Abstract
Monoclinic bismuth vanadate (m-BiVO4) has attracted many researchers as an advanced photocatalyst for hydrogen production via water splitting and degradation of organic contaminants. In this study, pure m-BiVO4 nanoparticles were fabricated by an easy reproducible solid state route at different temperatures (500 °C, 550 °C, 600 °C, 650 °C and 700 °C) for 2 h. The synthesized materials were characterized by X-ray Diffractometer where all the diffraction patterns reveal characteristic peaks corresponding to m-BiVO4 with space group C2/c. Obtained m-BiVO4 particles have the lattice parameters: a = 7.2477 Å, b = 11.6970 Å, c = 5.0900 Å and the volume of the unit cell is 309.23 (106 pm3). Fourier Transform Infrared spectroscopy exhibits formation of Bi–O bond in the prepared nano powders. Ultraviolet–Visible diffuse reflectance spectroscopy suggests that nanostructured BiVO4 particles possess strong energy absorption properties both in visible and ultraviolet region. The particles show red shift of band gap as the calcination temperature rises and possible reasons have been discussed. Energy-dispersive X-ray spectroscopy confirms presence of Bi, V, and O without any contaminant, while particle’s morphology was investigated using Field Emission Scanning Electron Microscope.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The hydrosphere is getting polluted because of high population growth and rapidly growing industrialization. On the contrary, the demand of pure water is soaring both for industrial and domestic use. Consequently, purification of contaminated water through a low cost green sustainable technique has emerged as a crucial issue. Another cardinal challenge is to meet the rising energy need which is now mostly dependent on fossil fuels. As fossil fuels are deleterious to environment, generation of hydrogen energy through utilization of solar energy is being reckoned as potential green energy source. So, it is high time to develop new semiconductor materials which can exploit solar energy by converting it into electrical and chemical energy [1,2,3,4]. Semiconductor photocatalysts have emerged as promising materials for water splitting and degradations of organic contaminants [5]. However, the performance of the photocatalyst material relies on several factors such as crystal structure, morphology and synthesis route [6]. Though various metal oxides like TiO2 [7, 8], SnO2 [9], ZnO [10, 11], Cu2O [12, 13], Ag3VO4 [14], InVO4 [14], BiVO4 [15] have been formulated to date, BiVO4 has drawn great attention to the scientific community owing to its unique properties like non-toxic nature, low band gap energy [16,17,18,19,20]. BiVO4 occurs naturally as the mineral pucherite with an orthorhombic crystal structure while synthetic BiVO4 has mainly three crystalline phases: tetragonal zircon, monoclinic scheelite and tetragonal scheelite [21, 22]. Monoclinic scheelite structure has been found to be the most suitable photocatalyst among the crystals of BiVO4 because of having narrow band gap (2.4 eV) [23,24,25]. The crystal system of m-BiVO4 has four unique lattice sites: Bi (4e), V (4e), O1 (8f), and O2 (8f) where O1 is aligned with one Bi and V, whereas O2 is aligned with 2 Bi and a single V [26]. This system possesses oxidation states of 3+, 5+ and 2− for Bi (5d10 6s2), V (3d0) and O (2p6) respectively and crystal distortion present in monoclinic phase increases lone pair effect of Bi 6s states resulting O 2p states moving upwards [27]. Consequently, band gap reduces to 2.4 eV in monoclinic phase compared to 2.9 eV in tetragonal phase which enables electronic transition by visible light from occupied O 2p states to the unoccupied V 3d states [27]. Furthermore, separation efficiency of photo induced electrons and holes is higher in m-BiVO4 due to distortion in Bi-O bond [27]. In this perspective, various methods have been employed for the preparation of m-BiVO4 nanoparticles such as hydrothermal process, molten salt method, solvothermal process, ultrasonic-assisted method, flame spray, microwave synthesis, sol–gel, mechanochemical synthesis, hybrid organic–inorganic route and co-precipitation method [28,29,30,31,32,33,34,35,36,37,38,39].
