Abstract
In this study, we report the synthesis of visible light active monoclinic structured bismuth vanadate (m-BiVO4) nanosheets (2D) by facile hydrothermal method. The morphology of the synthesized sample was governed by sodium dodecyl benzene sulfonate (SDBS) surfactant. Prepared samples were investigated by the characterization techniques such as ultraviolet visible (UV–Vis.) spectroscopy, fourier transform infra-red (FTIR) spectroscopy, x-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), and Raman spectroscopy. Monoclinic scheelite structure (m-BiVO4) was confirmed by XRD studies. The estimated direct band gap energy (Eg) 2.38 eV and monoclinic crystalline phase (XRD) clearly demonstrates a good visible light driven photocatalytic activity. The two-dimensional (2D) nanosheets of m-BiVO4 showed enhanced photocatalytic activity as studied by degradation efficiency (96% in 150 min) towards malachite green (MG) dye under natural sunlight irradiation in comparison with the synthesized BiVO4 nanoparticles. The analysis of all the measurements proposed that the nanosheets morphology with large surface area is an important factor in the amplification of photocatalytic reactions in m-BiVO4 nanosheets.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The most common organic dyes such as methyl orange; malachite green; rhodamine blue; and methylene blue; are heavily employed in textile, cosmetic, leather, food, drug, and plastic industries. However, they are toxic, indissoluble, and harmful for the health of environment and humans. Malachite green is a basic green dye, readily soluble in water and has been used as an antimicrobial agent, antifungal medication, and a topical antiseptic since 1930. But it has been classified as carcinogenic [1, 2]. The photocatalysis is the most widely used process, for resolving these types of environmental contaminations. Photocatalysis is the low-cost, sustainable, and ecofriendly technique for wastewater and other polluted water treatment. The photocatalytic materials have enormous potential to treat environmental problems by photocatalytic degradation of dyes and organic pollutants [3].
Monoclinic structured Bismuth vanadate (m-BiVO4) is a kind of photocatalytic material which shows excellent photocatalytic activity in the visible region for degradation of organic dyes and organic materials along with splitting of water for hydrogen and oxygen evolution due to its comparatively narrow band gap of < 2.4 eV, when compared with tetragonal phase (band gap about 3.1 eV) of BiVO4 [4, 5]. The hydrothermal synthesis has been considered a one-step convenient method of preparing metal oxides owing to its numerous benefits, namely particle size management, high purity, and high crystallinity [6].
In addition, the morphology-directing agents utilized in the synthesis method are considered as an imperative factor in varying morphology and improving surface area, which can enhance the photocatalytic activity of m-BiVO4. The presence of SDBS contributed to the crystal structure of BiVO4 and improved the photocatalytic activity for the degradation of RhB (N, N, N′, N′-tetraethylated rhodamine). The study of Lei et al. revealed the influence of pH in improving the phase and morphology of pure BiVO4. The monoclinic structured BiVO4 was fabricated at a low pH, while on increasing the pH value, the mixed phase containing monoclinic and tetragonal structured BiVO4 was obtained. The maximum activity of Rhodamine B (RhB) under visible light was shown by m-BiVO4 formed at pH 3.0 [7, 8].
2D (nanosheets, nanoplates, etc.) materials describe a developing class of materials that have sheet (or plate)-like structures containing the layer of only single or few atoms. The effort was shined by the invention of graphene, a single-layer carbon material with best thermal, electrical, and mechanical properties in 2004. Later, many graphene-like 2D photocatalyst materials became interesting topics in the photocatalysis field. 2D (sheets or plates-like) structures are described by weak van der Waals interaction between the planes, better electronic properties, strong in-plane bonds, and a large surface area [9,10,11,12].
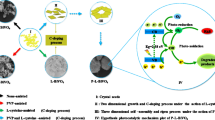
In the present work, we report the hydrothermal synthesis of highly crystalline two-dimensional (2D) m-BiVO4 nanosheets and nanoparticles. The crystal structure, functional group, band gap, morphology, and photocatalytic activity were investigated. The toxic malachite green (MG) dye was used for studying the comparative photocatalytic efficiency of BiVO4 nanosheets and nanoparticles. The m-BiVO4 nanosheets showed higher photocatalytic activity (efficiency 96%) and purified water by degrading hazardous malachite green (MG) dye in water bodies.
