Abstract
ataclastic rocks of Abu Rusheid area, Southeastern Desert of Egypt, were physically upgraded to obtain with producing a concentrate assaying 29.4% ZrO2, 1.55% Nb2O5, 3.13% RE2O3, 0.9% U3O8, and 0.66% ThO2. The corresponding minerals of these elements in the sample studied include zircon, columbite, samarskite, and uranothorite. Cassiterite is considered as an accessory mineral. The novelty of the suggested procedure is based on selective separation of niobium first from the aqueous liquor of the hydrous oxide cake obtained after alkali breakdown of the concentrate, leaving U, Th, Zr, and REEs in the residue. About 97% of niobium was selectively dissolved under the optimum conditions: weight ratio of concentrate sample to NaOH/KOH mixture 1 : 1.5, fusion time 2 h, and fusion temperature 500°C. This was followed by the recovery of Th, Zr, U, and REEs from the residue. The residue was treated with hydrochloric acid to achieve 98% leaching efficiency. From the two adequately obtained leach liquors, significant products have been prepared.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The Abu Rusheid area situated in the South Eastern Desert of Egypt is a distinctive occurrence of economically important rare-metal mineralization. The area is reached through a Marsa Alam–Baranes asphaltic road (160 km long) by a desert track 55 km long beginning from the asphaltic road (at km 52 from Marsa Alam) leading to W. El Gemal and W. Abu Rusheid [1]. Several mineral deposits have been found in this area, which can be grouped into two groups as follows: (1) ore mineral group including pyrite, brochanite, pyrolusite, Mn-franklinite, cassiterite, kasolite, thorite, thorianite, columbite-tantalite, and zircon, and (2) associated gangue mineral group containing fluorite, mica, garnet, amazonite, tourmaline, goethite, hematite, magnetite, jarosite, and thuringite [2, 3].
Thorium has immense potential for nuclear energy, because 232Th can be convertible to human-made fissile isotope 233U by absorbing slow neutrons [4]. Besides using Th as a nuclear energy resource, it has extensive use in various areas, including aeronautics, aerospace, optics, radio, metallurgy, chemical and nuclear industries, materials science [5, 6], and nuclear medicine. Thorium is found in several minerals, including thorianite (up to 90% ThO2), monazite (1–15% ThO2), and thorite (up to 80% ThO2). These minerals are less abundant compared to uranium, because thorium does not form secondary minerals. They occur principally in granites and pegmatites [7]. The leaching or dissolution of thorium is carried out through conventional or nonconventional techniques depending on the type of thorium minerals, ore grade, economy of the process, and environmental impact. Many techniques have been used for Th separation, including solvent extraction [8], precipitation [9], adsorption [10], membrane treatment [11], electrodeposition [12], and molecular imprinting [13].
Uranium has different applications including nuclear power plants, manufacture of nuclear weapons, electron microscopy, and glass and pottery glaze. It is present in ppm levels in the Earth’s crust, rocks, and soils; its content ranges from thousands of ppm to a few percents in minerals/rocks used in the mining industry [14–16]. In recent years, treatment of low-grade and complex refractory ore, of old abandoned deposits and other secondary resources related to mining activities in the past, and of the mine environments attracted increasing attention [17, 18]. Many leaching procedures have been developed [19, 20] to assess the related risks.
Zirconium and its compounds received increasing attention in recent years due to new fields of application. Most of zirconium is used as compounds for the ceramic industry, refractories, glazes, enamels, foundry mold, abrasive grits, and electrical ceramics. Zirconium metal is used almost entirely for cladding uranium fuel elements for nuclear power plants [21, 22]. Decomposition of zircon is a complicated process due to its high stability. Various decomposition methods were investigated including chemical breakdown using sodium hydroxide [23], sodium carbonate (Na2CO3) [24], calcium carbonate (CaCO3), [25] and potassium fluorosilicate [26], heat treatment in an arc furnace at 2000°C [23], or mechanochemical means.
