Abstract
The present work is concerned with selective dissolution of niobium from Gabal El Faliq concentrate sample by using fluoride-free process. The chemical analysis of concentrate sample revealed the presence of high amount of ZrO2, Nb2O5, Re2O3, and HfO2 assaying 33.6, 1.99, 4.8, and 1.2%w/w respectively. Several economic minerals including zircon, samarskite, fergusonite, and xenotime were recorded in the studied sample. About 98% of Nb content was selectively dissolved at the optimum conditions of 1:2 concentrate sample: KOH weight ratio, 2.5 h fusion time, and fusion temperature of 550 °C. Organic solvent extraction process using Aliquat 336S in xylene was effectively used for preparing pure Nb2O5 (98.95%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Gabal (G.) El-Faliq area, southeastern Desert of Egypt, is located between latitude 24° 35′ and 24° 42′ N and longitude 34° 28′ and 34° 35′ E. This area is covering about (250 km2) and it is composed mainly of ophiolitic mélange, gneisses, older, younger granitoids, post granitic dykes, and veins which include pegmatites [42]. The most important rock unite in the studied area is the post granitic dykes and veins. The younger granitoids and post granitic dykes and veins also attracted the interest of many authors because they are typically associated with anomalous concentrations of rare and some economically important elements such as Sn, W, Mo, U, Nb, Ta, REE, Zr, and Rb. It is worthy to mention herein that all the granitoid rocks of G. El Faliq area are cut and crossed by several pegmatite bodies. These bodies are trending (NNE-SSW) and ranging in length from 50 m to several meters. Also, they occur as pockets or lenses (10–20 m in length) at the margin and the core of the gneisses rocks as well as ophiolitic mélange. The present minerals of G. El Faliq area are classified into (a) secondary uranium minerals (uranophane and meta-autunite), (b) niobium–tantalum minerals (columbite, euxenite, and fergusonite), (c) sulphide minerals (pyrite and barite), and (d) accessory minerals (allanite and zircon) [35, 42, 43].
Niobium and tantalum are widely used in today’s technology due to their unique properties; they have been utilized widely in the steel, electronic, and other high-tech industries [7, 22]. Chemical separation or purification of these metals from their ores and concentrates has been studied using different methods. Among these methods, alkali fusion using sodium hydroxide and sodium carbonate (or the potassium salts) were used as leaching agents [6, 7, 12, 50, 53], and KHSO4 roasting [3]. Some niobium–tantalum ore types can also be processed using chlorination, fusion with ammonium fluoride and bifluoride [20, 25, 37], direct acidic dissolution with either H2SO4 [15], or a combination of H2SO4 and HF [23, 27, 39]. Also, most niobium–tantalum ores were dissolved with concentrated HF [14, 20, 40, 41]. Due to high volatility of HF, about 6–7% of it is lost during the decomposition process which is hazardous [10, 11, 21, 22]. Furthermore, a large amount of wastewater containing fluoride is generated that needs to be treated. More importantly, this method is only appropriate for high-grade niobium–tantalum ores [32].
With respect to pure Nb and Ta recovery from their leach liquors, many researchers studied in details the separation and purification of Nb and Ta via liquid–liquid extraction processes. In this regard, several solvents were applied for this purpose. One of the most important and widely industrially applied is tri-n-butyl phosphate (TBP) [4, 5, 20, 36]. Also, many workers as Salles [44], El-Hussaini [14], Agulyansky et al. [2], Nikolaev and Maiorov [34], Yang et al. [52], Toure et al. [47], and Htwe and Lwin [23] used methyl isobutyl ketone (MIBK) for separating pure compounds of Nb and Ta, while Mayorov and Nikolaev [31], Agulyansky et al. [2], and El-Hazek et al. [13] used octanol. In this target, Tertiary amines, e.g., trioctyl amine, Alamine 336, and Aliquat 336S, were effectively applied by Sato et al. [45, 46], Deblonde et al. [8], Mishra et al. [33], and Zhu and Cheng [54]. The following equation illustrates the extraction process of Nb and Ta from oxalate media with tertiary amines [54].
The main purpose of the present work is the selective alkaline dissolution of Nb from Gabal El Faliq concentrate sample depending essentially on the fluoride-free process with breakdown of the associated refractory minerals, e.g., zircon and xenotime. Finally, the preparation of pure Nb2O5 using Aliquat 336S in xylene was carried from the prepared leach solution.
2 Experimental
2.1 Materials
All the utilized chemicals in the experimental work were analytical grade including potassium hydroxide pellets (99%) [Prolabo], sulfuric acid (97%) [Adwic], oxalic acid (99%), ammonium hydroxide (30%) and xylene (99%) [Adwic], nitric acid (69%) [BDH], and Aliquat 336S (99%) [Sigma Aldrich]. On the other hand, the working concentrate sample of Gabal El Faliq area was subjected to both mineralogical and chemical specification and also to processing procedures.