2 Synthesis Method
All chemicals used for synthesis of pure bismuth vanadate were of scientific grade and were used as received without any alternation. The m-BiVO4 nanosheets were prepared by one-step hydrothermal technique. In this procedure, 5 mM of Bi(NO3)35H2O (bismuth nitrate penta hydrate) procured from (Central Drug House Pvt. Ltd., New Delhi) was added to 10 mL of 4 M HNO3 (nitric acid) solution, and 5 mM of NH4VO3 (ammonium vanadate) also procured from (Central Drug House Pvt. Ltd., New Delhi). All these were dissolved into 10 mL of 2 M sodium hydroxide (NaOH) solution. In the next step, 0.25 g of sodium dodecyl benzene sulphonate (SDBS) (Central Drug House Pvt. Ltd., New Delhi) was added to both the solutions and continuously stirred for 30 min separately. After 30 min the two solutions were mixed to get uniform yellow suspension and the pH value of the mixed solution was confirmed as 4.0 by adding 2 M NaOH solution and again stirred for 30 min. The solution was then shifted into a 50 mL teflon-lined hydrothermal autoclave and kept in the furnace at 200 °C for 1.5 h. After that the hydrothermal autoclave was left undisturbed to cool down at room temperature under natural conditions. The precipitate was subjected to filtration and washed with DI water several times and allowed to dry at 100 °C for 4 h in a vacuum oven. Finally collected sample was grinded using mortar and pestle.
3 Characterization
The crystal structure and phase determination of the powder samples was determined by using Smart Lab Guidance, Rigaku x-ray diffractometer instrument with X-ray source of Cu Kα radiation (λ = 1.540 Å) with monitoring interval of 0.5 and the scan 2θ ranging between 10° and 90°. Functional groups and bond structure on the surface of the sample were studied using fourier transform infrared (FTIR) spectroscopy from Bruker Tensor 37 FTIR spectrometer (range 400–4000 cm−1). Raman spectrum was measured using HR800 JY, Lab RAM HR in the region from 100 to 1200 cm−1 (Agilent Technologies). To investigate the morphology of the as-synthesized nanostructures, field emission scanning electron microscope (Zeiss, Sigma, FESEM) was used. UV–visible spectrometer (Cary 100 series, Agilent Technologies) was employed to analyze the optical absorption spectra at room temperature.
4 Results and Discussion
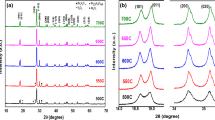
XRD pattern of the powder material is shown in Fig. 1a. The observed diffraction peaks are perfectly matched with (ICDD file No. 14-0688) [13]. The peaks indicate that the as-synthesized material has the structure of the phase-pure monoclinic scheelite BiVO4 (m-BiVO4) with high crystallinity. The high-intensity diffraction peak at 28.9° shows the specific orientation of (112) plane in the sample. No extra peaks are detected, inferred hence no impurities are present in the sample.
The diverse functional groups associated to pure BiVO4 was studied by FTIR measurements, as shown in Fig. 1b. In BiVO4 spectrum, the weak absorption at 1092 cm−1 is referred to the V=O stretching vibrations. The bands at 721 cm−1 is referred to the V–O–V stretching vibrations and band positioned at 617 cm−1 shows the absorption peak matching to the V–O–V stretching mode. The wavenumber of FTIR band vibration of Bi-O bending mode was recorded at 467 cm−1 [14, 15].
Raman spectroscopy is a useful tool for investigating the structure and bonding in materials by their vibrational features. In the m-BiVO4 nanosheets, the Raman spectrum as shown in Fig. 1c reveals six observable peaks at 124, 208, 324, 363, 708, and 823 cm−1 which are associated with the vibrational features of VO4 tetrahedron. The peak at 823 cm−1 is specified to symmetric stretching ʋs (VO), with a weak shoulder peak at 708 cm−1 which is attributed to asymmetric stretching ʋas (V–O). The asymmetric deformation modes δa (VO4−3) and δas (VO4−3) bands are observed at 324 and 363 cm−1. The rotational or translational bands are at about 213 and 124 cm−1 [16].
