostatic systolic state is examined by considering the following three values
For each solution, the value of c2 in Eq. 54 is determined by the solution procedure. Specifically, the numerical solutions for the two models discretize the integrals over the radial coordinate using the trapezoidal rule with 21 integration points, including the boundaries. Moreover, this value of λs was specified so that the HS-Ps solution with c1 = 0 causes the uniform systolic values of T𝜃𝜃 and Tzz to be identical, which is consistent with an isotropic state of stress in plane normal to the radial direction.
6.13 Solution procedure for the HS-Ps model
For the HS-Ps model, the value of c1 is specified by one of the values (94), the values of h1,h2, and p are determined for the 21 numerical integration points by solving (59) and (60), with the homeostatic stress given by Eq. 54 with Ph = Ps and the constitutive equation for \(\mathbf {T}^{\prime \prime }\) given by Eqs. 50 and 51. These equations include three unknown variables
which are determined by the Eqs. 65, 77, and 79. Once these values have been determined, the value of σs in Eq. 85 is known, use is made of Eqs. 69, 80, and 83 to determine the values of au,bu, and \(\tilde {\lambda }_{u}\) in the intact unloaded state. Similarly, use is made of Eqs. 70, 81, and 84 to determine the values of ad,bd, and \(\tilde {\lambda }_{d}\) in the diastolic state. For both of these solutions the pressure is determined by solving (76).
6.14 Solution procedure for the MF-c model
For the MF-c model, only one value of c1 = 0 is considered and the values of h1,h2, and p are determined for the 21 numerical integration points by solving scaled version of Eq. 59
with the pressure ph specified by a scaled version of Eq. 60
and α is a scale factor (0 ≤ α ≤ 1). In these equations, \(\mathbf {T}_{c}^{\prime \prime }\) and pc are the HS-Ps solution for the cut unloaded state and \(T_{rr}^{\prime \prime }\) and \(T_{\theta \theta }^{\prime \prime }\) are determined by substituting the values of \(m_{11}^{\prime }, m_{22}^{\prime }\), and \(m_{33}^{\prime }\) in Eq. 90 into the constitutive (50) with 𝜃 replaced by \(\tilde {\theta }\).
Next, the MF-c solutions for the intact unloaded, diastolic, and systolic states are determined by solving (80), (81), (82), (83), (84) and (89), for the eight unknowns
with σs determined by Eq. 85 and λs specified by Eq. 93. Again, the pressure is determined by solving (76).
7 Examples
For the solutions discussed in this section, the diastolic pressure Pd and systolic pressure Ps are specified by the typical values for a healthy human
7.1 The HS-Ps solution
Predictions of the HS-Ps model are shown for the cut unloaded state, the intact unloaded state, the diastolic state and the homeostatic systolic state in Figs. 2, 3, 4, and 5, respectively. These figures examine the influence of the constant c1 in Eq. 54, and the Fung solution (which is determined by the MF-c model with α = 0) is shown for comparison. Table 2 records the value of c2, the equivalent uniform axial stresses σd,σs in the diastolic and systolic states, the values of the inner and outer radii, the stretches predicted by the HS-Ps solution for each of theses states, and the minimum T𝜃𝜃(min) and maximum T𝜃𝜃(max) values of the circumferential stress T𝜃𝜃, with the stretches λu,λs,λd relative to the intact unloaded state defined by
Also, the value ΔT𝜃𝜃(max) in Table 2 represents the increase in the maximum circumferential stress relative to that predicted by the HS-Ps solution for c1 = 0 for each stress state. In addition, the original Fung solution is included in Table 2 for comparison.