Niobium and tantalum have a wide range of applications, including manufacture of alloys and superalloys used in nuclear and aerospace industries, production of microalloys used in the automotive, marine, mining, and transport industries, production of high-strength steels [27], and use in electronics for the construction of capacitors and conductors [28]. Chemical separation or purification of these metals from their ores and concentrates has been studied using different methods. The industrial methodology most studied and used for the treatment of materials containing niobium and tantalum is leaching with concentrated HF [29, 30], but large amount of hazardous wastewater containing fluoride is generated in the process [31, 32]. Alkali fusion using sodium hydroxide (or potassium salts) [33, 34] or ammonium fluoride and bifluoride [35] and KHSO4 roasting [36] were also used.
Rare earth elements (REEs), namely, scandium, yttrium, and lanthanides, play a critical role in the global economy due to their numerous applications [37]. REEs and their alloys are used in many ways, in particular, in memory storage devices, rechargeable batteries, mobile phones, catalytic converters, magnets, fluorescent bulbs, etc. [38]. About 95% of the REEs occur in only three minerals: monazite, bastnäsite, and xenotime. Several methods have been studied and industrially applied for the breakdown of the principal REE minerals; they require relatively severe conditions. Accordingly, economic treatment of these minerals should be achieved with high-grade concentrates obtained by prior physical beneficiation methods (gravimetric, magnetic, electrostatic, flotation, etc.). The solubilization of mixed rare earth oxides by acid solutions (HNO3, HCl, and H2SO4) has been studied recently [39].
The purpose of this work was to investigate the subsequent separation of different nuclear elements (U, Th, Nb, Zr, and REEs) from Abu Rusheid concentrate sample by breakdown of the refractory minerals present together in this sample. The method avoided the use of highly fluorine toxic media in which a mixture of NaOH and KOH alkaline fusion was applied. Moreover, preparation of Nb cake from the alkaline liquor while the residue was treated with hydrochloric acid to obtain Th, REE, Zr, and U products.
EXPERIMENTAL
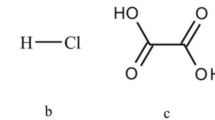
Materials. All the chemicals used in the experimental work were analytical grade, including potassium hydroxide pellets (Prolabo, 99%), sodium hydroxide pellets (Prolabo, 99%), sulfuric acid (Adwic, 97%), oxalic acid (Adwic, 99%), hydrochloric acid (Fluka, 37%), sodium sulfide (Adwic, 99%), and hydrogen peroxide (Merck, 30%). The working concentrate sample from Abu Rusheid was examined to determine the mineralogical and chemical composition before being processed.
Chemical and mineralogical specification. The study sample was collected from Abu Rusheid in South Eastern Desert, Egypt, and was put through a physical upgrade using a shaking table. A representative portion of the Abu Rusheid sample before and after concentration process was prepared by quartering and ground to a size of –200 mesh before being subjected to complete chemical analysis. This analysis included determination of the major and minor oxides, Nb, REEs, and Zr along with U and Th using X-ray fluorescence analysis (Philips X’Unique II spectrometer, Rh target tube). Analysis for Nb and Zr was performed by ICP (720 ICP-OES, Agilent Technologies). The U content was determined by oxidimetric titration against NH4VO3 in the presence of sodium diphenylamine-4-sulfonate as an indicator [40]. 0.05% Arsenazo III solution was used to quantify Th and REEs (using Y as a reference) at 655 and 654 nm, respectively [41]. The products were examined by scanning electron microscopy (CAM SCAN series 4 ISIS 200 E/X system with pentajet detector, SEM-EDX).
To study the mineralogical composition of the concentrate sample studied, heavy mineral separation procedures were applied. For this purpose, the sample was ground to –60 mesh size to liberate the heavy minerals. The ground sample was then washed with distilled water, and the slimes were removed by repeated decantation. The dry fractions were then sieved into the size intervals 0.4, 0.315, 0.2, and 0.1 mm. A heavy liquid separation procedure using bromoform (d = 2.8) was used for separating the heavy fraction. Under a binocular microscope, heavy mineral grains were selected from each of the produced heavy fractions. The separated mineral grains were then examined with an environmental scanning electron microscope (SEM) to identify the mineral crystal structure.
Leaching procedure. Two leaching methods were performed consecutively. First, fusion using NaOH (mp 323°C), KOH (mp 360°C), and NaOH/KOH mixture was performed to dissolve Nb and some of the associated elements, leaving behind REEs, Zr, Th and U in the residue. Then, hydrochloric acid leaching of the residue was performed to dissolve the unleached metal values.