In this report, a simple reproducible solid state reaction method was explored for the preparation of nanostructured pure m-BiVO4 from Bi2O3 and V2O5 as precursors at different temperatures (500 °C, 550 °C, 600 °C, 650 °C and 700 °C) for two hours. This study presents in depth analysis on structural formation, vibrational properties, optical band gap and morphology of pure m-BiVO4 particles obtained employing solid state route which should facilitate to carry out further research on nanostructured m-BiVO4.
2 Materials and Methods
In order to prepare m-BiVO4 nanoparticles, a facile solid state method was adopted where analytical grade bismuth oxide: Bi2O3 (99.9%, Inframat Advanced Materials) and vanadium pentoxide: V2O5 (99%, Loba Chemie) were used as precursors without any further purification. The precursors were mixed together at stoichiometric 1:1 (Bi:V) molar ratio by hand milling with mortar and pestle, followed by calcination at different temperatures (500 °C, 550 °C, 600 °C, 650 °C and 700 °C) for 2 h in a muffle furnace (model LT 15/11/C450, Nabertherm). Then the obtained particles were subjected to characterizations.
An X-Ray Diffractometer system (model Empyrean, PANalytical) with Cu target was used to determine the phases present and other structural information where High score plus data base and VESTA graphical interface software were used to analyze XRD data. FTIR spectra (model Spectrum Two, Perkin Elmer) were recorded in the wave number range 400–4000 cm−1 for observing bonding conformation of the synthesized powders. UV–Vis spectroscopy (model Lambda 1050, Perkin Elmer) was performed in diffuse reflection mode in an interval 200–800 nm in order to determine the absorption properties of the particles and the corresponding optical band gap. Morphology of the particles was examined by FESEM (model JSM 7600F, JEOL), while elemental compositions were determined using EDS coupled with FESEM.
3 Results and Discussion
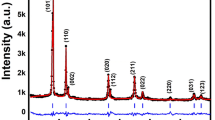
Room temperature XRD analysis was performed for the BiVO4 samples using Cu-Kα (λ = 1.541874 Å) radiation at 2θ values between 10° and 80° in order to estimate the crystalline parameters as a result of increasing synthesis temperature. Phase identifications have been carried out with high score plus database provided by PANalytical. As displayed in Fig. 1a, all the samples exhibit monoclinic clinobisvanite BiVO4 with space group C2/c (ICSD reference pattern code: 98-010-0605). Moreover, all diffraction patterns show the characteristic peak splitting at about 2θ = 18.5° and 35° (Fig. 1b). The presence of these distinctive phenomena in the XRD patterns obtained for the synthesized samples confirm the formation of monoclinic phase [40, 41]. For monoclinic BiVO4, the crystallographic parameters and atomic fractional coordinates of all atoms are listed in Table 1.
However, along with desired spectra, few extra peaks of unreacted precursors and intermediate phases become visible (Fig. 1a) for nanoparticles synthesized at 500 °C and 550 °C. It reveals that these lower temperatures are not sufficient to complete the reaction entirely. Moreover, quantitative analysis by reitveld refinement shows the presence of about 70% and 90% of monoclinic BiVO4 phase for 500 °C and 550 °C respectively elucidating effect of synthesis temperature on reaction yield. At higher temperatures above 550 °C, no additional diffraction peak of any intermediate phase has been detected, indicating the formation of single phase (m-BiVO4) structure.
From the crystal structure, it is evident that the unit cell of monoclinic BiVO4 is composed of VO4 tetrahedron and BiO8 dodecahedron. As shown in Fig. 2, the Bi site is bordered by eight oxygen atoms to form BiO8 dodecahedron and the V site by four oxygen atoms making a VO4 tetrahedron. Though the BiO8 dodecahedron shares edges with nearby BiO8 dodecahedra, each VO4 tetrahedron is separated and does not come into contact with the neighboring VO4 tetrahedron. By sharing an apex oxygen atom, the isolated VO4 tetrahedron is connected with BiO8 dodecahedron. The existence of four types of Bi–O bond and two types of V–O bond (Table 1) state that both VO4 tetrahedron and BiO8 dodecahedron are distorted.