From the results presented in these figures and Table 2, it is observed that c1 has a significant effect on the circumferential distribution of stress. The value of c1 = 15 has a smaller influence than c1 = − 15 on the value of c2, which controls the equivalent uniform axial stress. The predicted values of the compressive circumferential stress in Fig. 3b for the state (u) are similar for the HS-Ps model with c1 = 0,− 15 and the Fung model, but the large magnitude of the compressive circumferential stress for c1 = 15 seems unphysical. Also, the large magnitude of the compressive axial stress for c1 = − 15 in Fig. 3c seems unphysical. Furthermore, it is seen from Fig. 5b that the prediction of the circumferential stress using the HS-Ps model with c1 = − 15 is similar to that of the Fung model for the systolic state but the prediction for c1 = 15 has the opposite slope. From Table 2, it is observed that changes in c1 cause minor changes in the geometries of the predicted states. Moreover, it can be seen that the Fung solution predicts significantly larger values of the circumferential stress, especially for the systolic state.
7.2 The MF-c solution
Figures 6, 7, and 8 show results based on the MF-c model. Figures 6 and 7 show, respectively, the stress and stretch distributions for the cut unloaded homeostatic state and Fig. 8 shows the stress distributions predicted for the systolic state. For clarity, the values h1,h2, and h3 are denoted by hrc,h𝜃c, and hzc, respectively, in Fig. 7. Also, the value ΔT𝜃𝜃(max) in Table 3 represents the increase in the maximum circumferential stress relative to that predicted by the HS-Ps solution for α = 1.0 for each stress state. These figures and Table 3 examine the influence of residual stresses in the cut unloaded state on the predictions of the stress distributions in the other states. Specifically, when the scale parameter α = 0, the cut unloaded state has zero stress and the MF-c model predicts the standard Fung extrapolations for the intact unloaded, diastolic, and systolic states. However, when 0 < α ≤ 1, the predictions of the MF-c model show influences of the residual stresses in the cut unloaded state. In particular, it is seen that the results for the intact unloaded, diastolic, and systolic states in Tables 2 of the HS-Ps model for c1 = 0, which specifies the systolic state as the homeostatic state, are identical to those of the MF-c model in Table 3 with α = 1, which specified the cut unloaded state as the homeostatic state. This is because for both choices of the homeostatic state, the deformations from the homeostatic state are elastic. The original Fung solution with α = 0 is included in Table 3 for comparison.
Influence of the scale factor α on the stresses determined by the MF-c solution for the cut unloaded state. The value α = 0 corresponds the the original Fung solution and the value α = 1 calibrates the homeostatic state in the MF-c solution with the stress distribution in the cut unloaded state predicted by the HS-Ps model with c1 = 0
Influence of the scale factor α on the homeostatic stretches determined by the MF-c solution for the cut unloaded state. The value α = 0 corresponds the the original Fung solution and the value α = 1 calibrates the homeostatic state in the MF-c solution with the stress distribution in the cut unloaded state predicted by the HS-Ps model with c1 = 0
Influence of the scale factor α on the stresses determined by the MF-c solution for the systolic state. The value α = 0 corresponds to the original Fung solution and the value α = 1 calibrates the homeostatic state in the MF-c solution with the stress distribution in the cut unloaded state predicted by the HS-Ps model with c1 = 0
The elastic stretches shown in Fig. 7 indicate that this kinematic approximation of the cut unloaded state as a section of a circular tube with uniform axial stretch is not accurate. The predicted axial stretch is small so planes normal to ez would remain nearly planar but the planes normal to e𝜃 would tend to shear relative to e𝜃 if the bounding surfaces were actually traction free. This is qualitatively consistent with the work reported in [31] which observed that additional cuts of the cut unloaded state cause additional deformations of the cut sections due to stress relief.
From the results in Table 3 it can be seen that the predicted geometries of the other states are only slightly affected by the residual stresses in the cut unloaded state. However, small changes in these residual stresses can have a large influence on the predicted circumferential stress. For example, from the results in Table 3 for α = 0.2 it is seen that ΔT𝜃𝜃(max)=-3.47 [kPa] in the cut unloaded state causes ΔT𝜃𝜃(max)= 65.9 [kPa] in the systolic state. This same large sensitivity to the residual stresses in the cut unloaded state is also seen in Figs. 6b and 8b.