2.2 Chemical and Mineralogical Specification
The sample under study was selected from Gabal El-Faliq, southwestern Desert, Egypt, and was subjected to physical upgrading process using shaking table. The concentrate sample was subjected to major and minor oxide chemical analysis together with analysis of Nb, REE, Zr, and Hf besides U and Th elements using Axios advanced WDXRF-PANalytical (Netherland). In this regard, the analysis of Nb and Zr during all experimental work was carried out by Microwave Plasma Atomic Emission Spectrometer (4200 MP-AES, Agilent Technologies). The REE were estimated using 0.05% Arsenazo III at λ654 nm using Y as a reference ([29]), while U in all performed procedures was measured by oximetric titration against NH4VO3 in the presence of diphenyl amine-4-sulfonic acid sodium salt as an indicator [30]. The scanning electron microscopy (CAM SCAN series 4 ISIS 200 E/X system with pentajet detector) (SEM-EDX) was used for confirming the Nb cake and ICP (720 ICP-OES, Agilent Technologies) was used for identifying of the Nb pure product.
To study the mineralogical composition of the studied concentrate sample, the latter was subjected to X-ray diffraction analysis (PHILIPS PW 223/30) for identification of mineral content. Moreover, heavy mineral separation procedures were applied; for this purpose, the sample was ground to 60 mesh size to liberate the heavy minerals. The ground sample was then washed with distilled H2O for removing slimes; then, it was sieved into the size interval of 0.4, 0.3, 0.2, and 0.1 mm. Heavy liquid separation procedures were applied using bromoform (sp.gr. 2.8) to bring out the heavy fraction. The mineral grains of the same color in the heavy fraction were separately picked up under the binocular microscope. The separated mineral grains were then investigated by X-ray diffraction analysis to identify the mineral crystals structure.
2.3 Optimization of the Fusion Process
Several experiments were performed by mixing constant weight (5 g) of the fine ground concentrate sample with a solid KOH (melting point 360 °C) as the fused reagent in a porcelain crucible. The mixture was then fused at different temperatures ranged from 300 to 650 °C for different periods of times (1–3 h). After cooling, the fused matrix was leached with distilled H2O in S/L ratio of 1/4 for 30 min at room temperature. The spent residue was washed twice with distilled H2O. After filtration using filter paper, Nb in the prepared alkaline solution was analyzed and its leaching efficiency (%) was calculated using the equation:
2.4 Preparation of Impure Niobium Cake
Nb ion species in the prepared alkaline leach liquor was completely precipitated by neutralization process using diluted H2SO4 to pH 8.5. The precipitated Nb cake was then leached with oxalic acid solution for the dissolution of its Nb content with some associated impurities. Aliquat 336S diluted in xylene was used at ambient temperature 35 ± 5 °C for extraction of Nb from its oxalate leach liquor.
2.5 Optimization of Nb Extraction Process
Several relevant effective extraction parameters such as Aliquat 336S concentration, pH value, contact time, and phase volume ratios (O/A) were tested to optimize the loading process of Nb. The loaded Aliquat 336S was stripped with different mineral acids, e.g., H2SO4, HCl, and HNO3 of 0.4 M concentration at A/O ratio of 1/1 and contact time of 5 min to optimize the Nb stripping process. All the experiments were carried out in shaking funnels. Finally, the concentrated Nb strip solution was treated with an ammonia solution to precipitate Nb as Nb-hydroxide cake. The latter was carefully washed with distilled water and then ignited at 650 °C to prepare a pure Nb2O5 product which identified with XRD, while its purity was determined using ICP.
3 Results and Discussion
3.1 Chemical and Mineralogical Characterization of the Sample
Complete chemical composition of Gabl El-Faliq concentrate sample is represented in Table 1. From this table, it is clearly evident that the concentrate is mainly composed of ZrO2 together with SiO2 as well as Fe2O3 and Al2O3 which assaying 33.6, 39, 10, and 5.9%w/w, respectively. In the meantime, there are considerable concentrations of valuable economic oxides, e.g., Re2O3 [sum of all rare earth elements oxides (La2O3, CeO2, etc…)], Nb2O5, Ta2O3, and HfO2 assaying 4.8, 1.99, 0.045, and 1.2%w/w, respectively. Moreover, the present concentrate sample contains remarkable concentrations of radioactive nuclear elements, e.g., U and Th assaying 800 and 340 ppm, respectively.
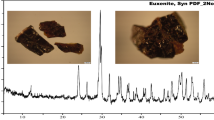
For mineral identification, the bulk concentrate sample was characterized by means of XRD analysis as shown in Fig. 1; it revealed the presence of zircon, silica, and hematite as main components, while the obtained data of XRD analysis of separated mineral grains revealed the presence of valuable minerals in close association with non-notable (gangue) minerals. Accordingly, the studied sample refers to the presence of the following mineral association (Figs. 2, 3, and 4).
