Abstract
This paper describes the present use of carbon capture and storage in industries such as iron and steel and cement industry. It also describes how implications of carbon capture and sequestration can lead to reduction in global warming and can reduce the hazardous effects of carbon on environment. The major processes involved in carbon capture and sequestration are pre-combustion, post-combustion, and oxy-fuel have also been discussed. Also, the paper highlights how different membranes can contribute towards carbon capture and sequestration. Polymer, carbon molecular sieve, and organic microporous membrane advantages and drawbacks have also been discussed. For gas separation, the major membranes discussed are polymer, carbon sieve, and microporous organic membrane along with their use in different industries. Major focus still relies on how advancement of membrane can be done to have an optimal strategy for carbon capture and sequestration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent studies, the top priority issue in the world of green science is greenhouse gas that is carbon dioxide (CO2) [1]. Carbon capture and sequestration (CCS) is one of the major way of reducing deleterious effects caused by carbon dioxide, which eliminates the impacts of carbon by isolating the molecules of carbon dioxide from emissions and infusing it into geological formations [2].Carbon capture and storage, also known by the acronym CCS, is a group of technology that restricts emissions of the CO2 from breaking into the atmosphere by storing them safely deep underground in dedicated geographical formations. It is important to highlight that CCS is not a new or futuristic technology. For millions of years, natural resources such as oil and gas have been trapped in the pores of the rocks of the earth’s cores. In the same way, the remains of CO2 have been stored deep underground in the pores of the rocks. It is a proven and safe technology that has been in commercial operation since the 1970s.CCS is an adaptable technology that can support global efforts to tackle climate change and decarbonize our economics. First, CCS is a powerful CO2 abatement option for emissions from industries and generation of the power. Support is provided by the CCS for the decarbonization of energy intensive industry including cement, fertilizers, steel, and petrochemicals as well as the electricity system. It allows the supply of clean hydrogen to fuel transport, heating, and industrial processes. Finally, CCS can also be an enabler of negative emissions through direct air capture and bio-energy with CCS. Recently, the technology emerged as the critical technology to avoid significant global warming with the release of the Intergovernmental Panel on Climate Change (IPCC) Special Report on Global Warming of 1.5°C in October 2018. Foremost, emissions of carbon products from industries and power plants cause malicious effects on human respiratory system and global warming and cause other mitigation problems. Also, carbon particles get trapped inside building structures and reduce the strength of infrastructure and it affects the way in which we use our present day fossil fuels. To safeguard our existing infrastructure and continue using our present abundant reserves of domestic fossil fuels, carbon capture and storage is one technology which allows us to reduce consequential greenhouse gas emission. The demand of CCS technology is increasing at brisk pace as it is helpful in the mitigation of carbon dioxide emissions. Initially, the capture of CO2 from ambient air was made materialistic as a pre-analysis for cryogenic air separation in 1950s [3]. Then in 1960s, hydrocarbon fuels were furnished using mobile nuclear power plants by providing carbon captured from air as a feedstock [3]. A wide range of technological options in order to capture carbon from air are available over a period of time, for example, adsorption, absorption, membrane separation, and cryogenic separation. However, current options of carbon storage are mainly restricted to geological storage, ocean storage, and mineralization [4]. Carbon capture and sequestration are a range of technologies that have the capabilities of confining up to 90% of CO2 emissions from nuclear power plants, cement, and steel industries. Carbon capture and storage consists of following main trapping methods: post-combustion, pre-combustion, and oxy-fuel [1, 4, 5]. In post-combustion method, an absorber medium which is filled with liquid solvents like ammonia is used in segregating carbon dioxide from exhaust gas of power plants. Pre-combustion method is mainly applied to cyclic power plant, which includes coal gasification as a process, and it results in the production of faux gas made from noxious carbon monoxide and hydrogen. In oxy-fuel, pure carbon dioxide is produced in the form of exhaust gas by burning coal, natural gas, or oil in an atmosphere where concentration of pure oxygen is unadulterated. However, the discussion about overall economic and environmental effectiveness of CCS system is a topic which can further be improved; main drawback of CCS technologies is the storage options that require further advancements rather than carbon capture technologies which have already been fortified. The abovementioned three processes post-combustion power plants are the major contributors to the greenhouse effects and it needs to be solved foremost. Various technologies which have been developed for carbon capture are physical adsorption, cryogenic distillation, chemical looping, physical and chemical absorption, and membrane separation [4, 6]. Chemical absorption is used widely for carbon separation and it is particularly used for capturing carbon at low partial pressure, and predominant solvents used for chemical absorption are amine and carbonate solutions. Although chemical absorption is mature technology for CO2 capture but there are various drawbacks of this process which are energy-intensive and it requires high cost that leads to major environmental disasters. In comparison with chemical absorption process, membrane technology is gaining more supremacy, and it is documented as competitive technology for separation of air and sweetening of natural gas since 2–3 decades.
In recent times, CCS technologies were studied by many researchers. The present status of carbon capture technologies were reviewed by Zheng and Xu, Pires et al., Tan et al., Leung et al. [7,8,9,10,11,12], and Figueroa et al. which was associated to the progress of certain rules for the application of life cycle assessment (LCA) to CCS that have been provided by Strazza et al. Capturing of carbon can be carried out by combustion process in varying phases [13]. Lior Rao and many others have studied available procedures of post-combustion systems [14, 15]; Jansen et al. reviewed pre-combustion systems and oxy-fuel capture systems, shown in diverse studies [16]. Gas separation membranes, biological separation, cryogenic distillation, adsorption, and absorption process were the most significant CO2 separation techniques used to fulfill the previously mentioned procedures [9, 17,18,19,20,21,22].
The tool used for carbon capture are membranes as they have pore-like structure which helps in trapping significant amount of carbon particles. In simple words, membrane can be defined as a thin, soft, flexible sheet or layer especially derived from animal or plant origin. In carbon capture process, membrane is used as a primary or secondary barrier between two adjoining phases which helps to facilitate movement of substances between adjacent phases. Foremost, advantages of membrane technology are easy adaptability, operational simplicity, low capital cost, and environmental friendly and its design efficiency is good and easy to establish in remote area. However, the membranes which are commercialized at present are not suitable for absorption using amine solutions as they have low flux. Also, it has short life span when it has been exposed to atmosphere of gas stream which contains harmful impurities of acid gases such as NOx and SO2 [4]. Two major types of membranes used are organic and inorganic membranes. Further, organic membranes are divided into different categories which are PES (polyehtersulfone), PVDF (polyvinylidenedifloride), PAN (polyacrylonitrile), and other polymeric membranes. Also, polymeric membranes are extensively used in markets due to their low economical cost. But polymeric membranes endure low mechanical stability and fouling problems which lowers the effectiveness of this category of membrane. To overcome this problem of polymeric membrane, inorganic membrane have been commercialized which possessed high chemical, mechanical, and thermal stability which helps the user to use inorganic membrane in extreme conditions such as corrosive and high-temperature environment. Membranes are used over a diverse range of markets—from medicines to chemical industry and are widely used in medical devices and water treatment. Future predictions on membrane market is estimated to reach 11.95 billion USD by 2021 at a CAGR of 10.3%, and the base year considered for this prediction is 2015 and forecasted period is from 2016 to 2021.