Optimization of fusion process. A constant amount (5 g) of the finely ground concentrate sample was fused with a NaOH/KOH mixture (melting point of the eutectic 118°C) [42] in a porcelain crucible. The sample to alkali weight ratio, fusion temperature, and fusion time were varied. After cooling, the fused matrix was leached with distilled H2O (S/L = 1/5) for 1 h at room temperature to dissolve its Nb content.
To recover Nb(V) ions, after optimizing the fusion parameters, a 100-g portion of the concentrate sample was used to prepare 1 L of the alkaline leach liquor. Nb(V) ions in the liquor were completely precipitated by neutralization with dilute H2SO4 to pH 8.0.
Acid agitation leaching. Leaching with hydrochloric acid was used to dissolve the unleached elements Zr, U, Th, and REEs from the residue obtained after alkaline fusion process. The obtained hydrous oxide cake was washed and dried at 100°C before being subjected to agitation leaching with 7 M HCl at 90°C for approximately 1 h [43]. 100 g of the residue was taken to prepare 1 L of the chloride solution used for the extraction of Th, Zr, REEs, and U. The purity of the final precipitate cake of Th, Zr, REEs, and U was evaluated using SEM-EDX analysis.
RESULTS AND DISCUSSION
Chemical and Mineralogical Characterization of the Sample
The chemical nature of the concentrate sample was studied to determine the type of treatment employed. The results are given in Table 1. Low level of the loss on ignition (about 0.5%) reflects its low organic matter content. The sample was rich in ZrO2, SiO2, Fe2O3, and Al2O3, assaying 29.4, 36.72, 14.41, and 6.25 wt %, respectively. Also, there were significant concentrations of valuable oxides, e.g., ΣRE2O3 (total rare earth elements oxides + Y2O3), Nb2O5, Ta2O3, HfO2, and ThO2, assaying 3.13, 1.55, 0.036, 1.03, and 0.656 wt %, respectively. The U content was 0.902 wt %.
Mineralogical analysis was performed to confirm the grade of the sample under study, on the one hand, and determine whether the sample contains high concentrations of refractory minerals and/or deleterious gangues, on the other hand. For mineral identification, separated mineral grains were characterized by SEM-EDX (Fig. 1). The analysis revealed the presence of valuable minerals in close association with gangue minerals. Namely, monazite [(Ce,La,Nd,Th)(PO4,SiO4)], zircon (ZrSiO4), samarskite (Y,Er,Ce,U,Fe,Th)(Nb,Ta,Ti)2O6, cassiterite (SnO2), columbite [(Fe,Mn)(Nb,Ta)2O6], and uranothorite [(Th,U)SiO4] were detected. In fact, the presence of these minerals together reflects the refractory nature of the concentrate sample during processing.
Alkaline Fusion of the Concentrate Sample
Due to the refractory nature of the Abu Rusheid concentrate sample under study, it was found necessary to apply both the alkaline breakdown and acidic leaching agitation. The relevant factors for each of these techniques have been properly studied to find the optimum conditions for processing this sample. Alkaline breakdown using NaOH, KOH, and NaOH/KOH mixture led to dissolution of Nb, about 1% of REEs, and less than 10% of U away from Zr, Th and remaining REEs due to formation of water-soluble Na (K) niobates [16]. The other metal values remained in the residue in the form of insoluble hydrous oxides.
Some of the interactions can be summarized by the following equations representing the chemical reactions between the molten NaOH/KOH mixture and the sample studied [25, 34, 44]:
Nb2O5 + 6MOH → 2M3NbO4 + 3H2O,
ZrSiO4 + 4MOH → M2ZrO3 + M2SiO3 + 2H2O,
ZrSiO4 + 6MOH → M2ZrO3 + M4SiO4 + 3H2O,
RE(PO4) + 3MOH → RE(OH)3 + M3PO4,
SnO2 + 2MOH → M2SnO3 + H2O,
UO2 + 2MOH → M2UO4 + H2O,
Fe2O3 + 2MOH → K2Fe2O4 + H2O,
SiO2 + 2MOH → M2SiO3 + H2O
(M = Na, K).