3.1.1 Zircon Mineral
Zircon (ZrSiO4) is a common accessory mineral in igneous, metamorphic, and sedimentary rocks. It may form large well-developed crystals in granites and pegmatites. This mineral is extremely resistant to chemical and physical weathering. Zircon was identified by XRD analysis (Fig. 2) in association with xenotime and separated in abundant amounts from pegmatite sample of G. El Faliq. Some crystals show corrosion, zonation, and overgrowth phenomena; they have varying shades of honey color due to the effect of radioactivity.
3.1.2 Nb, Ta, and REE Minerals
Samarskite is common and locally abundant enough that it has the potential to be a valuable source of REE. It is usually associated with fergusonite as Nb, Ta, and REE mineral. Samarskite has dark pitchy to velvety black to dark brown color, irregular massive grains with sub-conchoidal fracture. XRD data confirmed its presence in the separated grains from the pegmatite of G. El Faliq (Fig. 3).
3.1.3 Rare Earth Minerals
In the study area, fergusonite (Y, Er) (Nb, Ta, Ti) O4 and xenotime were recorded as parent REE minerals in pegmatite of G. El Faliq. It is worthy to mention herein that fergusonite is mainly associated with samarskite. Fergusonite-Formanite series of chemical formula (Y, Er) (Nb, Ta) O4-(Y, Er) (Nb, Ta) O4 have brown color with no specific crystal shape but usually metamicted (Fig. 3). Fergusonite end member is the most common mineral. The chemical heterogeneity of fergusonite is represented by the variation in Re2O3 and Nb2O5 [49]. Fergusonite, ideally YNbO4, occurs as an accessory mineral in granitic pegmatites, mostly in rare earth element (REE)-enriched pegmatites [17, 18, 38], and often in combination with one or more Y, Th, Nb, Ta, and Ti oxide accessory minerals [28]. The XRD data confirmed the detection of fergusonite mineral (Fig. 4).
On the other hand, xenotime is an (YPO4) REE-phosphate mineral; Eu, Er, Tr, and Yb, as well as metal elements such as Th and U (all replacing Y), are the expressive secondary components of xenotime. Xenotime is used chiefly as a source of yttrium and HREE (Dy, Yb, Er, and Gd). It is found in pegmatites and other igneous rocks, as a minor accessory mineral. Associated minerals include biotite, quartz, and zircon; xenotime is typically translucent to opaque in shades of brown to brownish yellow and has a variable habit. It may form as minute grains or as extremely thin (less than 10 μ) coatings on detrital zircon grains (Fig. 4).
In this context, oxy–hydroxy minerals are either primary phases (e.g., magnetite) or oxidation products of the mineralized rocks occurring at different ratios in the studied area (e.g., hematite and goethite) in close association with quartz and kaolinite minerals and also with the valuable minerals which refer to hematization process.
3.2 Alkaline Fusion of the Concentrate Sample
The mentioned chemical and mineralogical composition of Gabl El-Faliq concentrate sample reflected the significant high-grade type of mineralization and reflected also the importance of the processing of such concentrate sample for recovering its valuable elements. According to the huge assemblage of mineral constituents in the working concentrate, it was found necessary to apply the alkaline breakdown using KOH due to:
-
*Selective dissolution of Nb away from Zr and REE, where it forms water-soluble K-niobates and insoluble (Zr- REE) hydrous oxides.
-
**Molten KOH forms with zircon mineral (ZrSiO4), a water-soluble K-silicates and K-zirconate. It also forms with xenotime mineral (YPO4), soluble K-phosphate, and acid soluble RE-hydroxide.
All of these can be summarized by the following equations which represented the chemical reactions between the molten KOH and the studied sample [1, 19, 50].
In this context, several relevant factors such as sample/KOH mixed weight ratios, fusion time, and fusion temperature were properly studied in a manner to optimize the Nb dissolution process.
3.2.1 Effect of Concentrate Sample/KOH Weight Ratios
The effect of concentrate sample/KOH weight ratios upon the leaching efficiency of niobium was studied in the range between 1/1 to 1/2.5, while the other fusion conditions were fixed at 2 h as fusion time and fusion temperature of 550 °C. The obtained data (Fig. 5) showed that Nb dissolution efficiency improved obviously with decreasing the concentrate sample/KOH weight ratio, where it increased from 64.5 to 91.9% with decreasing the ratio from 1/1 to 1/2. However, further decreasing in the weight ratio more than 1/2 reflected very slight improvement in Nb dissolution efficiency. This may be attributed to the consumption of KOH in the dissolution of interfering metal ions especially Si4+ during the dissolution of niobium [50]. Thus, 1/2 weight ratio is preferred as the optimum ore concentrate/KOH weight ratio.
3.2.2 Effect of Fusion Temperature
Data in Fig. 6 indicated the effect of changing fusion temperature from 400 to 650 °C upon Nb dissolution efficiency from the working ore material concentrate at ore concentrate/KOH weight ratio of 1/2 and fusion time of 2 h. It was observed that the best Nb dissolution efficiency (95.4%) was achieved at the suitable fusion temperature of 550 °C. Also further increase in fusion temperature up to 650 °C did not show any significant change in Nb dissolution efficiency, where the reaction between Nb2O5 and KOH occurred much more readily than other reactions and was complete at only 550 °C [50].