The agenda of this paper is to analyze what can be the carbon capture and sequestration policy. Integrated assessment models have been used by the CSS technology users which have influenced a significant amount of empirical studies. The main governing forces which help us in determining the feasible CSS policy for a simple economy has been considered using a non-realistic model that has been illustrated in this paper. On the contrary, general abatement option can take numerous forms, such as legalizing of forests or reducing the amount of pollution at the host site; in this paper, we have mainly considered carbon capture and sequestration, and we also introduce the miniscule size and a surplus cost of the reservoir. In addition to carbon capture and sequestration, this paper will discuss major feature of different categories of membranes having potential for molecular separation, inspect the drawback that membrane materials may face during their application for intensifying a particular process, and will also help in furnishing ideas for future research and development. This paper will also provide information regarding carbon capture and storage from different industries and power plants.
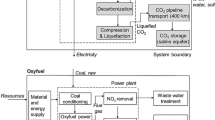
The flow diagram depicts the carbon capture and storage (CCS) process from different industries such as power plant, cement plant, steel works, and petro-chemical plant (Fig. 1). To begin with, carbon generation from different fossil fuels is considered. Coal, oil, and natural gas are the fossil fuels which are major contributors of carbon. The first step in the CCS technique is capturing the carbon from different power plants and industries such as petrochemical plant, steel and iron plants, cement plant, and other modern power plants. The next step involved in the process is treating the captured carbon through pipelines and ships and carrying this carbon to storage facilities. Two types of storage facilities are available for storing carbon and they are the following: on-shore geological storage and off-shore geological storage. In this way, carbon can be treated from different industries and plants, and we can live in a safer milieu.
2 Membrane materials
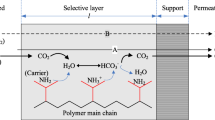
Recent developments in carbon capture and sequestration has led to the utilization of membrane-based processes as the most preferred process. In comparison with the scrubbing process which is used since long time and employs concentrated solutions of amine, membrane-based processes have diverse operational advantages [23,24,25]. The advantage which is of primary importance is that in membrane-based processes there is no need of handling corrosive and noxious solvents which leads to reduce the extra cost required for additional equipment and engineering to shun emissions released by solvents and their byproducts (1). A diverse range of membrane materials have evolved overtime; however, each of the membrane material has its own advantages and drawbacks linked to lifetime, cost, and separation performance. In order to reduce carbon capture cost, advancements in membrane materials are of primary importance. Different membranes which have been developed for carbon separation are fixed-site-carrier (FSC) membranes, mixed material membranes (MMMs), microporous organic polymers, nano membranes, inorganic membranes, and carbon molecular sieve membranes [4, 26]. Many studies have been reported for CO2 capture on various materials such as hybrid organic–inorganic, polymeric, microporous, and facilitated transport membranes [27]. The two major prospects which a membrane should consist in order to operate as required are the following:
A high selectivity so high that purity is achievable undergoing single stage process.
A high permeability so that cross-section area of working can be reduced [27].
Overall, membrane separation technology is a process where semi-permeable membranes are used for separating gas mixtures consisting of two or more components. Advantages of membrane separation are ease of operation, ease of application in isolated places, low capital cost, and low energy consumption. The chemical requirements for membrane separation process are also less as compared with conventional scrubbing process employing amine for use [28]. Aaron and Tsouris analyzed different methods for carbon capture from industries where flue gas is produced using various membranes, solid sorbents, and cryogenic distillation in order to find out the appropriate membrane material to capture carbon from flue gas. We found out that the most favorable method for carbon separation is liquid absorption employing monoethanolamine (MEA), but on the contrary with recent developments, metallic and ceramic membranes are significantly more efficient for membrane diffusion [28]. Each membrane material has varying properties such as thermal, chemical, mechanical, and separation property. To recapitulate, each of the membrane materials have superior separation quality as well as relatively low cost but with these advantages there are some drawbacks like their stability decreases when exposed to adverse environment conditions such as high temperature and pressure, and a decline in the stability of membrane is also evident when exposed to acid gases [4]. To overcome such problems of operation under adverse conditions, inorganic membranes have been developed. But still there are problems of constructions of such modules and also sealing for high temperature applications and cost required is also high. At present, novel membranes such as FSC and MMMs are gaining interest in membrane’s milieu which is established on the basis of transport mechanism and a combination of both inorganic and polymeric material properties. So, on the basis of various studies, a suitable membrane should possess the following characteristics: flow rate, process operating conditions, feed gas composition, material properties, and separation requirements [4, 29]. Recent trends have shown major advancements in membrane performance whose success is mainly attributed to membrane community [4]. Also, stupefying developments have led to the discovery of carbon molecular sieve membranes. Carbon molecular sieve membranes are used along with polymer and microporous membranes in various industries such as coal, steel, and iron. Carbon molecular sieve membranes are usually developed with the help of cellulose derivatives and polymides such as polyacrylonitile and phenylene oxide by the process of carbonization. Further information regarding carbon molecular sieve membrane has been provided in our paper. Wang et al. outlined the performance of single-stage membrane in upper bond plots for CO2/CH4 and also CO2/N2; he also observed that as compared with commercial polymers, ultrathin polymer membranes remained more closer to the upper bonds; this outcome demonstrated their great future for carbon capture applications [30]. In this paper, our major aim will be related to polymeric, carbon molecular sieve and microporous membranes for carbon capture applications. A little while back, Roussanaly et al. undertook a study considering 1600 varying combinations of membranes on the basis of their selectivity and permeability. The study was undertaken by him to design an optimized version of the present two-stage process for each membrane, and the study also included differentiating present membrane process with amine process as a reference. The study was carried out in the Robeson plot for N2/CO2 separation. The main point upon which their analysis was based comprised of a simple cascade strategy and involving no recycles, and after the analysis got completed, they were able to find the state in which the present day membranes can emulate with conventional membrane processes on the basis of cost. The most economical value of selectivity for a particular permeability was discovered (e.g., 40 at 3500 GPU); however, their study was confined to a binary flue gas and a simple cascade strategy [31, 32]. In the future, it is likely that further advancements in membranes process will be discovered.