FESEM is used to examine the morphology of the powder sample. Figure 2a–d shows the FESEM images of m-BiVO4 nanoparticles and nanosheets at magnifications of 1 µm and 500 nm. Figure 2a, c demonstrated that m-BiVO4 is an agglomeration of nanoparticles, and Fig. 3b, d depicted m-BiVO4 clusters of well-defined nanosheets with a smooth, asymmetrical, and dense pattern, and a surface layer containing multiple folds of 2D nanosheets. The optical characteristics of the as-synthesized m-BiVO4 nanosheets were characterized by using the UV–visible absorbance spectroscopy. Figure 3a shows the absorption spectra. In the nanosheets, band gap energy (Eg) was successfully calculated with help of tauc plot (Fig. 3b), which is found to be 2.38 eV (Eg) [16]. This band gap lies in the absorption range of visible light, supporting the hypothesis that the sample is in fact photoactive in the visible light range [6].
5 Photocatalytic Activity Test
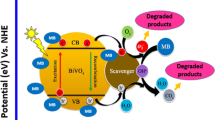
The photocatalytic activity experiment was carried out using synthesized nanosheets under sunlight irradiation. For the performance of tests for photocatalysis, 0.025 g photocatalyst was added to 100 ml of aqueous solution of malachite green (10 mg/L). The solution was stirred in dark chamber for half an hour to establish uniform equilibrium between the molecules of dye and the surface of as-synthesized sample. Then, the solution was irradiated under solar light. The photocatalytic behaviour was studied by taking 1 mL solution from MG mixture solution at interval of 30 min for 2 h and 30 min and the collected samples were analyzed using UV–visible spectrometer. The relative absorbance curve of BiVO4 nanospheres and BiVO4 nanosheets at UV–visible range wavelengths are depicted in Fig. 4a, b, respectively. Figure 4c the relative absorbance plot C/C0 versus time are shown in Fig. 4c. It is observed that m-BiVO4 nanosheets are more efficient in degradation when compared with BiVO4 nanoparticles. The steep C/C0 curve is the result of enhanced photocatalytic degradation of MG solution. The degradation efficiency of photocatalyst is shown in Fig. 4d which was evaluated by using relation (1) [17]. It is observed that m-BiVO4 nanosheets show more photodegradation efficiency (η = 96% in 150 min) when compared with m-BiVO4 nanoparticles (η = 53% in 150 min). It can be suggested that the BiVO4 nanosheets have a good ability for the purifying water by removing toxic dye (MG) in water bodies. The photodegradation efficiency (%) of the nanosheets (2D) and nanoparticles (3D) were calculated with the help of equation:
where C and C0 denote the concentration at time t and initial concentrations of MG solution, after visible light irradiation.
The mechanism of photodegradation of malachite green dye solution under the influence of BiVO4 nanosheets can be attributed to the following equations [1, 18].
6 Conclusion
Two-dimensional (2D) monoclinic-BiVO4 nanosheets have been prepared by facile hydrothermal technique with the help of the morphology-directing agent SDBS, for the photocatalytic degradation of organic water pollutant. The morphology of the as-synthesized material is well-defined nanosheets clearly observed by FESEM images. The nanosheets are monoclinic structured with a preferred (112) plane orientation. UV–visible absorption spectra determine the band gap 2.38 eV indicating that m-BiVO4 nanosheets have good capacity to consume sunlight in the range of visible light region. The results revealed that 2D m-BiVO4 nanosheets (η = 96% in 150 min) exhibits enhanced photocatalytic activity than the m-BiVO4 nanoparticles (η = 53% in 150 min) for the photodegradation of malachite green (MG) dye. The increased efficiency of nanosheets (2D) over nanoparticles (3D) is due to large surface area, higher number of active sites, and good absorption of visible light when compared with nanoparticles. This study presents an excellent visible light induced photocatalytic activity of 2D m-BiVO4 nanosheets for purification of water.