3.2.3 Effect of Fusion Time
This effect was studied in the time periods ranged between 1 to 3 h, where the other parameters were fixed at fusion temperature of 550 °C and concentrate sample/KOH weight ratio of 1/2. Data represented in Fig. 7 emphasized that Nb dissolution efficiency reached its maximum value (97.9%) at the time period of 2.5 h.
3.3 Preparation of the Alkaline Leach Liquor
In the light of the foregoing mentioned alkaline fusion process of Gabal El-Faliq concentrate sample, it could be concluded that the optimum fusion conditions required for dissolving 97.9% of niobium are as follows:
-
Concentrate sample/KOH weight ratio: 1/2
-
Roasting time: 2.5 h
-
Roasting temperature: 550 °C
On the other hand, it is worthy to mention herein that the other associated metal values, e.g., REE, Zr, U, and Th, were left in the spent residue as insoluble hydrous cake. These determined optimum fusion conditions were used to prepare the pregnant leach liquor for subsequent recovery of niobium. Accordingly, 200 g of the concentrate sample was used to yield 2 L of alkaline leach liquor. The latter was found to assay 1.36 g/L of Nb and 0.03 g/L of Ta with chemical composition as shown in Table 2 and its pH value higher than 12. Yttrium showed small solubility with potassium hydroxide (11.3%) and this may be attributed to the technical viability of the alkaline leaching of yttrium [48, 51]. The prepared alkaline leach liquor was first subjected to silica gel precipitation. This was carried out by adjusting the pH value of the prepared leach liquor via H2SO4 (97%) to be 10. Almost Si4+ ions were precipitated as gelatinous Si(OH)4 with precipitation efficiency of 93%, while small amounts of Nb5+ ions (not exceed 0.95% of total Nb content) were lost. After filtration, the precipitate of gelatinous Si(OH)4 was then ignited at 850 °C for 1 h and identified using XRD (Fig. 8).
3.4 Extraction and Purification of Niobium
The filtrate of the working alkaline solution which contains almost niobium (1.34 g/L) and the remains of silicon (0.8 g/L) was subjected for further acidification to pH 8.5, where almost Nb5+ ions (99.8%) were already precipitated via hydrolysis process [50]. After filtration and washing, the prepared Nb cake was collected and a small amount of the cake was examined using SEM-EDAX, where it revealed that its purity attained only 55.3% with the interfering impurities of Si, Fe, K, and Y as shown in Fig. 9.
Therefore, further purification using solvent extraction Aliquat 336S in xylene was needed. For this purpose, the previously prepared hydrous Nb cake was completely dissolved in 8% oxalic acid solution and stirring for 30 min at 80 °C to form the corresponding stable anion Nb-oxalate complexes [Nb(C2O4)2]− and/or [Nb(C2O4)3]3− at pH 0.3–4.3 [24]. Complete dissolving of Nb, Fe, and small portion of Si was attained, while Y was kept as insoluble Y-oxalate [16]. The prepared Nb-oxalate solution of pH 2.8 was found to assay 3.15 g/L of Nb and 0.39 g/L of Fe as well as 0.25 g/L of Si with leaching efficiencies of 98.9, 84.7, and 14.3% respectively.
3.4.1 Optimization of Loading Step
The maximum loading of [Nb(C2O4)3]3− anion complex from the aqueous phase of Nb-oxalate solution to the organic phase of Aliquat 336S required the investigation of several effective loading parameters as follows:
Effect of Aliquat 336S Concentration
This effect was already studied using the Aliquat 336S concentrations ranged between 2 and 5% in xylene from the prepared oxalate leach liquor of pH 3 at O/A volume ratio of 1/1 and contact time of 10 min at ambient temperature (35 ± 5). After separation, Nb and Fe were analyzed in the raffinate to calculate their extraction efficiencies. The obtained data conducted in triplicate with low standard deviation of 1 ± 0.4% (Fig. 10) revealed that Nb and the interfering Fe loading efficiencies were progressively improved from 69.2 and 48.4% to 92.9 and 91.8%, respectively, as the Aliquat 336S concentration increased from 2–5%, where further increase to 6% showed a limit increase in the Nb loading efficiency to 94.1%, while that of the interfering Fe increased to 96.5%. Indeed, Aliquat 336S concentration of 5% was very suitable to apply for Nb extraction process. Thus, the optimum conc. of Aliquat 336S was 5%.