3 Polymer membrane
Over the past 3 decades, it has been seen that polymer membrane has been considerably applied in petrochemical refinery and CO2 separation on industrial scale [33]. Polymer membrane has been mostly the preferred media for capturing CO2 from post-combustion as it is easy for operation and scaling up is easy and for reliability in performance. Gas separation using polymer membrane falls largely into three different categories: (1) hollow fiber membrane; (2) facilitated transport; and (3) physically selective membrane. These membranes consist some common merits and demerits [34]. They are cost-effective, linear scale up, higher mass transfer rate per volume, more adaptable, foreseeable, and compacted as compared with tray columns. In comparison with the liquid adsorbent scrubbing, separation through membrane has the low driving force as concentration of CO2 in flue gas is low, high transport resistance, smaller flow rate, and devours more energy [35]. For CO2 capture, other prime concerns are the higher temperature and the probable smearing membrane of flue gas [36]. For minimizing cost and bringing reasonable performance of CO2 capture, vacuum, pressure, and dual/multi stages of membrane are employed [37, 38].
Based upon the properties of membrane which are physical and chemical structures, the polymeric membranes implement their process. During the process, interaction between components, membrane, and nature of gas occurs [39]. The polymeric membrane can be categorized into two types: non-porous membrane and porous membrane [40].
Non-porous membrane consists of a dense film and it is also known as dense membrane. Under the main driving force of pressure or concentration gradient, permeate molecules which are absorbed during the process are diffused through polymer matrix. Transport of gases through non-porous membrane is very low, so these membranes are highly demanding [40].
Porous membrane is a distinctly voided structure with casually scattered interconnected pores and having a structure similar to a standard filter. Porous membranes are generally rigid in nature. Based on the distribution of the pore size and molecular size of polymer, separation is reliant on [40].
On the basis of polymer material, polymeric membranes can be classified as follows:
Glassy polymer-The molecules are frozen in an amorphous region of a polymer in a glassy state at very low temperature. These frozen molecules do not have any segmental motion apart from some molecular vibrations. Generally, this glassy form is hard, rigid, similar to crystalline solid, and brittle [41].
Rubber polymer-After heating the material, polymer eventually will reach a glass transition temperature due to which the amorphous region becomes rubbery. At this rubbery state, the polymer will be soft and flexible [42].
The suggestion made by Van der Ent et al. of the grouping of non-porous polymer membranes for enantioseparation is as follows: sorption-selective vs. diffusion-selective [43]. During diffusion, the differentiation of the chiral had been supposed “chiral interactions summation” so that diffusion of one enantiomer becomes faster than the variant. Neglecting some sorption selectivity which existed in those membranes is not because of one to one interaction between the molecules. Further research on sorption selective membrane will be based on performance data and examining result of flux for diffused selective membrane. Nevertheless, effective results can be obtained if non-selective diffusion through the membrane is decreased and selectively absorbed occupants of molecules is maximized. Increasing in “mobility” is observed with increasing enantioselectivity of the membranes with increasing transmembrane potential gradient [44].
Hillmyer et al. had found that hexagonally packed nanocylinders of polylactide (PL) in polystyren (Pst) can be formed from polystyren-block-polylactide copolymers [45, 46]. Pores can be formed in hexagonally packed cylinders of PL by selective hydrolysis of the PL. Ordered and highly porous monoliths can be prepared on the basis of his work with connected and hydrophilic pores (e.g., average spacing ~ 30 nm, average pore diameter ~ 20 nm) [47]. Here, base material for monoliths with a low polydispersity was a PL-poly (N,N-dimethylacrylamide)-PSt triblock copolymer. By cooling from the melt in a channel, die alignment of the phase-separated polymer was fulfilled. Eventually, quantitative polylactide can be removed; the hydrophilic polyacrylamide will cover the pore surface while leaving the PSt matrix.
Triblock copolymer polyisopren-block-poly (2-cinnamoylethyl methacrylate)-block-poly (tert-butyl acrylate) (ABC)-ordered nanochannel film was prepared by Liu et al. [48]. The homopolymerpoly (tert-butyl acrylate) (homo-C) had been mixed with copolymer, and films were formed. In common solvent, these films were casted from solutions. After annealing and drying, the block “B” of the “AB” phase could be utilized for UV-cross-linking. Thereafter, the “homo-C” had been extracted, and a regular pore morphology had been visualized by TEM. The measurements of gas permeability confirmed the films of highly porous nature, but the scarcity of the water permeability proposed that on a macroscopic level the nano channels might be discontinuous, e.g., due to the presence of “grain boundaries” in the film.
The cross-linking of polybenzimidazole had been detailed by Young et al. for improved mechanical properties [49]. Reasonable selectivity and permeability for carbon dioxide separation from methane has been provided in the past patents of unmodified polybenzimidazole [50, 51]. The mechanical properties can be improved by cross-linking of the polymer, by increasing the yield stress of the membrane, which also results in increased performance of separation. Patented example with permeation of CO2 of 7.9 barrer with a selectivity over nitrogen of 27, when cross-linking of the polymer occurred, compared with 0.3 barrer of permeability of CO2 with a selectivity of 18 for unmodified linear polybenzimidazole, tested by single gases at 23°C. The linking agent is responsible for the effect of cross-linking on performance [52].
Solvent resistant polymeric membranes have been presented by Wang and Yeager that can reduce the effects of plastification of hydrocarbons in the feed stream [53]. The patent covers polyether ketone, polyphenylenesulphide, polyimide, polyketone, polyarylene, and polyetherimide which contains intrinsic solvent inertness and hence can withstand organic rich operation conditions. The controlled annealing of hollow fiber polyimide membranes has been described by Ekiner and Simmons [54]. For the polyimide-based membrane, the patented annealing conditions to prevail under a vacuum less than 15 in. of mercury between 100 and 250°C for 6–30 h. Improved chemical resistance can be obtained with these annealing conditions and also ensures that the resulting hollow fibers have required mechanical strength for applications of high temperature and pressure. However, annealing conditions are relatively known for polymeric membranes [55].