8 Conclusions
The nonlinear orthotropic invariants developed in [27] for themoelastic response of soft materials have been generalized to include the inelastic effect of homeostasis. The evolution (14) for the elastic dilatation Je and Eq. 16 for the distortional microstructural vectors \(\mathbf {m}_{i}^{\prime }\) are Eulerian formulations which are insensitive to arbitrariness of the specifications of: a reference configuration, an intermediate configuration, a total deformation measure, and an inelastic deformation measure. This Eulerian formulation is naturally suited to model growth, remodeling and homeostasis of tissues since the evolution equations depend only on state variables which can be determined in the present configuration.
As an example, the stress distributions in a human carotid artery are studied. The inelastic process of homeostasis is used to specify the residual homeostatic stress state and the inelastic process of homeostasis is assumed to be slow relative to physiological times for cardiac cycles from diastolic to systolic pressures and relative to unloading to cut unloaded and intact unloaded states. Consequently, homeostasis during these processes is ignored and the artery is modeled as an incompressible, orthotropic nonlinear hyperelastic material. Due to the incompressibility constraint, the special features of the orthotropic invariants developed in the generalized model are not needed and a Fung-type exponential strain energy function can be used directly.
The HS-Ps model developed here specifies the systolic state as the homeostatic state and deformations from this loaded systolic state are used to analyse the stresses in the cut unloaded, intact unloaded and diastolic states and the influence of different homeostatic stress states is considered. In addition, a modified Fung model MF-c is developed which uses the cut unloaded state with residual stresses as a reference state for predicting stresses in the other intact states and the influence of a family of residual stress states is analyzed.
The results indicate that:
-
The geometry of the cut unloaded state does not uniquely determine the stresses in other intact states
-
The circumferential stress in the artery is particularly sensitive to the distribution of residual stress in the cut unloaded state
-
Extrapolation should be limited to the physiological pressure range to improve the accuracy of the predicted stress distributions
These conclusions are consistent with those in previous work on a rabbit thoracic artery in [1, 34]. In addition, the results in Table 3 indicate that relatively small changes in the residual stresses in the cut unloaded state, which cause significant changes in the circumferential stress in the systolic state, have little effect on the geometries of the diastolic and systolic states. However, measured differences in the in vivo geometries of the diastolic and systolic states of an artery can be used to validate constitutive equations. Also, from Figs. 4b,c and 5b,c, it is interesting to note that the engineering assumption of uniform circumferential and axial stresses of equal values in the systolic homeostatic state cause the circumferential and axial stresses to remain of the same order in the diastolic state.
Improved constitutive equations for modeling of biological tissues should be inspired by in vivo experimental measurements of mechanical response and mechanobiological processes that control homeostasis and the homeostatic state. Also, differences in the mechanical properties, homeostasis and the homeostatic states of the media and adventitia layers of the artery, which were not considered here, need to be investigated.