Effect of pH Value
Data given in Fig. 11 represented the effect of increasing pH values of the prepared oxalate solution from pH 3 to pH 5.5 upon Nb and Fe loading efficiencies using 5% (v/v) Aliquat 336S at O/A volume ratio of 1/1 and contact time of 10 min. It was noticed that Nb loading efficiency increased up to 95.3% at the pH 3.5 and decreased gradually up to 61.3% at pH 5.5. This may be explained that the present soluble anion Nb complex [Nb(C2O4)3]3− would be hydrolyzed to [Nb(C2O4)2(OH)2]3− precipitated, at higher pH values more than pH of 4.5 [54]. In an opposite manner, the loading efficiency of Fe increased gradually with increasing the solution pH values. So, the pH value 3.5 was considered the optimum value due to the highest extraction efficiency of Nb.
Effect of Contact Time
The effect of contact time (shaking time) upon the extraction efficiencies of Nb and Fe from the oxalate leach liquor adjusted to pH of 3.5 was investigated by contacting 5% (v/v) Aliquat 336S at different contact time ranging from 5 to 10 min at O/A ratio 1/1. The obtained results (Fig. 12) emphasized that the loading efficiency of Nb achieved its maximum value (97.9%) at the contact time of 10 min [26]. It was also noticed that further increase in the contact time to 12 min had an opposite effect. With respect to Fe loading, efficiency was found inversely proportional to the contact time.
Effect of Organic/Aqueous Volume Ratio and Constriction of Macabe-Thiele Diagram
This effect was studied at different O/A ratios 3/1,…1/1,…. 1/3 using 5% Aliquat 336S at a contact time of 10 min and pH of 3.5. From the obtained data (Table 3 and Fig. 13), it was observed that the Nb loading efficiency at O/A ratios 2/1 (98.7%) and 1/3 (99.8%) was higher than O/A of 1/1 (97.9%). But these ratios were not applied because they yielded lower Nb concentrations in the organic phase. On the other hand, the Nb loading efficiencies at O/A ratios 1/2 and1/3 are 80.1 and 69.7%, respectively, which were lower than that of 1/1 (97.9%). The same manner was already found for the loading efficiency of Fe; it was 98.9% and 97.5% at O/A ratios of 2/1 and 3/1 and lower to be 65.8% and 48.3% at the ratios 1/2 and 1/3 (Table 4 and Fig. 14). Indeed, the suitable O/A ratio applied for the best Nb and Fe loading efficiencies was 1/1.
From the above study, the co-extraction of both of Nb and Fe was already attained with achieved loading efficiencies of 97.9 and 93.8%, respectively, from the prepared Nb-oxalate solution adjusted to pH 3.5 using 5% Aliquat 336S at O/A ratio of 1/1 and contact time of 10 min. The loaded organic solvent was then directed to the stripping process to bring out the loaded Nb and Fe to the aqueous solution until regeneration of the utilized organic solvent to be reused.
3.4.2 Optimization of Stripping Process
The stripping process was conducted to attain the maximum Nb stripping efficiency from one hand and for its separation from the co-extracted Fe from the other hand. For this purpose, the following stripping parameters would be investigated.
Effect of Stripping Reagent Types
After separation and determination of both Nb and Fe in the strip solution, the obtained data (Fig. 15) showed that 0.4 M H2SO4 acid gave very low Nb stripping efficiency (4.3%) with high Fe stripping efficiency (55.5%) from the loaded Aliquat 336S. Therefore, H2SO4 acid would be firstly utilized and its effective stripping factors would be studied for selective stripping of Fe ([9]), while both HCl and HNO3 acids yielded co-stripped Nb and Fe strip solution.
Effect of H2SO4 Acid Concentrations
This effect was conducted via contacting the loaded solvent with H2SO4 acid solution in different concentrations ranging from 0.2 to 1 M at A/O volume ratio of 1/1 and contact time of 5 min. A remarkable improvement of Fe stripping efficiency from 51.4 to 87.8% was attained with increasing H2SO4 concentration from 0.2 to 0.7 M with maximum Nb stripping efficiency not exceeding 8.2% as presented in Fig. 16, while the further increase in the strip solution concentration to 1 M showed improvement in both Fe stripping efficiency to 91.5% and Nb stripping efficiency to 20.3%. Indeed, 0.7 M H2SO4 acid was already chosen to remove almost of Fe from the loaded solvent.
Effect of Contact Time
Figure 17 represents the influence of varying contact time periods from 3 to 9 min when 0.7 M H2SO4 solution was contacted with the loaded Aliquat 336S at A/O ratio of 1/1. It was actually noticed that not less than 90.5% of the co-extracted Fe was removed with the smallest Nb efficiency (8.2%) which was attained at a contact time of 5 min.
It was concluded from the above study that 0.7 M H2SO4 acid concentration should be firstly applied for removing 90.3% of Fe with a minimum amount of Nb (not exceeding 8.2%) at a contact time of 5 min and A/O ratio of 1/1. Another stripping process would be subsequently applied using HNO3 solution for Nb stripping. Different Nb effective stripping factors should be studied as follows.
Effect of HNO3 Acid Concentration
The effect of HNO3 acid concentration upon Nb stripping efficiency was studied in the range from 0.5 to 1.2 M with shaking time for 5 min at A/O ratio 1/1. Data (Fig. 18) reflected the great improvement of Nb stripping efficiency from 87.2 to 95.4% when HNO3 acid concentration increased from 0.5 to 0.8 M, while the further increase in strip solution concentration more than 0.8 M had very limited improvement in stripping efficiency which increased to 96.2%.