4 Microporous organic polymeric material
In addition to membrane materials, microporous materials are one of the applications used in CCS (carbon capture and storage). A microporous material is a material containing pores with diameters less than 2 nm (nanometer). Such materials with small dimensions are capable of holding large surfaces areas, normally 300–1500 m2 g−1. In industry, many types of microporous materials are operated but there are four main materials which are widely used. These are the following: (1) crystalline zeolites (aluminosilicates), (2) activated carbons [56], (3) thermally rearranged (TR) [57,58,59,60,61], and (4) polymers of intrinsic microporosity (PIMs) [62,63,64,65]. As a catalyst or an assisting carrier, however, the size of the pore in crystalline zeolites is too nanoscopic to scatter organic reactant in pore passage, whereas the size of mesophorous (pore diameters between 2 and 50 nm) is too substantial to hold the magnitude of selective properties in catalyzed response. Therefore, super-microporous materials having consistent size holes in 1.0–2.0 nm; sizes are being suited for the technological-based applications [66]. Also, activated carbon is a resourceful material with large surface area, good thermal stability, ample of pore size distribution, and varying functional group at the exterior. Further development on microporous materials was prepared by Park et al. who introduced that polymide-based TR polymers have 0.4–0.9 nm pore size and have also a normal pore size distribution. Moreover, for CO2-related separation processes, TR polymer membranes were found to manifest excellent gas separation performance, for example, at high pressure natural gas sweetening process CO2/CH4 were separated [57, 60]. Another type of microporous materials are polymers of intrinsic microporosity (PIMs), as reported by Budd et al., that show a high surface area (600–900 m2/g) [63] and a high fractional free volume (22–24%) [67]. PIMs engaged in great endorsement due to their high gas permeability, and their relatively slow physical aging. In the last few years, varieties of application have been developed over the decades such as energy storage, adsorption, super capacitor (electrochemical application), and catalysis regarding usage of microporous material in CCS [68].
Based on above information, Shujie, Ke Song, Jingqi Guan, and Qiubinkan have performed a research based on super-microporous material with upgrade on hydrothermal stability. According to the operations reported in the literature, purely siliceous MCM-41 was composed. Many solutions were added and stirred under room temperature to obtain the final proportion MCM-41-T. If MCM-41-T and water were prepended in a glass flask with reflux device, then the sample will be dried out at 110°C for characterization. Also, when characterized powdered catalyst undergo X-ray diffraction, they recorded pattern-like structure. However, XRD patterns of the samples before and after high-temperature treatment show some changes on the framework of mesoporous which has some contraction. Similarly, the results of hydrothermal stability studies suggest that the pore structures have completely destroyed as there is no diffraction peak when fluxed in boiling water. To conclude, comparison of MCM-41 and MCM-41-T suggests that the hydrothermal stability of MCM-41-T has been enhanced remarkably and can make an extensive use in chemical industry [69].
Wei Zhou, Lin Zhang, Putewu, Yaohuicai, Xiao Zhao, and Chunpingyao have prepared a study practically on permeability stability of sand-based microporous ceramic filter membrane. Membrane filtration is an effective process for eliminating organic matter, particles, and microorganisms from drain water [65, 70]. In addition, a number of methods such as chemical vapor deposition, electrochemical deposition, chemical etching and electrospinning have been introduced [71,72,73,74] but such processes are rarely used in large-scale industries because of the complications involved in this processes, and also they are expensive. However, considering a theoretical perspective, few studies have approached to enhance the stability of the permeate flux of the microporous ceramic filter membrane. For sample, preparation, standard sand, and river sand were used where different types of additives were added. After several operations of heating at persistent temperature, ceramic filter membrane was obtained by cooling. Also, there has been a modification in the permeate flux of RS and SS series microporous ceramic filter membranes with respect to seepage time. Similarly, microporous ceramic filter membrane shows a certain impact on the flow rate under definite working pressure. However, there have been some factors that affect changes in permeate flux such as porosity, microstructure, and phase composition but by adjusting porosity, improving surface properties of materials, selecting an appropriate matrix material, and pore former improvement can be brought in permeate flux of ceramic membrane. To conclude, the development of the water film in the inner barrier of the pore is a very convoluted and interesting method [65].
4.1 Carbon molecular sieve membrane
To begin with, the use of carbon molecular sieve membrane for separation of gas gained some interest in 1980s with the effort of Koresh and Soffer [75,76,77]. As discussed earlier, carbon molecular sieve membranes (CMSMs) are prepared by the process of carbonization of polymide and cellulose derivatives under restraint atmospheric conditions backing a specific temperature. Carbon molecular sieve membranes are used successfully since a long time as adsorbents in area of gas separation as they are effective in separation process with molecules having the same structural dimensions [78,79,80]. They are widely used in industries as carbon molecular sieve membranes have excellent mechanical strength and an average modulus due to their turbostratic and graphitic structures as compared with conventional graphitized fibers [4, 81]. With advancements in membrane society, newer and better carbon molecular sieve membranes have been developed with extraordinary gas separation features, and this was possible with the help of pyrolysis of polymeric materials [78, 82, 83]. While carbon sieve membranes have atypical separation properties with purest form of feed gas, the properties of carbon possess some malicious drawbacks to compounds found in some industries [78]. Non-polar surfaces of carbon make them organophilic. This property of carbon makes them exceptional adsorbents for removing harmful particles from process streams, but on the contrary it also possesses some problems in membrane applications [78]. The mechanism employed in carbon molecular sieve membrane is based on kinetic diameter difference in the gaseous molecules [4]. Also, CO2 molecules have the least kinetic difference as compared with N2 and O2 which enhances its separation performance [4]. In the separation process using CMSMs, atoms of varying sizes (essentially hydrogen and oxygen) that had form polymeric chains get progressively detached and form amorphous and porous carbon skeleton, leading to disarray graphene layers [84]. Pores are formed due to the distorted graphene layers which are accountable for molecular sieving effect [84]. A feature of CMSMs that is of utmost importance is that it is possible to alter the pore network in carbon molecular sieve membrane by different heat treatment methods or employing set of pre and post-heat treatment methods [84]. The selectivity and permeability properties of carbon membrane are different from that of polymer membrane.
The following are the advantages of carbon membrane as compared with polymer membrane:
- 1)
Selectivity and permeability combinations available are better than any known polymer membrane.
- 2)
Carbon membranes have the ability to resist high pressure deviations and they have good mechanical strength for given a wall thickness.
- 3)
Carbon membranes have good stability at high temperatures [85].
Although CMSMs have many advantages but there are some drawbacks which cannot be neglected. CMSMs are brittle, because of this reason their module construction is difficult and also their operation cost is high [4, 83, 86,87,88,89,90,91].Taking into considerations future commercial applications of carbon molecular membrane, significant and stupefying advancements need to be developed in the membrane sector. Especially developments in carbon membranes bonded by tubular ceramic and asymmetric hollow fiber carbon membranes need to be evolved [4]. Xu et al. carried out a study in which for olefin/paraffin and ethylene/ethane separations he developed asymmetric hollow fiber carbon membrane. And for organic separations employing liquid as a medium, PVDF-based asymmetric hollow fiber carbon membranes were delineated [4, 92,93,94]. The results of their investigations exhibited that carbon membranes are widely used in energy-related procedures. Also, recently, Richter et al. patented a carbon membrane which was braced by high flux ceramic having a selectivity and permeance of 30 and 0.6 m3 (STP), respectively. The membrane developed was used to remove CO2 from natural gas. Still, cost of membrane and up-scaling of it needs to be further scrutinized [95]. Barsema et al. carried out an experiment by incorporating Ag particles into CMS matrix. From his experiment, he found out that integrating nano size Ag particles into CMSMs membrane leads to the increase in the selectivity and permeability as compared with Ag free CMSMs membrane [85].