Factors affecting the Nb dissolution. Several relevant factors such as sample to NaOH/KOH mixture weight ratio, fusion time, and fusion temperature have been properly studied to optimize the Nb dissolution process.
Effect of the type of alkali. A 5-g portion of an alkali fusion reagent (R: NaOH, KOH, or NaOH/KOH mixture) was mixed in a porcelain crucible with the concentrate sample (S) in weight ratio S/R = 1/1 and was fused at 500°C for 1 h. The fused matrix was then cooled and washed with distilled H2O. The filtrate obtained was analyzed to determine the Nb dissolution efficiency. The data obtained (Table 2) emphasized that the NaOH/KOH mixture was the most effective reagent for Nb dissolution. This mixture gave the maximum Nb dissolution efficiency of 86%.
Effect of the sample/reagent ratio. The effect of concentrate sample/reagent (S/R) weight ratios on the Nb leaching efficiency of Nb was studied at the other fusion conditions kept constant: fusion time 1 h, fusion temperature 500°C, –200 mesh size and muffle furnace. After cooling, each fused matrix was washed with distilled H2O, and the content of dissolved Nb(V) ions in the filtrate was determined. The data obtained (Fig. 2a) showed that the S/R weight ratios of 1/1.5 and 1/2 ensured the maximum value of the Nb dissolution efficiency. In going from 1/1.5 to 1/2 ratio, the Nb dissolution efficiency increased insignificantly. Thus, 1/1.5 mass ratio was preferred as the optimum ore concentrate/reagent weight ratio.
Effect of the fusion temperature. The fusion temperature was varied from 400 to 600°C with the other parameters kept constant. The results (Fig. 2b) showed that the best Nb dissolution efficiency was achieved at the fusion temperature of 500°C. Further increase in the fusion temperature to 550°C led to only a slight increase in the dissolution. Thus, from the economic viewpoint 500°C was chosen as the optimum temperature.
Effect of the fusion time. The fusion time was varied from 0.5 to 2.5 h, with the other parameters kept constant: fusion temperature 500°C, concentrate to NaOH/KOH weight ratio 1/1.5 and –200 mesh size. The results (Fig. 2c) emphasized that the Nb dissolution efficiency reached its maximum (97%) in 2 h.
Preparation of the alkaline leach liquor. Thus, the optimum conditions for dissolving 97% of Nb from the Abu Rusheid concentrate sample can be summarized as follows: concentrate sample to NaOH/KOH weight ratio 1/1.5, fusion temperature 500°C, and fusion time 2 h. On the other hand, it should be noted that less than 10% of uranium content was dissolved with niobium, and the other associated metal values, e.g., REEs, Zr, and Th, remain in the residue as insoluble hydrous cake. One liter of the alkaline leach liquor for the niobium recovery was prepared from 100 g of the concentrate sample. The liquor was found to assay 0.99 g/L Nb, 0.01 g/L Ta, and 0.05 g/L U; its pH was higher than 12. Uranium and REEs showed small solubility (less than 10% and about 1%, respectively), in agreement with the published data [43]. The prepared alkaline leach liquor was first partially neutralized with H2SO4 (97%) to pH 10 [16]. Gelatinous Si(OH)4 with the precipitation efficiency of 96% was obtained with small amounts of Nb(V) ions (not exceeding 1.2% of the total Nb content).
Extraction of niobium. The working alkaline solution (free from most of Si ions) containing Nb(V) ions and a small amount of U ions was further acidified to pH 8.0 to precipitate Nb(V) via hydrolysis [44]. After filtration and washing, the prepared Nb cake was collected and examined using SEM-EDAX; its purity was 61% with the interfering impurities of Si, Fe, U, and Mn (Fig. 2d).
Acidic Agitation of the Concentrate Residue
As mentioned above, niobium was successfully recovered from the concentrate sample of Abu Rusheid with a small amount of uranium. The remaining metal values (Th, Zr, REEs, and remaining U) were left in the residue. The results of complete chemical analysis of the residue are given in Table 3; ZrO2 was found to assay 24.93 wt %, with RE2O3 (total rare earth elements oxides + Y2O3), ThO2, and U3O8 assaying 3.64, 0.93, and 1.21 wt %, respectively.