References
Safadi, M.M., Rubin, M.B.: A new analysis of stresses in arteries based on an Eulerian formulation of growth in tissues. Int. J. Eng. Sci. 118, 40 (2017)
Humphrey, J., Rajagopal, K.: A constrained mixture model for growth and remodeling of soft tissues. Mathematical models and methods in applied sciences 12, 407 (2002)
Ateshian, G.A., Costa, K.D., Azeloglu, E.U., Morrison, B., Hung, C.T.: Continuum modeling of biological tissue growth by cell division, and alteration of intracellular osmolytes and extracellular fixed charge density. J. Biomech. Eng. 131(10), 2009 (2009)
Ambrosi, D., Ateshian, G.A., Arruda, E.M., Cowin, S., Dumais, J., Goriely, A., Holzapfel, G.A., Humphrey, J.D., Kemkemer, R., Kuhl, E., et al.: Perspectives on biological growth and remodeling. J. Mech. Phys. Solids 59, 863 (2011)
Ateshian, G.A., Morrison, IIIB., Holmes, J.W., Hung, C.T.: Mechanics of cell growth. Mech. Res. Commun. 42, 118 (2012)
Sciumè, G., Shelton, S., Gray, W.G., Miller, C.T., Hussain, F., Ferrari, M., Decuzzi, P., Schrefler, B.: A multiphase model for three-dimensional tumor growth. New J. Phys. 15, 015005 (2013)
Green, A.E., Naghdi, P.M.: A dynamical theory of interacting continua. Int. J. Eng. Sci. 3, 231 (1965)
Green, A.E., Naghdi, P.M.: A theory of mixtures. Arch. Ration. Mech. Anal. 24, 243 (1967)
Ateshian, G., Humphrey, J.: Continuum mixture models of biological growth and remodeling: Past successes and future opportunities. Ann. Rev. Biomed. Eng. 14, 97 (2012)
Hsu, F.H.: The influences of mechanical loads on the form of a growing elastic body. J. Biomech. 1, 303 (1968)
Cowin, S., Hegedus, D.: Bone remodeling I: Theory of adaptive elasticity. J. Elast. 6, 313 (1976)
Skalak, R.: Growth as a finite displacement field. In: Proceedings of the IUTAM symposium on finite elasticity, pp 347–355 (1981)
Skalak, R., Dasgupta, G., Moss, M., Otten, E., Dullemeijer, P., Vilmann, H.: Analytical description of growth. J. Theor. Biol. 94, 555 (1982)
Cowin, S.C.: Wolff’s law of trabecular architecture at remodeling equilibrium. J. Biomech. Eng. 108, 83 (1986)
Rodriguez, E.K., Hoger, A., McCulloch, A.D.: Stress-dependent finite growth in soft elastic tissues. J. Biomech. 27, 455 (1994)
Taber, L.A.: Biomechanics of growth, remodeling, and morphogenesis. Appl. Mech. Rev. 48, 487 (1995)
Lubarda, V.A., Hoger, A.: On the mechanics of solids with a growing mass. Int. J. Solids Struct. 39, 4627 (2002)
Volokh, K.: Stresses in growing soft tissues. Acta Biomater. 2(5), 493 (2006)
Kuhl, E.: Growing matter: a review of growth in living systems. J. Mech. Behav. Biomed. Mater. 29, 529 (2014)
Rajagopal, K., Srinivasa, A.: Mechanics of the inelastic behavior of materials—Part 1, Theoretical underpinnings. Int. J. Plast. 14, 945 (1998)
Eckart, C.: The thermodynamics of irreversible processes. IV. The theory of elasticity and anelasticity. Phys. Rev. 73(4), 373 (1948)
Leonov, A.I.: Nonequilibrium thermodynamics and rheology of viscoelastic polymer media. Rheologica acta 15(2), 85 (1976)
Rubin, M.B.: Plasticity theory formulated in terms of physically based microstructural variables - Part I. Theory. Int J. Solids Struct. 31(19), 2615 (1994)
Rubin, M.B., Safadi, M.M., Jabareen, M.: A unified theoretical structure for modeling interstitial growth and muscle activation in soft tissues. Int. J. Eng. Sci. 2015, 2015 (2015)
Rubin, M.B., Jabareen, M.: Physically based invariants for nonlinear elastic orthotropic solids. J. Elast. 90(1), 1 (2008)
Rubin, M.B., Jabareen, M.: Further developments of physically based invariants for nonlinear elastic orthotropic solids. J. Elast. 103(2), 289 (2011)