5 CCS in industries
As mentioned earlier, there is a widespread use of CCS in various industries. The industries which are considered in our paper are mainly steel, iron, and cement. The time in which carbon gets captured and cost of capturing carbon are the applications on which CCS technology depends [96]. In order to have a fiscal transportation and storage, dilute streams of CO2 need to be concentrated to a low level and this requires ancillary CO2 separation technologies applied mainly to industries such as boilers, iron and steel, turbines, furnaces, and cement. Modifications required in such cases are difficult to achieve. Foremost, we will consider carbon emissions from iron and steel industries and carbon capture in those industries. Around 80% of the total carbon emissions are attributed to power plants and the remaining 20% carbon is emitted by different industries such as iron and steel and cement and refineries. Iron and steel industry discharges around 5% of the total carbon emissions [33, 97]. The percentage included both direct source and indirect source which are used to run the mills. However, the advantages of using steel and iron balance out the emissions caused by this industries [5]. On an average, around 3.5MtCO2/year is emitted by integrated steel mills as compared with 3.9MtCO2/year of power plants [97, 98]. But on the contrary considering the percentage of emission in steel and iron industry is less than that in sectors such as forestry, agriculture, and transport [5]. The reasons which have led to significant amount of carbon emissions in iron and steel industries are to begin with, there is need of some kind of energy to manufacture steel and this energy is often generated by high carbon emitting fossil fuels; further, steel needs to be produce from iron ore and there is a requirement of reducing agent to do so, usually the reducing agent used is carbon of coal as it is available easily and economically [5]. Integrated steel mills are used for steel production which is situated near to a large river or lake which receives the ore required to manufacture coal through sea transport. Enormous plants are used wherein raw materials are mixed and prepared to meet the enumeration for steel production; within this plant the sinterization of ore is carried out followed by pyrolyzing of coal into metallurgical coke [5]. Blast furnace is used for steel production where coke is the main component. As the raw materials get burnt inside the furnace, a by-product of this reaction is formed known as blast furnace gas which is rich in CO and is used as an energy carrier in steel production. Carbon, in the form of CO and CO2, enters inside the steel mill through blast furnace gas which is utilized on the site and it is the major source of CO2 emissions [97]. Furthermore, coke production consequence is a coke oven gas which is rich in carbon and it ultimately leads to CO2 emissions. Recent advancements in technologies have helped researchers to come up with strategies to reduce carbon emissions in intricate BF+BOF-derived steel mills; however, the results are not satisfactory as compared with total CO2 emissions from the factories. It is possible to capture carbon from integrated iron and steel mills by using flue gases or combustible process gases which are the by-products of combustion process. Few drawbacks are likely to be encountered while using this process as the composition of gases may differ and hence it is not possible to furnish flue gas compositions and impurity levels. Both post and pre-combustion technologies can be applied to flue gas for carbon capture. However, there are some problems of applying this process like variations in composition and impurity levels of carbon emissions. CCS still has to face many precariousness concerning technology preferences, cost, and efficiency.CO2 emissions from iron and steel industries depend upon the type of process used as the emissions of carbon are very site-specific. Iron and steel mills situated in countries like Sweden, UAE, France, and Germany are trying to investigate new processes for CCS through small-scale exhibitions of CO2 seizure such as oxy-fuel, TGR, and DRI [96]. Recently, a large project regarding CCS in iron and steel industry was undertaken by a European company; the project was named Ultra low CO2 steelmaking (ULCOS).The nucleus of the project was to develop better steel production technology with less amount of carbon emissions; their main goal was to reduce the carbon emissions from steel industry by 50% until 2030 (base year 2004) [7]. Lie et al. researched that FSC membranes which are blend of PVAm/PVA have the necessary characteristics required to capture carbon from flue gas in iron and steel-producing industries. Also, Roussanaly et al. delineated a process with different membrane materials in an iron and steel factory, and he was able to find an economical carbon capture process in comparison to other capturing process. Yet the experiment was carried out on feed gas which only includes CO2 and N2; H2 and CO tends to be neglected which do exist in the industry [6]. Hence, the research needs to be carried out again with precise composition of feed gas. Cement is widely used as binder at construction site, when mixed along with sand and rock forms, a binding material is known as concrete. At present, cement production is around 3.3 billion per year and it is likely to increase to 4.5 billion per year by 2050 [99]. Sand, limestone, and clay are used as raw materials to produce cement. Cement industry is liable for around 5–7% of the anthropogenic CO2 emissions globally. Reduction in carbon emissions has been carried out by cement industry by employing methods such as reduction of clinker factor, energy efficiency, and usage of alternative fuels [100]. However, employing such reduction methods are likely to work over a short period of time and also they are not cost-effective. The amount of CO2 emissions which can be reduced depends upon the production process involved in the manufacturing of cement, this is because only 42% of the CO2 emissions are caused by combustion of fuel and the rest of the carbon emission is caused by the chemical reaction between raw materials such as heating of limestone to high temperatures in the presence of oxygen or air. Hence, from the recent trends, it can be said that carbon capture and sequestration (CCS) will play a major role in cement industry in the future. CO2 emissions are released both directly and indirectly by cement industries: limestone calcination is a direct way while burning of fuel is an indirect way of carbon emission. Pre-combustion, post-combustion, and oxy-combustion are the processes by which carbon can be captured in both old and new plants. New cement plants usually utilize pre-combustion process for carbon capture coherent with gasification technologies. In post-combustion method, CO2 is separated from fuel gas leaving behind clinker kiln, and in oxy-combustion method, CO2 is captured by using oxygen of pure form [100]. There is still skepticism about when will the methods for carbon capture be commercially available or it will not until 2020.It has also been researched that CCS will increase cost of production from 40 to 90%. Oxy-fuel combustion process is considered as the most convincing process as it will increase efficiency of cement production. Until now, only a single small-scale project has been demonstrated using post-combustion method. Dry sorbent CO2 technologies were planned by CEMEX with taking economic help from the Department of Energy (DOE), USA. CEMEX reported that wary attention must be given to reduce CO2 emission from cement industry and also reduce cost of production in cement industry [96].