Effect of Stripping Time
The effect of stripping time upon Nb stripping efficiency was studied in the range from 5 to 10 min using 0.8 M HNO3 solution at A/O ratio of 1/1. The obtained results (Fig. 19) revealed that the stripping time 6 min is sufficient to regenerate not less than 99.1% of the loaded Nb.
Effect of Aqueous/Organic (A/O) Ratio and Constriction of Macabe-Thiele Diagram
The equilibrium state of Nb stripping process from the loaded Aliquat 336S was investigated using 0.8 M HNO3 solution at stripping time of 6 min via applying different A/O ratios of 2/1, 1/1, 21/2, and 1/3. Table 5 and Fig. 20 clearly reflected that almost 100% of the loaded Nb was stripped at A/O ratio of 2/1 compared with 99.1%, 63.9%, and 52.6% at A/O ratios of 1/1, 1/2, and 1/3, respectively. Indeed, the ideal applied A/O volume ratio was 1/1 which is also beneficial for Nb stripping with efficiency of 99.1%.
3.4.3 Preparation of Nb Product
From the previous data, it was found that the maximum value 99.1% of Nb stripping efficiency was achieved using 0.8 M HNO3 solution at stripping time of 6 min and applied A/O ratio of 1/1. The strip nitrate solution rich in Nb was treated with 30% NH4OH solution where Nb was completely precipitated at pH 7.5 [13]. After filtration and washing, the obtained Nb hydroxide cake was ignited at 650 °C for 1 h. A small portion of the product was confirmed by XRD analysis (Fig. 21) and chemically analyzed for its purity. The final Nb2O5 product was achieved with global recovery of 86% and purity of 98.95% with low concentration of associated impurities, e.g., 0.9% SiO2, 0.05% Fe2O3, and 0.1% TiO2.
4 Conclusion
An alkaline fusion process using KOH followed by water leaching was applied for a selective dissolving of Nb as water-soluble potassium niobates from Gabal El-Faliq ore material concentrate, where 97.9% of the total Nb content was already dissolved at 1/2 concentrate sample/KOH weight ratio and fusion temperature of 550 °C for a fusion time of 2.5 h. The prepared alkaline solution of pH higher than 12 was firstly neutralized at pH of 10 to remove almost of Si4+ as Si(OH)4 gel, while complete precipitation of Nb and other associated impurities, e.g., Fe, Ti, and Y, were attained by further neutralization at pH 8.5.
The precipitated Nb-hydrous cake assaying 55.5% of Nb was dissolved in oxalic acid solution and subjected to Nb purification process using Aliquat 336S in xylene. The co-extracted Nb and Fe with loading efficiencies 97.9% and 93.8%, respectively, were attained using 5% Aliquat 336S concentration at pH 3.5 and shaking time of 10 min at O/A ratio of 1/1. Two subsequent stripping processes were performed using both 0.7 M H2SO4 for removing 90% of Fe away from the Aliquat 336S and 0.8 M HNO3 for stripping 97.9% of Nb. Finally, this process was successfully applied to prepare Nb2O5 product with purity of 98.95% and global recovery of 86% as summarized in the proposal flowsheet (Fig. 22).
References
Abdelkader AM, Daher A, El-Kashef E (2008) Novel decomposition method for zircon. J Alloys Compd 460:577–580
Agulyansky A, Agulyansky L, Travkin VF (2004) Liquid-liquid extraction of tantalum with 2-octanol. Chem Eng Process 43:1231–1237
Amer TE, El-Assay IE, Rezk AA, El-Kammar AA, El-Manawy AW, Abu Khoziem HA (2014) Geometallurgy and processing of North Ras Mohamed ore deposits, South Sinai, Egypt. Int J Miner Process 129:12–21
Bhattacharyya SN, Ganguly B (1984) Solvent extraction separation of niobium and tantalum. Solvent Extr Ion Exch 2(4-5):699–740
Campderrós ME, Marchese J (2000) Facilitated transport of niobium (V) and tantalum (V) with supported liquid membrane using TBP as carrier. J Membr Sci 164:205–210
Deblonde GJ-P, Chagnes A, Bélair S, Cote G (2015) Solubility of niobium(V) and tantalum(V) under mild alkaline conditions. Hydrometallurgy 156:99–106
Deblonde GJ-P, Weigel V, Bellier Q, Houdard R, Delvallée F, Bélair S, Beltrami D (2016a) Selective recovery of niobium and tantalum from low-grade concentrates using a simple and fluoride-free process. Sep Purif Technol 162:180–187
Deblonde GJ-P, Chagnes A, Roux M-A, Weigelb V, Cote G (2016b) Extraction of Nb(V) by quaternary ammonium-based solvents: toward organic hexaniobate systems. Dalton Trans 45:19351–19360
Deblonde, G., Roux M, Weigel, V., Changes, A, Cote, G, 2017. Hydrometallurgical method for separating and purifying tantalum and niobium, w o 2017/085404 Al.