The average crystallite size (D) was calculated using Scherrer formula: D = K λ/(βsizecosθ) where K is the crystal shape factor, λ is the wavelength of Cu-Kα radiation used, θ is the bragg angle. Lattice strain was determined from the tangent formula: Lattice strain = βstrain/(4*tanθ). In the above two equations, β describes the structural broadening in radians which is the difference between integral profile width of standard and test sample: βsize = βobs − βstd and βstrain = Square root (β 2obs − β 2std )
From Table 2, it is observed that crystallites become larger with increasing synthesis temperature which may be due to the crystallization process through accelerating the Bi3+ ions diffusive into VO43− anions [42,43,44]. However, value of lattice strain varies inversely with temperature. The synthesized BiVO4 at 700 °C shows lowest strain which validates the higher degree of crystallization of BiVO4 at higher temperature [42]. In addition, a reverse relation exists between crystallite size and lattice strain resembling the previously reported work [3, 45,46,47,48,49]. Indeed, the grain boundaries of nanostructured materials are relatively more disordered and inherently contain a certain amount of excess volume in the form of vacancies and vacancy clusters [50,51,52,53,54]. According to elasticity theory [55, 56], these excess volumes may create stress field which exerts lattice strain in nanocrystalline materials. Furthermore, for smaller crystallites, the internal pressure exerted by the surface tension on the nano materials may produce a stress field resulting lattice strain. However, as the crystallite size increases, the grain boundary stress field becomes weak causing decrease of lattice strain [46, 57]. So, the excess volume near grain boundaries as well as the crystallite interface both highly contribute to stress field and could be the plausible sources of internal strain with decreasing crystallite size [46, 48].
FTIR spectra (Fig. 3) analyzed at room temperature demonstrate strong and broad IR band at 718 cm−1 with shoulder at 802 cm−1 which is a characteristic of m-BiVO4. In addition, a small IR band is observed at 466 cm−1. According to literature, the strong IR band at 718 cm−1 is due to anti-symmetric stretching vibration from VO4 and the weak IR band at 466 cm−1 results from absorption of Bi-O bond [58]. Powder synthesized at 500 °C shows V–O stretching of V2O5 (1009 cm−1) and presence of V2O5 at low temperature (500 °C) is also confirmed by XRD analysis [59].
The diffuse reflectance of the BiVO4 samples was measured in the wavelength range of 200–800 nm from which absorbance versus wavelength plot was constructed (Fig. 4a). The diffuse reflectance data were converted to Kubelka–Munk function given by F(R) = (1 − R)2/2R (where R is the diffuse reflectance value) to construct the [F(R) hʋ]1/n vs. hʋ (photon energy) plots for the fabricated samples (Fig. 4b) [60]. The electronic structure as well as sharp fall of the graph of m-BiVO4 indicate that it possesses features of direct band gap semiconductor and we used n = 1/2 for direct electronic transition. Here, the intersection of the tangent line with [hʋ F(R)]2 = 0 represents the optical band gap energy, e.g. The band gap energies obtained for the samples are quite consistent with the reported band gap values of the monoclinic BiVO4. From the plot, red shift of band gap is observed (2.49–2.37 eV) as the fabrication temperature rises (500–700 °C). The absorption edge of the prepared m-BiVO4 also reveals red shift and their corresponding values (absorption edge and band gap) with respect to temperature are summarized in Table 3. When crystallite size decreases to nanoscale the number of atoms becomes smaller in a crystal which results less overlapping of energy levels and width of each band (valence and conduction) starts to narrow. Consequently, band gap increases as crystallite size decreases. Furthermore, at higher calcination temperature all active electronic regions at the surface of the particles are subdued along with their size increase and so absorption spectra show this phenomenon [58].