6 Future scope
At present, the deployment and utilization of CO2, from anthropogenic sources, resulted as very low. However, characterization of CO2 capture in industries and power plant plays an important role in removing or reducing CO2 emissions [101]. Also, a number of technologies are employed considering plant location, separation required, and flue gas composition [101]. The result in this research has many advantages not only in CO2 sequestration but also in other gas separation techniques such as solar-to-fuel conversion, H2 production, etc. [102]. New challenges are being developed after utilizing new materials such as development in modern characterization, development in molecular control, and several computational methods which will guide, support, and yield for further refinement to most CCS structure [103].
Furthermore, CO2-to-fuels technology is lacking behind from commercial positioning; to compete with other resources or unprecedented methods, liquid fuels have to be produced, such as coal-to-liquids (CTL) and gas-to-liquids (GTL). Also, high efficiency battery and ultra-capacitors are required for storing and utilizing energy. To conclude, CO2-to-fuels technology remains unresolved because of the flows generated and also lowers thermodynamic efficiency [104].
7 Conclusion
To recapitulate, the primary information provided by this review paper is regarding carbon capture and storage as there is an increase in the amount of pollution all around the world. Carbon capture and storage is on the verge of being demonstrated throughout the world and is a key climate change mitigation technology. At present, various technologies ranging from amine scrubbing to technologies used during 2nd and 3rd generations have been employed with supercilious thermodynamics such as carbonate or chemical looping. A number of technologies have been suggested to capture CO2 directly from air or to utilize the capture CO2 in order to produce useful products. However, extreme care must be taken while evaluating the climate benefits of such process. A number of empirical studies have been motivated by CCS via different models. The findings always indicate that the existing technologies allow sequestrating a fraction of carbon emissions and reach towards a conclusion that early implementation of sequestration can lead to decrease in malicious caused by carbon. Many countries have started deploying CCS in their countries seeing its significant role in controlling CO2 emissions. The major amount of carbon emissions occurs from various industries, and preliminary steps are being taken at base level in order to reduce carbon emissions. The major carbon emitting industries are iron, steel, and cement which have been discussed in this paper. Also, steps taken by these industries with funding from various institutes have also been discussed. In order to capture carbon from these industries, a material known as membrane is used and it has pores to capture carbon within it. Major membranes used at present are polymer membrane, carbon molecular sieve membrane, and microporous organic polymer. The field of membrane is interdisciplinary from the very beginning. Chemical synthesis and structure characterization for membrane materials, model design, biology, membrane formation and modification, and membrane characterization are the major inspirations of membrane formation. At present, absorption is the most widely used method but membrane separation has its own benefits as this process is less energy-intensive and more affordable in comparison to the latter. Different types of membrane can be manufactured depending on fabrication technique and separation method such as polymeric, hollow fiber, or carbon molecular sieve membrane. This diversity feature of membrane allows them to adapt to any type of feed gas depending upon their temperature, pressure, and CO2 concentration. This paper also discusses advantages and drawbacks of polymer, microporous, and carbon molecular membrane. Although there has been stupefying developments to reduce carbon from industries and membranes used, there is still a large room for improvement within industries to reduce carbon emissions to a lower level by taking help from developed countries and organizations.
References
A. Sood, S. Vyas, A review: carbon capture and sequestration (CCS) in India. Int J Mech Eng Technol 8(2), 1–7 (2017)
D. Cebrucean, V. Cebrucean, I. Ionel, CO2 capture and storage from fossil fuel power plants. Energy Procedia 63, 18–26 (2014)
D.W. Keith, G. Holmes, D. St. Angelo, K. Heidel, A process for capturing CO2 from the atmosphere. Joule 2(8), 1573–1594 (2018). https://doi.org/10.1016/j.joule.2018.05.006
X. He, A review of material development in the field of carbon capture and the application of membrane-based processes in power plants and energy-intensive industries. Energy Sustain Soc 8, 34 (2018)
Birat, J.P., 2010 Carbon dioxide (CO2) capture and storage technology in the iron and steel industry Developments and innovation in carbon dioxide (CO2) capture and storage technology, 492–521
X. He, Q. Yu, M.B. Hägg, in Encyclopedia of Membrane Science and Technology, ed. by H. EMV, V. V. Tarabara. CO2 Capture (Wiley, 2013b), pp. 1–2390
J.L. Míguez, J. Porteiro, R. Pérez-Orozco, M.A. Gómez, Technology evolution in membrane-based CCS. Energies 11, 1–18 (2018)
Figueroa, J.D., Fout, T., Plasynski, S.,McIlvried, H., Srivastava, R.D., 2008. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int J Greenh Gas Control.2, 9–\
D.Y.C. Leung, G. Caramanna, M.M. Maroto-Valer, An overview of current status of carbon dioxide capture and storage technologies. Renew Sust Energ Rev 39, 426–443 (2014)
J.C.M. Pires, F.G. Martins, M.C.M. Alvim-Ferraz, M. Simões, Recent developments on carbon capture and storage: an overview. Chem Eng Res Des 89, 1446–1460 (2011)
B. Zheng, J. Xu, Carbon capture and storage development trends from a techno-paradigm perspective. Energies 7, 5221–5250 (2014)