The residue was washed with distilled water several times and dried at 100°C before being subjected to agitation leaching with 7 M HCl at 90°C for approximately 1 h; the 97% Zr dissolution efficiency was reached [21], and Th, REEs, and uranium were dissolved completely [21].
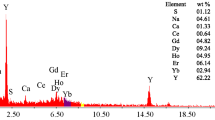
Thorium recovery from the chloride leach liquor. Thorium was selectively separated from the prepared chloride solution by precipitation with oxalic acid at pH 0.35 [46, 47]. Thorium was precipitated selectively because of the great difference in the solubility products of Th [48] and REE [39] oxalates. The precipitate was filtered off, washed, dried, and ignited at 800°C for 1 h to obtain ThO2. SEM-EDAX analysis of the Th product cake showed its approximately 89% purity (Fig. 3).
Direct precipitation of zirconium with sodium sulfide (Na2S). The parameters affecting the precipitation process such as pH, sodium sulfide (Na2S) concentration, time, and temperature were investigated.
Effect of pH. The pH was varied from 1.5 to 3.2 with the other conditions kept constant: stirring time 15 min, room temperature, and 10% Na2S. The results obtained (Fig. 4a) showed that the Zr precipitation increased with increasing pH to 2.8. However, further increase in pH had no remarkable effect. Thus, pH 2.8 was chosen as an optimum.
Effect of Na2S concentration. The effect of the Na2S concentration on the selective Zr precipitation from the chloride leach liquor was studied at the other conditions fixed: room temperature, pH 2.8, and precipitation time 15 min. The Na2S concentration was varied from 5 to 25%. The results obtained (Fig. 4b) showed that the efficiency of the Zr precipitation increased with increasing the Na2S concentration to 15%. Further increase in the Na2S concentration led to only a slight increase in the Zr precipitation efficiency. Thus, 15% Na2S was chosen as an economically optimal concentration for zirconium precipitation.
Effect of time. The time was varied from 5 to 25 min, keeping the other factors constant: pH 2.8, 15% Na2S concentration, and room temperature. The results obtained (Fig. 4c) showed that the Zr precipitation efficiency increased with increasing the precipitation time from 5 to 15 min, but further increase in the precipitation time had no effect on the precipitation efficiency. Thus, the optimum contact time is 15 min.
Effect of temperature. The temperature was varied from room temperature to 80°C. The other factors were fixed: pH 2.8, 15% Na2S concentration, and 15 min. The results obtained (Fig. 4d) showed that the precipitation at room temperature did not ensure precipitation of more than 60.5% of Zr, whereas increasing the temperature to 75°C led to precipitation of approximately 98.9% of Zr. However, further increase in the temperature to 80°C decreased the precipitation efficiency because of precipitation of some other impurities.
Thus, the optimum conditions for precipitating about 98.9% of Zr from its combined U and REEs acidic leach liquor are as follows: pH 2.8, 15% Na2S concentration, 15 min, and 75°C. The obtained zirconium sulfide was washed with distilled water, ignited at 800°C for 1 h, and identified by SEM-EDX analysis (Fig. 5).
Direct precipitation of rare earth elements. Selective precipitation of REEs from the leach liquor free from Th and Zr was performed by gradually adding 10% oxalic acid with continuous stirring for 1 h at pH 1.2. The precipitation of REEs as oxalate cake was almost complete (99.3%) [20, 49]. After filtration, washing, and drying, the oxalate cake obtained was ignited at 800°C for 1 h. The RE2O3 cake prepared was cooled, washed, dried, and identified by SEM-EDX analysis (Fig. 6).
Direct precipitation of uranium. The leach liquor free from Th, REEs, and Zr was subjected to uranium precipitation. The presence of excess sulfide ions was effectively overcome by adding hydrogen peroxide, which leads to the oxidation of U(IV) to the extractable U(VI) form and of sulfide to sulfur [50]. The solution was treated with H2O2. Then, the pH of the solution was adjusted to approximately 13 with 1 M NaOH solution. After filtration, washing, and drying, the oxalate cake obtained was ignited at 800°C for 1 h. The sodium diuranate precipitate was cooled, washed, dried, and identified by SEM-EDX analysis (Fig. 7).