The FESEM micrographs shown in Fig. 5a–e, reveal that the microstructures contain agglomerated particles. The agglomeration occurs mainly due to the effects of electrostatic forces at the interfaces and Van der Waals interactions [3]. The particles are nearly spherical in shape with non-uniform size distribution when synthesized up to 550 °C. However, at higher temperatures above 550 °C, the BiVO4 samples show particles with irregular shape morphology. It is observed that particle size increases with increasing synthesis temperature (Fig. 5). Figure 6a–e show EDS spectra of the particles and Table 4 gives the elemental compositions. The characteristic X-ray radiation of each element has different energy values: bismuth Mα = 2.419 keV, vanadium Kα = 4.949 keV and oxygen Kα = 0.525 keV. It is evident that no pollutant is present in the prepared particles and atomic ratio of Bi, V and O is nearly 1:1:4.
4 Conclusion
In this research, a simple method of producing pure m-BiVO4 nanoparticles has been presented. XRD patterns confirm formation of highly crystalline m-BiVO4. The yield of m-BiVO4 increases as reaction temperature rises and single phase m-BiVO4 is found in all samples calcined above 550 °C. Besides, it is evident that crystallite size increases (employing Scherrer formula) and red shift of band gap occurs (2.49–2.37 eV), in the synthesized particles with respect to temperature rise (500–700 °C). The vibrational properties measured by FTIR spectra also show conformity with m-BiVO4. FESEM micrographs reveal that at lower temperatures (500 °C and 550 °C) the particles are nearly spherical, whereas they are irregular in shape at higher temperatures (600 °C, 650 °C and 700 °C). The above study on structural, optical and morphological properties of the prepared m-BiVO4 particles suggests them as promising photocatalyst.
References
D.W. Chen, A.K. Ray, Appl. Catal. B: Environ. 23, 143–157 (1999)
H. Zhou, D.W. Smith, J. Environ. Eng. Sci 1, 247–264 (2002)
V. Rajalingam, Synthesis and characterization of BiVO4 nanostructured materials: application to photocatalysis. Dissertation, Universit´e du Maine (2014)
F.M. Toma, J.K. Cooper, V. Kunzelmann, M.T. McDowell, J. Yu, D.M. Larson, N.J. Borys, C. Abelyan, J.W. Beeman, K. Man, J. Yang, L. Chen, M.R. Shaner, J. Spurgeon, F.A. Houle, K.A. Persson, I.D. Sharp, Nat. Commun. 7, 12012 (2016)
K. Ordon, Functionalized semiconducting oxides based on bismuth vanadate with anchored organic dye molecules for photoactive applications. Dissertation, Université du Maine (2018)
M.J. Madiabu, J. Gunlazuardi, AIP Conf. Proc. 2023, 020079 (2018)
A. Fernandez, G. Lassaletta, V.M. Jimenez, A. Justo, A.R. Gonzalez Elipe, J.M. Herrmann, H. Tahiri, Y. Aitichou, Appl. Catal. B: Environ. 7, 49–63 (1995)
C. Minero, E. Pelizzetti, P. Pichat, M. Sega, M. Vincenti, Environ. Sci. Technol. 29, 2226–2234 (1995)
S.S. Wu, H.Q. Cao, S.F. Yin, X.W. Liu, X.R. Zhang, J. Phys. Chem. C 113, 17893–17898 (2009)
L. Gao, L.Q. Jiang, Mater. Chem. Phys. 91, 313–316 (2005)
M.A. Gondal, K. Hayat, M.M. Khaled, S. Ahmed, A.M. Shemsi, Appl. Catal. A 393, 122–129 (2011)