Y. Tan, W. Nookuea, H. Li, E. Thorin, J. Yan, Property impacts on carbon capture and storage (CCS) processes: a review. Energy Convers Manag 118, 204–222 (2016)
C. Strazza, A. Del Borghi, M. Gallo, Development of specific rules for the application of life cycle assessment to carbon capture and storage. Energies 6, 1250–1265 (2013)
Y. Li, Q.M. Wang, P.B. Wang, Evaluation of post-combustion CO2 capture technologies. Adv Mater Res 734–737, 1881–1886 (2013)
A.B. Rao, E.S. Rubin, A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ Sci Technol 36, 4467–4475 (2002)
D. Jansen, M. Gazzani, G. Manzolini, E.V. Dijk, M. Carbo, Pre-combustion CO2 capture. Int J Greenh Gas Control 40, 167–187 (2015)
C. Alie, L. Backham, E. Croiset, P.L. Douglas, Simulation of CO2 capture using MEA scrubbing: a flow sheet decomposition method. Energy Convers Manag 46, 475–487 (2005)
J. Gomes, S. Santos, J. Bordado, Choosing amine-based absorbents for CO2 capture. Environ Technol 36, 19–25 (2015)
N.S. Kwak, J.H. Lee, I.Y. Lee, K.R. Jang, J.G. Shim, A study of the CO2 capture pilot plant by amine absorption. Energy 47, 41–46 (2012)
F. Vega, A. Sanna, B. Navarrete, M.M. Maroto-Valer, V.J. Cortés, Degradation of amine-based solvents inCO2 capture process by chemical absorption. Greenh Gases Sci Technol 4, 707–733 (2014)
G. Xu, F. Liang, Y. Yang, Y. Hu, K. Zhang, W. Liu, An improved CO2 separation and purification system based on cryogenic separation and distillation theory. Energies 7, 3484–3502 (2014)
Y. Tan, W. Nookuea, H. Li, E. Thorin, J. Yan, Evaluation of viscosity and thermal conductivity models forCO2 mixtures applied in CO2 cryogenic process in carbon capture and storage (CCS). Appl Therm Eng 123, 721–733 (2017)
J.A. Swisher, A.S. Bhown, Analysis and optimal design of membrane-based CO2 capture processes for coal and natural gas-derived flue gas. Energy Procedia 63, 225–234 (2014)
N. Du, H.B. Park, M.M. Dal-Cin, M.D. Guiver, Advances in high permeability polymeric membrane materials for CO2separations. Energy Environ Sci 5(6), 7306–7322 (2012)
T.K. Carlisle, E.F. Wiesenauer, G.D. Nicodemus, D.L. Gin, R.D. Noble, Ideal CO2/light gas separation performance of poly(vinylimidazolium) membranes and poly(vinylimidazolium)-ionic liquid composite films. Ind Eng Chem Res 52(3), 1023–1032 (2012)
X. He, Q. Yu, M.-B. Hägg, in Encyclopedia of Membrane Science and Technology, ed. by E. M. V. Hoek, V. V. Tarabara. CO2 Capture (Wiley, 2013b)
B. Belaissaoui, E. Favre, Membrane separation processes for post-combustion carbon dioxide capture: state of the art and critical overview. Oil Gas Sci Technol – Rev IFP Energiesnouvelles 69(6), 1005–1020 (2014)
Khalilpour, R., Mumford, K., Zhai, H., Abbas, A., Stevens, G., 2015. Rubin, E.S., Membrane-based carbon capture from flue gas: a review. J Clean Prod 103, 286–300
K. Ramasubramanian, W.S.W. Ho, Recent developments on membranes for post-combustion carbon capture. Curr Opin Chemeng 1(1), 47–54 (2011)
M. Wang, J. Zhao, X. Wang, A. Liu, K.K. Gleason, Recent progress on submicron gas-selective polymeric membranes. J Mater Chem A 5(19), 8860–8886 (2017)
M.C. Ferrari, D. Bocciardo, S. Brandani, Integration of multi-stage membrane carbon capture processes to coal-fired power plants using highly permeable polymers. Green Energy Environ 1(3), 211–221 (2016)
S. Roussanaly, R. Anantharaman, K. Lindqvist, H. Zhai, E. Rubin, Membrane properties required for post-combustion CO 2 capture at coal-fired power plants. J Membr Sci 511, 250–264 (2016)
J.L. Li, B.H. Chen, Review of CO2 absorption using chemical solvents in hollow fiber membrane contractors. Sep Purif Technol 41(2), 109–122 (2005)
G.H. Bakeri, A.F. Ismail, M. Sharialy-Miassar, T. Matsuaro, Effect of polymer concentration on the structure and performance of polyetherimide hollow fiber membrane. J Membr Sci 363(1–2), 103–111 (2010)
E. Favre, Membrane processes and post combustion carbon dioxide capture: challenges and prospects. Chem Eng J 171(3), 782–793 (2011)
Khalilpour, R., Abbas, A., Lai, Z.P., Pinnau, I., Modelling and parametric analysis of hollow fiber membrane system for carbon capture from multicomponent flue gas processing system engineering, 58(5), 1550–1561
R. Wang, H.Y. Zhang, P.H.M. Feronand, D.T. Liomg, Influence of membrane writing on CO2 capture in microporous hollow fiber membrane contactors. Sep Purif Technol 46(1–2), 33–40 (2005)
X. Wang, X. Han, Application of polymeric membrane in CO2 capture from post combustion. Adv Chem Eng Sci 2, 336–341 (2012)
M. Duval, B. Folkers, M.H.V. Mulder, G. Desgrandchamps, C.A. Smolders, Absorbent filled membranes for gas separation, properties of polymeric membranes by incorporation of microporous absorbent. J. Member. Sci. 80(1), 189–198 (1993)
R. Abedini, A. Mezhadmagshadam, Application of membrane in gas separation processes, its suitability and mechanisms. Petrol Coal 52, 69–80 (2010)
Faculty.uscupstate.edu/elever/polymer%20resources/Glasstrans.html, Accessed 31 Jan 2001
A. Mushtaq, H.B. Mukhtar, A.M. Shariff, H.A. Mannan, A review: development of polymeric blend membrane for removal of CO2 from natural gas. Int J Eng Technol 13(2), 53–60 (2013)
L.Y. Chu, Thermo-responsive membranes for chiral resolution. Smart Membr Mater Syst, 121–143 (2011). https://doi.org/10.1007/978-3-642-18114-6_5
M. Yoshikawa, K. Murakoshi, T. Kogita, K. Hanaoka, M.D. Guiver, G.P. Robertson, Chiral separation membranes from modified polysulfone having myrtenal-derived terpenoid side groups. Eur Polym J 42, 2532–2539 (2006)
M.A. Hillmyer, Nanoporous materials from block copolymer precursors. Adv Polym Sci, 137–181 (2005). https://doi.org/10.1007/12_002
A.S. Zalusky, R. Olayo-Valles, J.H. Wolf, M.A. Hillmyer, Ordered nanoporous polymers from polystyrene–polylactide block copolymers. J Am Chem Soc 124, 12761–12773 (2002)
J. Rzayev, M.A. Hillmyer, Nanoporous polystyrene containing hydrophilic pores from an ABC triblock copolymer precursor. Macromolecules 38(1), 3–5 (2005)