CONCLUSIONS
The suggested alkaline procedure followed by water leaching for the processing of the Abu Rusheid concentrate sample has successfully been applied for selective dissolution of Nb with its separation from Zr, Th, REEs, and U. About 97% of the total Nb content was dissolved at a concentrate sample to NaOH/KOH weight ratio of 1/1.5, a fusion temperature of 500°C, and a fusion time of 2 h. To remove silicate ions, the alkaline solution obtained was adjusted to pH 10. Complete precipitation of Nb was ensured by the second pH adjustment of the alkaline solution to pH 8.0.
The obtained residue was treated with 7 M hydrochloric acid at 90°C for 1 h, which ensured 98% leaching of Zr and quantitative leaching of Th, U, and REEs. From the prepared chloride solution, Th, Zr, REE, and U products could be obtained. The products were ignited at 800°C and examined by SEM-EDX analysis. The proposed process flowsheet is shown in Fig. 8.
REFERENCES
Ibrahim, M.E., Saleh, G.M., Ibrahim, I.H., Azab, M.S., Khamies, A.A., Oraby, F., Abu El-Hassan E.A., and Ragab, A.A., Geologic and ground spectrometric prospecting of the Abu Rusheid–Sikeit shear zones, South Eastern Desert, Egypt, Seventh Arab Conf. on the Peaceful Uses of Atomic Energy, Sanaa, 2004.
El-Afandy, A.H., El-Feky Mohamed, G., Taha, S., El Minyawi, S.M., and Sallam, H.A., Greener J. Geol. Earth Sci., 2016, vol. 4, no. 3, pp. 056–069. https://doi.org/10.15580/GJGES.2016.3.120916213
Mansour, G.R., Geological and geophysical investigations of mineralized structures in Wadi Abalea and its surroundings, Abu Rusheid area, South Eastern Desert, Egypt, PhD Thesis, Mansoura Univ., Egypt, 2005, p. 139
Huang, H., Ding, S., Su, D., Liu, N., Wang, J., and Tan, M., Sep. Purif. Technol., 2014, vol. 138, pp. 65–70. https://doi.org/10.1016/j.seppur.2014.10.008
Mastren, T., Radchenko, V., Owens, A., Copping, R., Boll, R., Griswold, J.R., Mirzadeh, S., Wyant, L.E., Brugh, M., Engle, J.W., Nortier, F.M., Birnbaum, E.R., John, K.D., and Fassbender, M.E., Sci. Rep., 2017, vol. 7, ID 8216. https://doi.org/10.1038/s41598-017-08506-9
Mastren, T., Radchenko, V., Engle, J.W., Weidner, J.W., Owens, A., Wyant, L.E., Copping, R., Brugh, M., Nortier, F.M., Birnbaum, E.R., John, K.D., and Fassbender, M.E., Anal. Chim. Acta, 2018, vol. 998, pp. 75–82. https://doi.org/10.1016/j.aca.2017.10.020
Afifi, S.Y., Saad, M.Z., Yousef, L.A., and Ismail, A.H., Arab J. Nucl. Sci. Appl., 2018, vol. 51, no. 2, pp. 10–19. http://doi.10.21608/AJNSA.2018.6506
Chung, K.W., Yoon, H-S., Kim, C.J., Lee, J.-Y., and Jyothi, R.K., J. Ind. Eng. Chem., 2020, vol. 83, pp. 72–80. https://doi.org/10.1016/j.jiec.2019.11.014
Kul, M., Topkaya, Y., and Karakaya, I., Hydrometallurgy, 2008, vol. 93, no. 3, p. 129. https://doi.org/10.1016/j.hydromet.2007.11.008
Hu, C., Liu, H.J., Peng, L., Sun, Y.K., and Long, W., J. Radioanal. Nucl. Chem., 2016, vol. 308, no. 1, p. 251. https://doi.org/10.1007/s10967-015-4306-z
Chellam, S. and Clifford, D.A., J. Environ. Eng., 2002, vol. 128, no. 10, pp. 942–952. https://doi.org/10.1061/(ASCE)0733-9372(2002)128:10(942)
Kuruc, J., Strisovska, J., Galanda, D., Dulanska, S., Matel, L., Jerigova, M., and Velic, D., J. Radioanal. Nucl. Chem., 2012, vol. 292, pp. 973–981. https://doi.org/10.1007/s10967-012-1670-9
Sharma, S. and Balasubramanian, K., RSC Adv., 2015, vol. 5, pp. 31732–31741. https://doi.org/10.1039/C5RA02861B
Stegnar, P. and Benedik, L., Arch. Oncol., 2001, vol. 9, pp. 251–255. https://inis.iaea.org/collection/NCLCollectionStore/_Public/34/047/34047643.pdf?r=1&r=1
The Health Hazards of Depleted Uranium Munitions, London: Royal Soc., 2001.