W.Z. Wang, H.L. Xu, W. Zhu, J. Phys. Chem. B 110, 13829–13834 (2006)
K. Rajeshwar, S. Somasundaram, C.R.N. Chenthamarakshan, N.R. de Tacconi, Int. J. Hydrog. Energy 32, 4661–4669 (2007)
M.A.A. Mamun, A.F.M.M. Hossain, M. Hasan, M.M. Rahman, Hydrothermal Synthesis and Characterization of Bismuth Vanadate Photocatalyst, in Proceedings of the 1st International Conference on Engineering Materials and Metallurgical Engineering, Bangladesh Council of Scientific and Industrial Research, Dhaka, 22–24 December 2016
H. Cai, L. Cheng, F. Xu, H. Wang, W. Xu, F. Li, R. Soc, Open Sci. 5, 180752 (2018)
A. Fujishima, K. Honda, Nature 238(5358), 37–38 (1972)
A. Kudo, K. Ueda, H. Kato, I. Mikami, Cat. Let. 53, 229 (1998)
P.H. Le, N.T. Kien, C.N. Van, Recent Advances in BiVO 4 - and Bi 2 Te 3 -Based Materials for High Efficiency-Energy Applications (Intech Open, London, 2018)
S. Dolic, D. Jovanovic, L. Zur, M. Cincović, M. Ferrari, M. Dramićanin, Synthesis, multifunctional properties and applications of bivo4 nanoparticles (Conference Presentation), in Proceeding of SPIE 10683, Fiber Lasers and Glass Photonics: Materials through Applications, 106831G (23 May 2018)
F. Rullens, A. Laschewsky, M. Devillers, Chem. Mater. 18, 771 (2006)
M.F. Rahman, M.S. Haque, M.H. Rizvi, M.A. Matin, M.A. Hakim, M.F. Islam, in Abstracts of the International Conference on Nanotechnology and Condensed Matter Physics, Bangladesh University of Engineering and Technology, Dhaka, 11–12 January 2018
M. Noor, M.A.A. Mamun, M.A. Matin, M.F. Islam, S. Haque, F. Rahman, M.N. Hossain, M.A. Hakim, Effect of pH Variation on Structural, Optical and Shape Morphology of BiVO4 Photocatalysts, in 10th International Conference on Electrical and Computer Engineering (IEEE, Dhaka, 20–22 December, 2018). https://doi.org/10.1109/icece.2018.8636721
H. Zhao, F. Tian, R. Wang, R. Chen, Rev. Adv. Sci. Eng. 3, 3–27 (2014)
Z. Wang, W. Luo, S. Yan, J. Feng, Z. Zhao, Y. Zhu, Z. Li, Z. Zou, Cryst. Eng. Commun. 13, 6674–6679 (2011)
P. Madhusudan, J. Yu, W. Wang, B. Cheng, G. Liu, Dal. Trans. 41, 14345–14353 (2012)
A. Walsh, Y. Yan, M.N. Huda, M.M. Al-Jassim, S.H. Wei, Chem. Mater. 21, 3 (2009)
Z. Zhao, Z. Li, Z. Zou, Phys. Chem. Chem. Phys. 13, 4746–4753 (2011)
U.M.G. Perez, S.S. Guzman, A.M. de la Cruz, J. Peral, Int. J. Electrochem. Sci. 7, 9622–9632 (2012)
D.P. Dubal, K. Jayaramulu, R. Zboril, R.A. Fischer, P.G. Romero, J. Mater. Chem. A 6, 6096 (2018)
T.L. Kim, M.J. Choi, H.W. Jang, Boosting interfacial charge transfer for efficient water-splitting photoelectrodes: progress in bismuth vanadate photoanodes using various strategies. MRS Commun. 8, 3 (2018)
M. Guo, Q. He, W. Wang, J. Wu, W. Wang, J. Wuhan Univ. Technol.-Mater. Sci. Edit. 31, 791 (2016). https://doi.org/10.1007/s11595-016-1447-z
M. Peng, J. Shi, Z. Wang, L. Li, Penglong CHEN Improvement of synthesis experiment of bismuth vanadate pigment by pH optimization. Univ. Chem. 33(8), 26–31 (2018)
M.V. Malashchonak, E.A. Streltsov, D.A. Kuliomin, A.I. Kulak, A.V. Mazanik, Monoclinic bismuth vanadate band gap determination by photoelectrochemical spectroscopy. Mater. Chem. Phys. (2017). https://doi.org/10.1016/j.matchemphys.2017.08.053