Deblonde GJ-P, Bengio D, Beltrami D, Belair S, Cote G, Chagnes A (2019a) A fluoride-free liquid-liquid extraction process for the recovery and separation of niobium and tantalum from alkaline leach solutions. Sep Purif Technol 215:634–643
Deblonde GJ-P, Bengio D, Beltrami D, Belair S, Cote G, Chagnes A (2019b) Niobium and tantalum processing in oxalic-nitric media: Nb2O5·nH2O and Ta2O5·nH2O precipitation with oxalates and nitrates recycling. Sep Purif Technol 226:209–217
Eckert, J., 1995. Hydrometallurgical processing of Ta/Nb compounds. Present state of the art. Proceedings of international symposium on tantalum and niobium, Germany. Tantalum–Niobium International Study Center, Belgium, 51-64.
El-Hazek MN, Amer TE, Abu El-Azm MG, Issa RM, El-Hady SM (2012) Liquid–liquid extraction of tantalum and niobium by octanol from sulfate leach liquor. Arab J Chem 5:31–39
El-Hussaini OM (2001) Extraction of niobium and tantalum from nitrate and sulfate media by using MIBK. Miner Process Extr Metall Rev 22:633–650
El-Hussaini OM, Mahdy MA 2002. Sulfuric acid leaching of Kab Amiri niobium-tantalum bearing minerals, Central Eastern Desert, Egypt, Hydrometallurgy 64(3), 219-229
El-Hussaini, OM, Saalman H, Mahmoud M, 2014. Extraction of yttrium from ferruginous sandstone, SouthWeastern Sinai, TMS (The Minerals, Metals & Materials Society).
Ercit TS (2005) Identification and alteration trends of granitic-pegmatite-hosted (Y, REE, U, Th)–(Nb, Ta, Ti) oxide minerals: a statistical approach. Can Mineral 43:1291–1303
Ervanne H (2004) Uranium oxidation states in allanite, fergusonite and monazite of pegmatites from Finland. Neues Jb Mineral Monat 7:289–301
Gomes YF, Ribeiro S, Costa CB, Motta FV (2018) Optimization of the process of obtaining Re2O3 from xenotime using statistical design. Ceramica 64:79–85
Gupta CK, Suri AK (1994) Extractive metallurgy of niobium. CRC Press, Boca Raton, p 105
He C-y, Liu Z-m, Zhang H-j (1998a) Treatment of fluorine-containing waste gas from hydrometallurgy of tantalum and niobium ore. Nonferrous Metals 50(4):141–142 (in Chinese)
He J, Zhang Z, Xu Z (1998b) Hydrometallurgical extraction of tantalum and niobium in China. Tantalum–Niobium Int Study Centre Bull 93:1–6
Htwe HH, Lwin KT (2008) Study on extraction of niobium oxide from columbite–tantalite concentrate. World Acad Sci Eng Technol 46:133–135
Jeng J-M, Waches IS (1991) Niobium oxide solution chemistry. J Raman Spectrosc 22:83–89
Kabangu MJ, Crouse PL (2012) Separation of niobium and tantalum from Mozambican tantalite by ammonium bifluoride digestion and octanol solvent extraction. Hydrometallurgy 129-130:151–155
Karve MA, Khopkar SM (1993) Liquid-liquid extraction of niobium(V) in the presence of other metals with high molecular mass amines and ascorbic acid. Talanta 40(6):913–917
Krismer B, Hoppe A, 1984. Process for recovering niobium and/or tantalum compounds from such residues further containing complexes of uranium, thorium, titanium and/or rare earth metals. U.S. Patent 4,446,116.
Lumpkin GR (1998) Rare-element mineralogy and internal evolution of the Rutherford pegmatite, Amelia County, Virginia: a classic locality revisited. Can Mineral 36:339–353
Marczenko Z (2000) Spectrophotometric determination of elements. John Wiley and Sons, Harwood
Mathew KJ, Burger S, Ogt SV, Mason PM Narayanan UI, 2009. Uranium assay determination using Davies and Gray titration. Processing the eighth international conference on methods and applications of radioanalytical chemistry (Marc Viii) Kaailua-Kona, Hawaii, P.5M.J.