Li, P., Zhun, B., Wang, X., Liao, P., Wang, G., Wang, L., Guo, Y., and Zhang, W., Environ. Sci. Technol., 2017, vol. 51, no. 24, pp. 14368–14378. https://doi.org/10.1021/acs.est.7b05288
Yao, L., Min, X., Xu, H., Ke, Y., Liang, Y., and Yang, K., Int. J. Environ. Res. Public Health, 2018, vol. 15, no. 9. https://doi.org/10.3390/ijerph15091863
Yin, S., Wang, L., Kabwe, E., Chen, X., Yan, R., An, K., Zhang, L., and Wu, A., Minerals, 2018, vol. 8, no. 2, p. 32. https://doi.org/10.3390/min8020032
Quevauviller, Ph., Methodologies for Soil and Sediment Fractionation Studies, Cornwall, UK: Royal Soc. Chem., 2002.
Patra, A.C., Sumesh, C.G., Mohapatra, S., Sahoo, S.K., Tripathi, R.M., and Puranik, V.D., J. Environ. Manag., 2011, vol. 92, pp. 919–925. https://doi.org/10.1016/j.jenvman.2010.10.046
Zolfonoun, E., Monji, A.M., Taghizadeh, M., and Ahmadi, S.J., Miner. Eng., 2010, vol. 23, no. 9, pp. 755–756. http://doi.org/10.1016/j.mineng.2010.05.005
Abdel-Rehim, A.M., Int. J. Miner. Process., 2005, vol. 76, pp. 234–243. https://doi.org/10.1016/j.minpro.2005.02.004
Blumenthal, W.B., Zirconium and zirconium compounds, Kirk–Othmer Encyclopedia of Chemical Technology, Howe-Grant, M., Ed., New York: Wiley, 1998.
Manhique, R., Optimization of Alkali Fusion Process for Zircon Sands: A Kinetic Study of the Process, MSc Thesis, Faculty of Natural and Agricultural Sciences, Univ. of Pretoria, 2003.
Daher., A.M., Isot. Radiat. Res., 2008, vol. 40, no. 1, pp. 91–105.
Kwela, Z.N., Alkali-Fusion Processes for the Recovery of Zirconia and Zirconium Chemicals from Zircon Sand, MSc Thesis, Faculty of Natural and Agricultural Sciences, Univ. of Pretoria, 2000.
Deblonde, G.J.-P., Weigel, V., Bellier, Q., Houdard, R., Delvallée, F., Bélair, S., and Beltrami, D., Sep. Purif. Technol., 2016, vol. 162, pp. 180–187. https://doi.org/10.1016/j.seppur.2016.02.025
Rodriguez, M.H., Rosales, G.D., Pinna, E.G., and Suarez, D.S., Hydrometallurgy, 2016, vol. 159, pp. 60–64. https://doi.org/10.1016/j.hydromet.2015.10.033
El-Hussaini, O.M., Miner. Process. Extr. Metall. Rev., 2001, vol. 22, pp. 633–650. https://doi.org/10.1080/08827509808962519
Rodriguez, M.H., Rosales, G.D., Pinna, E.G., and Suarez, D.S., Hydrometallurgy, 2015, vol. 156, pp. 17–20. https://doi.org/10.1016/j.hydromet.2015.05.006
Deblonde, G.J.-P., Bengio, D., Beltrami, D., Belair, S., Cote, G., and Chagnes, A., Sep. Purif. Technol., 2019, vol. 215, pp. 634–643. https://doi.org/10.1016/j.seppur.2019.01.052
Deblonde, G.J.-P., Bengio, D., Beltrami, D., Belair, S., Cote, G., and Chagnes, A., Sep. Purif. Technol., 2019, vol. 226, pp. 209–217. https://doi.org/10.1016/j.seppur.2019.05.087
Abdel Wahab, G.M., Abdellah, W.M., Yousif, A.M., and Mubark, A.E., Min., Metall. Explor., 2022, vol. 39, pp. 833–846. https://doi.org/10.1007/s42461-019-00136-1