A.N. Zulkifili, A. Fujiki, S. Kimijima, Appl. Sci. 8, 216 (2018)
S.D. Dolića, D.J. Jovanovića, K. Smitsb, B. Babićc, M.M. Cincovića, S. Porobića, M.D. Dramićanina, Ceram. Int. 44, 17953–17961 (2018)
V. Sivakumar, R. Suresh, K. Giribabu, V. Narayanan, Cogent. Chem. 1, 1074647 (2015)
A. Kudo, K. Omori, H. Kato, J. Am. Chem. Soc. 121(49), 11459–11467 (1999)
S. Khademinia, M. Behzad, H.S. Jahromi, RSC Adv. 5, 24313–24318 (2015)
J. Yu, Y. Zhang, A. Kudo, J. Solid State Chem. 182, 223–228 (2009)
S.M. Thalluri, C.M. Suarez, M. Hussain, S. Hernandez, A. Virga, G. Saracco, N. Russo, Evaluation of the parameters affecting the visible-light-induced photocatalytic activity of monoclinic BiVO4 for water oxidation. Ind. Eng. Chem. Res. 52, 17414–17418 (2013). https://doi.org/10.1021/ie402930x
Y.K. Kho, W.Y. Teoh, A. Iwase, L. Maedler, A. Kudo, R. Amarl, ACS. Appl. Mater. Interfaces 3(6), 1997–2004 (2011)
C. Ravidhas, A.J. Josephine, P. Sudhagar, A. Devadoss, C. Terashima, K. Nakata, A. Fujishima, A.M.E. Raj, C. Sanjeeviraja, Mater. Sci. Semicond. Process. 30, 343–351 (2015)
Q. Jia, K. Iwashina, A. Kudo, Proc. Natl. Acad. Sci. 109, 11564–11569 (2012)
K. Rajeshwar, N.R. Tacconi, Chem. Soc. Rev. 38, 1984–1998 (2009)
S. Obregón, A. Caballero, G. Colón, Appl. Catal. B: Environ. 117–118, 59–66 (2012)
P.M. Shafi, A.C. Bose, AIP Adv. 5, 057137 (2015)
W. Qin, J.A. Szpunar, Phil. Mag. Lett. 85, 653 (2005)
K. Reimann, R. Wurschum, J. Appl. Phys. 81, 7186 (1997)
T.R. Malow, C.C. Koch, Acta Mater. 45, 2177 (1997)
W. Qin, T. Nagase, Y. Umakoshi, J.A. Szpunar, Philos. Mag. Lett. 88(3), 169–179 (2008)
D.H. Ping, D.X. Li, H.Q. Ye, J. Mater. Sci. Lett. 14, 1536 (1995)
K. Lu, Mater. Sci. Eng. R. 16, 161 (1996)
K. Lu, R. Lück, B. Predel, Mater. Sci. Eng. A. 179–180, 536 (1994)
P.P. Chattopadhyay, P.M.G. Nambissan, S.K. Pabi et al., Phys. Rev. B. 63, 054107 (2001)
W. Qin, J.A. Szpunar, Philos. Mag. Lett. 85(12), 649–656 (2005)
J.W. Christian, The Theory of Transformations in Metals and Alloys, Part 1 (Pergamon Press, Oxford, 2002), pp. 202–203
M. Dapiaggi, C.A. Geiger, G. Artioli, Am. Miner. 90, 506 (2005)
R. Venkatesan, S. Velumani, A. Kassiba, Mat. Chem. Phys. 135, 842–848 (2012)
H.D. Telpande, D.V. Parwate, J. Appl. Chem. 8(5), 28–37 (2015)
P. Kubelka, F. Munk, EinBeitrag ZurOptik Der Farbanstriche. Zeitschriftfür Technische Physik. 12, 593–601 (1931)
Acknowledgements
The authors would like to thank Department of Glass and Ceramic Engineering, Bangladesh University of Engineering and Technology (BUET) for providing assistance regarding characterization and preparation of the specimen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest in writing and publishing the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahman, M.F., Haque, M.S., Hasan, M. et al. Fabrication of Bismuth Vanadate (BiVO4) Nanoparticles by a Facile Route. Trans. Electr. Electron. Mater. 20, 522–529 (2019). https://doi.org/10.1007/s42341-019-00144-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42341-019-00144-4