Mayorov VG, Nikolaev AI (2002) Tantalum (V) and niobium (V) extraction by octanol. Hydrometallurgy 66:77–83
Miller GL (1959) Tantalum and niobium. In Butterworths Scientific Publications, London, pp 17–66
Mishra PK, Chakravotty V, Dash KC, Das NR, Bhattacharyya SN (1992) Extraction and separation of Zr, Nb and Hf by Aliquat 336 and its mixtures with TOPO from acidic thiocyanate media. J Radioanal Nucl Chem 162(2):289–298
Nikolaev AI, Maiorov VG (2007) New approaches to niobium and tantalum extraction technology. Dokl Chem 415(1):167–169
Noweir AM, Sewifi BM, Abu El Ela AM (1990) Geology, petrography, geochemistry and petrogenesis of the Egyptian younger granites. Qatar Univ Sci Bull 10:363–393
Okada T (2001) Manufacturing of special niobium oxides for optical and ceramic applications. International Symposium on Niobium 2001, Orland
Purcell W, Potgieter H, Nete M, Mnculwane H (2018) Possible methodology for niobium, tantalum and scandium separation in ferrocolumbite. Miner Eng 119:57–66
Reto G, Terry W, Richard W, Katja R (2009) Metamict fergusonite (Y) in a spessartine-bearing granitic pegmatite from Adamello, Italy. Chem Geol 261:333–345
Ritcey GN (2006) Niobium (columbium) and tantalum. In: Solvent extraction—principles and applications to process metallurgy, 2nd edn. G.M. Ritcey and Associates Incorporated, Ottawa, p 2
Rodriguez M, Quiroga O, Del M, Ruiz C (2007) Kinetic study of ferrocolumbite dissolution in hydrofluoric acid medium. Hydrometallurgy 85(2-4):87–94
Rodriguez MH, Rosales GD, Pinna EG, Suarez DS (2015) Extraction of niobium and tantalum from ferrocolumbite by hydrofluoric acid pressure leaching. Hydrometallurgy 156:17–20
Saleh GM, Salem IA, Darwish ME, Mostafe DA (2014) Gabal El Faliq granitoid rocks of the southeastern Desert, Egypt: geochemicalconstraints, mineralization and spectrometric prospecting. World J Earth Planet Sci 1(1):1–12
Saleh GM, Hassan SF, Mohmoud MAM, Rashed MA (2016) Geology and radiometric measurments of granitiod rocks of Gabal El Faliq and Gabal El Harami, Southeastern Desert, Egypt. Arab J Earth Sci 3(1):57–78
Salles JJC (2000) Production of niobium and tantalum from the Pitinga hard rock tin mine. TIC Bulletin 101:4–7
Sato D, Murakami M, Ooe K, Haba H, Goto S, Kudo H, 2014. Extraction behavior of Nb and Ta with Aliquat 336 in HF solutions. Radiochem Nuclear Chem 47.
Sato D, Murakami M, Ooe K, Motoyama R, Haba H, Komori Y, Toyoshima A, Mitsukai A, Kikunaga H, Goto S, Kudo H, 2016. Solid-liquid extraction of Nb and Ta with Aliquat 336 resin from HF solutions for chemical experiment of element 105, Db. Radiochem Nuclear Chem 49.
Toure M, Arrachart G, Duhamet J, Pellet-Rostaing S (2018) Tantalum and niobium selective extraction by alkyl-acetophenone. Metals. 8(9):1–12
Vasconcellos ME, Rocha SMR, Pedreira WR, Queiroz CA, Abr˜ao A (2006) Enrichment of yttrium from rare earth concentrate by ammonium carbonate leaching and peroxide precipitation. J Alloys Compd 418:200–203
Wang RC, Wang DZ, Zhao GT, Lu JJ, Chen XM, Xu SJ (2001) Accessory mineral record of magma-fluid interaction in the Laoshan I- and A-type granitic complex, Eastern China. Phys. Chem. Earrh (A) 26(9-10):835–849
Wang X, Zheng S, Xu H, Zhang Y (2009) Leaching of niobium and tantalum from a low-grade ore using a KOH roast–water leach system. Hydrometallurgy 98:219–223
Wang Z, Dai S, Zou J, French D, Graham IT (2019) Rare earth elements and yttrium in coal ash from the Luzhou power plant in Sichuan, southwest China: concentration, characterization and optimized extraction. Int J Coal Geol 203:1–14
Yang XL, Wang XH, Wei C, Zheng SL, Sun Q, Wang D (2013) Extraction kinetics of tantalum by MIBK from pulp using Lewis cell. Hydrometallurgy 131:34–39
Zhou HM, Yi DQ, Zhang Y, Zheng SL (2005) The dissolution behavior of Nb2O5, Ta2O5 and their mixture in highly concentrated KOH solution. Hydrometallurgy 80(1-2):126–131
Zhu Z, Cheng CY (2011) Solvent extraction technology for the separation and purification of niobium and tantalum: a review. Hydrometallurgy 107:1–12
Acknowledgments
The authors wish to thank Dr. Soliman A. Abdallah for the provision of Gabal El-Faliq concentrate sample and Dr. Hanaa A. Abu Khoziem for helping us in experimental work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel Wahab, G.M., Abdellah, W.M., Yousif, A.M. et al. Preparation of Pure Nb2O5 from Gabal El-Faliq Pegmatite, South Eastern Desert, Egypt. Mining, Metallurgy & Exploration 39, 833–846 (2022). https://doi.org/10.1007/s42461-019-00136-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-019-00136-1