Wang, X., Zheng S., Xu, H., and Zhang, Y., Hydrometallurgy, 2009, vol. 98, pp. 219–223. https://doi.org/10.1016/j.hydromet.2009.05.002
Purcell, W., Potgieter, H., Nete, M., and Mnculwane, H., Min. Eng., 2018, vol. 119, pp. 57–66. https://doi.org/10.1016/j.mineng.2018.01.031
Amer, T.E., El-Assay, I.E., Rezk, A.A., El-Kammar, A.A., El-Manawy, A.W., and Abu Khoziem, H.A., Int. J. Min. Process., 2014, vol. 129, pp. 12–21. https://doi.org/10.1016/j.minpro.2014.04.005
Mubarak, A.E., Chemical Processing of Some Valuable Elements from Gabal El-Faliq Mineralizations, South Eastern Desert, Egypt, PhD Thesis, Faculty of Science, Menoufia Univ., 2019.
Krishnamurthy, N. and Gupta, C.K., Extractive Metallurgy of Rare Earths, CRC, 2004.
Horlait, D., Clavier, N., Szenknect, S., Dacheux, N., and Dubois, V., Inorg. Chem., 2012, vol. 51, pp. 3868–3878. https://doi.org/10.1021/ic300071c
Mathew, K.J., Burger, S., Ogt, S.V., Mason, P.M., and Narayanan, U.I., Uranium assay determination using Davies and Gray titration, Proc. Eighth Int. Conf. on Methods and Applications of Radioanalytical Chemistry (MARC VIII), Kaailua-Kona, Hawaii, 2009.
Marczenko, Z., Spectrophotometric Determination of Elements, Harwood, New York: Wiley, 2000.
Abdelkader, A.M., Daher, A., and El-Kashef, E., J. Alloys Compd., 2007, vol. 460, pp. 577–580. https://doi.org/10.1016/j.jallcom.2007.06.032
El-Nadi, Y.A., Daoud, J.A., and Aly, H.F., Int. J. Miner. Process., 2005, vol. 76, pp. 101–110. https://doi.org/10.1016/j.minpro.2004.12.005
Wang, X., Zheng, S., Xu, H., and Zhang, Y., Hydrometallurgy, 2009, vol. 98, pp. 219–223. https://doi.org/10.1016/j.hydromet.2009.05.002
Sharma, B.K., Nuclear Reaction Chemistry Book, GOEL, 2011, 7th ed., p. 348
Amer, T.E., El-Sheikh, E.M., Gado, M.A., Abu-Khoziem H.A., and Zaki, S.A., Sep. Sci. Technol., 2017, vol. 53, no. 10, pp. 1522–1530. https://doi.org/10.1080/01496395.2017.1405039
Kobayashi, T., Sasaki, T., Takagi, I., and Moriyama, H., J. Nucl. Sci. Technol., 2009, vol. 46, no. 11, pp. 1085–1090. https://doi.org/10.1080/18811248.2009.9711619
Crouthamel, C.E. and Martin, D.S.Jr., Ames Laboratory, 1950. http://lib.dr.iastate.edu/ameslab_iscreports/3
Habashi, F., A Textbook of Hydrometallurgy, Quebec, Canada, 1993.
El-Nadi, Y.A. and Daoud, J.A., J. Nucl. Radiochem. Sci., 2004, vol. 5, no. 1, pp. 11–15. https://doi.org/10.14494/jnrs2000.5.11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
El-Afandy, A.H., Yousif, A.M. & Mubark, A.E. Subsequent Separation of Niobium (Nb), Thorium (Th), Rare Earth Elements (REEs), Zirconium (Zr), and Uranium (U) from Abu Rusheid Cataclastic Concentrate, South Eastern Desert, Egypt. Radiochemistry 64, 257–267 (2022). https://doi.org/10.1134/S1066362222020175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1066362222020175