Rubin, M.B.: A new approach to modeling the thermomechanical, orthotropic, elastic-inelastic response of soft materials. Mech. Soft Mater. 1(1), 3 (2019)
Chuong, C.J., Fung, Y.C.: Residual stress in arteries. In: Frontiers in biomechanics, pp 117–129. Springer (1986)
Fung, Y.C.: What are the residual stresses doing in our blood vessels?. Ann. Biomed. Eng. 19 (3), 237 (1991)
Holzapfel, G.A., Gasser, T.C., Ogden, R.W.: A new constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elast. Phys. Sci. solids 61(1-3), 1 (2000)
Holzapfel, G.A., Sommer, G., Auer, M., Regitnig, P., Ogden, R. W.: Layer-specific 3D residual deformations of human aortas with non-atherosclerotic intimal thickening. Ann. Biomed. Eng. 35(4), 530 (2007)
Holzapfel, G.A., Ogden, R.W.: Constitutive modelling of arteries. Proc. R. Soc. A Math. Phys. Eng. Sci. 466, 1551 (2010)
Humphrey, J., Eberth, J., Dye, W., Gleason, R.: Fundamental role of axial stress in compensatory adaptations by arteries. J. Biomech. 42, 1 (2009)
Safadi, M.M.: An Eulerian theoretical structure for modeling growth, remodeling and morphogenesis of soft tissues. Ph.D. thesis, Technion-Israel Institute of Technology (2016)
Emuna, N., Durban, D., Osovski, S.: Sensitivity of arterial hyperelastic models to uncertainties in stress-free measurements. J. Biomech. Eng. 140(10) (2018)
Flory, P.J.: Thermodynamic relations for high elastic materials. Trans. Faraday Soc. 57, 829 (1961)
Zeinali-Davarani, S., Choi, J., Baek, S.: On parameter estimation for biaxial mechanical behavior of arteries. J. Biomech. 42(4), 524 (2009)
Eddhahak-Ouni, A., Masson, I., Mohand-Kaci, F., Zidi, M.: Influence of random uncertainties of anisotropic fibrous model parameters on arterial pressure estimation. Appl. Math. Mech. 34(5), 529 (2013)
Heusinkveld, M.H., Quicken, S., Holtackers, R.J., Huberts, W., Reesink, K.D., Delhaas, T., Spronck, B.: Uncertainty quantification and sensitivity analysis of an arterial wall mechanics model for evaluation of vascular drug therapies. Biomech. Model. Mechanobiol. 17(1), 55 (2018)
von Hoegen, M., Marino, M., Schröder, J., Wriggers, P.: Direct and inverse identification of constitutive parameters from the structure of soft tissues. Part 2: dispersed arrangement of collagen fibers. Biomech. Model. Mechanobiol. 18, 897 (2019)
Keyes, J.T., Lockwood, D.R., Utzinger, U., Montilla, L.G., Witte, R.S., Geest, J.P.V.: Comparisons of planar and tubular biaxial tensile testing protocols of the same porcine coronary arteries. Ann. Biomed. Eng. 41, 1579 (2013)
Kamenskiy, A.V., Dzenis, Y.A., Kazmi, S.A.J., Pemberton, M.A., Pipinos, I.I., Phillips, N.Y., Herber, K., Woodford, T., Bowen, R.E., Lomneth, C.S., et al.: Biaxial mechanical properties of the human thoracic and abdominal aorta, common carotid, subclavian, renal and common iliac arteries. Biomech. Model. Mechanobiol. 13(6), 1341 (2014)
Acknowledgements
The author would like to acknowledge helpful discussions with MM Safadi and N Emuna.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A: Details of some the mathematical expressions
Appendix A: Details of some the mathematical expressions
1.1 A.1 Details of the functions N i and A i
The scalar functions Ni and Ai in Eq. 26 are defined by
1.2 A.2 Details of the tensors \(\mathbf {B}_{i}^{\prime \prime }\)
The deviatoric tensors \(\mathbf {B}_{i}^{\prime \prime }\) in Eq. 26 are defined by
Rights and permissions
About this article
Cite this article
Rubin, M. Modeling orthotropic elastic-inelastic response of growing tissues with application to stresses in arteries. Mech Soft Mater 3, 5 (2021). https://doi.org/10.1007/s42558-021-00035-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42558-021-00035-w