References
Ashraf W, Bansal S, Singh V, Barman S, Khanuja M (2020) BiOCl/WS2 hybrid nanosheet (2D/2D) heterojunctions for visible-light-driven photocatalytic degradation of organic/inorganic water pollutants. RSC Adv 10(42):25073–25088
Mittal H, Khanuja M (2020) Nanosheets-and nanourchins-like nanostructures of MoSe2 for photocatalytic water purification: kinetics and reusability study. Environ Sci Pollut Res 27(19):23477–23489
Kumar A, Singh S, Khanuja M (2020) Temperature based morphological study of graphitic carbon nitride for photocatalytic application. AIP Conf Proc 2276(1):020029
Pookmanee P, Paosorn S, Phanichphant S (2020) Chemical synthesis and characterization of bismuth vanadate powder. Adv Mater Res 93:153–156
Singh S, Ruhela A, Rani S, Khanuja M, Sharma R (2018) Concentration specific and tunable photoresponse of bismuth vanadate functionalized hexagonal ZnO nanocrystals based photoanodes for photoelectrochemical application. Solid State Sci 76:48–56
Xi G, Ye J (2010) Synthesis of bismuth vanadate nanoplates with exposed 001 facets and enhanced visible-light photocatalytic properties. Chem Commun 46(11):1893–1895
Zhang A, Zhang J, Cui N, Tie X, An Y, Li L (2009) Effects of pH on hydrothermal synthesis and characterization of visible-light-driven BiVO4 photocatalyst. J Mol Catal A Chem 304(1–2):28–32
Lei B-X, Zeng L-L, Zhang P, Sun Z-F, Sun W, Zhang X-X (2014) Hydrothermal synthesis and photocatalytic properties of visible-light induced BiVO4 with different morphologies. Adv Powder Technol 25(3):946–951
Trinh DTT, Khanitchaidecha W, Channei D, Nakaruk A (2019) Synthesis, characterization and environmental applications of bismuth vanadate. Res Chem Intermed 45(10):5217–5259
Tan C, Cao X, Wu X-J, He Q, Yang J, Zhang X, Chen J et al (2017) Recent advances in ultrathin two-dimensional nanomaterials. Chem Rev 117(9):6225–6331
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306(5696):666–669
Low J, Cao S, Yu J, Wageh S (2014) Two-dimensional layered composite photocatalysts. Chem Commun 50(74):10768–10777
Sharma R, Singh S, Verma A, Khanuja M (2016) Visible light induced bactericidal and photocatalytic activity of hydrothermally synthesized BiVO4 nano-octahedrals. J Photochem Photobiol B Biol 162:266–272
Sivakumar V, Suresh R, Giribabu K, Narayanan V (2015) BiVO4 nanoparticles: preparation, characterization and photocatalytic activity. Cogent Chem 1(1):1074647
Sajid MM, Amin N, Shad NA, Javed Y, Zhang Z (2019) Hydrothermal fabrication of monoclinic bismuth vanadate (m-BiVO4) nanoparticles for photocatalytic degradation of toxic organic dyes. Mater Sci Eng B 242:83–89
Zhang A, Jiezhang Z (2009) The effect of hydrothermal temperature on the synthesis of monoclinic bismuth vanadate powders. Mater Sci Poland 27:14573
Ashraf W, Khan A, Bansal S, Khanuja M (2022) Mechanical ball milling: a sustainable route to induce structural transformations in tungsten disulfide for its photocatalytic applications. Phys E Low-Dimens Syst Nanostruct 140:115152
Aslam Z, Rahman RS, Shoab M, Khan ZMSH, Zulfequar M (2022) Photocatalytic response of CuCdS2 nanoparticles under solar irradiation against degradation of methylene blue dye. Chem Phys Lett 804:139883
Acknowledgements
Dr. Manika Khanuja (one of the authors) is appreciative of Science and Engineering Research Board [(SERB) (No. ECR/2017/001222)] for their support. The use of characterization facility at Centre for Nanoscience and Nanotechnology, Jamia Millia Islamia, New Delhi, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Jahan, F., Ashraf, W., Siddiqui, A.M., Khanuja, M. (2023). Enhanced Photocatalytic Activity of Hydrothermally Synthesized Nanostructured Monoclinic BiVO4 Nanosheets. In: Khan, Z.H., Jackson, M., Salah, N.A. (eds) Recent Advances in Nanotechnology. ICNOC 2022. Springer Proceedings in Materials, vol 28. Springer, Singapore. https://doi.org/10.1007/978-981-99-4685-3_35
Download citation
DOI: https://doi.org/10.1007/978-981-99-4685-3_35
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-4684-6
Online ISBN: 978-981-99-4685-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)