G. Liu, J. Ding, A. Guo, M. Herfort, D. Bazzett-Jones, Macromolecules 30, 1851 (1997)
Young, J.S., Berchtold, K.A., Eric, P., Greenberg, A.R. James, A., Onorato, F., Hopkins, S., 2005. Novel polymeric-metallic composite membranes for CO2 separations at elevated temperatures. 2005 AIChE spring National Meeting, conference proceedings. 1489-1494
Peinemann, K., Nunes, S.P., 2007. Membranes for energy conversion. Wiley-VCH Verlag GmbH & Co. KGaA. 2, 1–286
C. Dye, Robert & Jorgensen, Betty & Pesiri, David. 2004. Meniscus membranes for separations
M. Ulbricht, Advanced functional polymer membranes. Polymer 47(7), 2217–2262 (2006)
Wang, H., Yeager G.W.:US20070556901 2007
Ekiner, O.M., Del, W., 1999. Gas separation membranes of blends of polyethersulfones with aromatic polyimides. 1-10
S.E. Kentish, C.A. Scholes, G.W. Stevens, Carbon dioxide separation through polymeric membrane systems for flue gas applications. Recent Patent Chem Eng 1(1), 52–66 (2008)
G.A. Grant, P.R. Fisher, J.E. Barrett, P.C. Wilson, Removal of paclobutrazol from irrigation water using granular-activated carbon. Irrig Sci 2018(36), 159–166 (2018)
S.H. Han, H.J. Kwon, K.Y. Kim, J.G. Seong, C.H. Park, S. Kim, C.M. Doherty, A.W. Thornton, A.J. Hill, A.E. Lozano, K.A. Berchtold, Y.M. Lee, Tuning microcavities in thermally rearranged polymer membranes for CO2 capture. Phys Chem Chem Phys 14(13), 4365–4373 (2012)
S. Kim, Y. Lee, Thermally rearranged (TR) polymer membranes with nanoengineered cavities tuned for CO2 separation. J Nanopart Res 14(7), 1–11 (2012)
H.B. Park, S.H. Han, C.H. Jung, Y.M. Lee, A.J. Hill, Thermally rearranged (TR) polymer membranes for CO2 separation. J Membr Sci 359(1–2), 11–24 (2010)
H.B. Park, C.H. Jung, Y.M. Lee, A.J. Hill, S.J. Pas, S.T. Mudie, E. Van Wagner, B.D. Freeman, D.J. Cookson, Polymers with cavities tuned for fast selective transport of small molecules and ions. Science 318(5848), 254–258 (2007)
N.B. McKeown, Polymers of intrinsic microporosity. ISRN Mater Sci 2012, 16 (2012)
P.M. Budd, E.S. Elabas, B.S. Ghanem, S. Makhseed, N.B. McKeown, K.J. Msayib, C.E. Tattershall, D. Wang, Solution-processed, organophilic membrane derived from a polymer of intrinsic microporosity. Adv Mater 16(5), 456–459 (2004)
J. Ahn, W.J. Chung, I. Pinnau, J. Song, N. Du, G.P. Robertson, M.D. Guiver, Gas transport behavior of mixed-matrix membranes composed of silica nanoparticles in a polymer of intrinsic microporosity (PIM-1). J Membr Sci 346(2), 280–287 (2010)
W.F. Yong, F.Y. Li, Y.C. Xiao, T.S. Chung, Y.W. Tong, High performance PIM-1/Matrimid hollow fiber membranes for CO2/CH4, O2/N2 and CO2/N2 separation. J Membr Sci 443, 156–169.R (2013)
N.B. McKeown, P.M. Budd, K.J. Msayib, B.S. Ghanem, H.J. Kingston, C.E. Tattershall, S. Makhseed, K.J. Reynolds, D. Fritsch, Polymers of intrinsic microporosity (PIMs): bridging the void between microporous and polymeric materials. Chem Eur J 11(9), 2610–2620 (2005)
N. Du, H.B. Park, G.P. Robertson, M.M. Dal-Cin, T. Visser, L. Scoles, M.D. Guiver, Polymer nanosieve membranes for CO2-capture applications. Nat Mater 10(5), 372–375 (2011)
W. Zhou, L. Zhang, P. Wu, Y. Cai, X. Zhao, C. Yao, Study on permeability stability of sand-based, microporous, ceramic filter membrane. Materials 12, 1–14 (2019)
S. Wu, K. Song, J. Guan, Q. Kan, Synthesis and characterization of super-microporous material with enhanced hydrothermal stability. Bull Mater Sci 34(4), 979–983 (2011)
Y.S. Do, W.H. Lee, J.G. Seong, J.S. Kim, H.H. Wang, C.M. Doherty, A.J. Hill, Y.M. Lee, Thermally rearranged (TR) bismaleimide-based network polymers for gas separation membranes. Chem Commun 52(93), 13556–13559 (2016)
B.S. Karnik, S.H. Davies, K.C. Chen, D.R. Jaglowski, M.J. Baumann, S.J. Masten, Effects of ozonation on the permeate flux of nano-crystaline ceramic membranes. Water Res 39, 728–734 (2005)
A. Reina, X. Jia, J. Ho, D. Nezich, H. Son, V. Bulovic, M.S. Dresselhaus, J. Kong, Large area, few-layer. Graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett 9, 30–35 (2009)
S. Milenkovic, A.J. Smith, A.W. Hassel, Single crystalline molybdenum nanowires, nanowire arrays and nanopore arrays in nickel-aluminium. J Nanosci Nanotechnol 9, 3411–3417 (2009)
M. Li, J. Zhai, H. Liu, Y. Song, L. Jiang, D. Zhu, Electrochemical deposition of conductive superhydrophobic zinc oxide thin films. J Phys Chem B 2003(107), 9954–9957 (2003)
M.E. Boot-Handford, J.C. Abanades, E.J. Anthony, M.J. Blunt, S. Brandani, N. Mac Dowell, J.R. Fernandez, M.C. Ferrari, R. Gross, J.P. Hallett, R.S. Haszeldine, P. Heptonstall, A. Lyngfelt, Z. Makuch, E. Mangano, R.T.J. Porter, M. Pourkashanian, G.T. Rochelle, N. Shah, J.G. Yao, P.S. Fennell, Carbon capture and storage update. Energy Environ Sci 7(1), 130–189 (2014)
M.C. Campo, F.D. Magalhães, A. Mendes, Carbon molecular sieve membranes from cellophane paper. J Membr Sci 350(1–2), 180–188 (2010)
J.E. Koresh, A. Soffer, Molecular-sieve carbon perm-selective membrane. 1. Presentation of a new device for gas–mixture separation. Sep Sci Technol 18(1983), 723–734 (1983)
J.E. Koresh, A. Soffer, Mechanism of permeation through molecular-sieve carbon membrane. Part 1. The effect of adsorption and the dependence on pressure. J Chem Soc Faraday Trans 1(82), 2057–2063 (1986)
C.W. Jones, W.J. Koros, Carbon molecular sieve gas separation membranes-II. Regeneration following organic exposure. Carbon 32(8), 1427–1432 (1994)
J. Koresh, A. Soffer, Study of molecular sieve carbons. Part 1.—Pore structure, gradual pore opening and mechanism of molecular sieving. Journal of the chemical society, faraday transactions 1: Physical chemistry in condensed phases 76, 2457 (1980)
A. Kapoor, R.T. Yang, Kinetic separation of methane—carbon dioxide mixture by adsorption on molecular sieve carbon. Chem Eng Sci 44(8), 1723–1733 (1989)
H. Marsh, F. Rodriguez-Reinoso (eds.), Science of Carbon Materials (Universidad de Alicante, Secretariado de Publications, Spain, 2000), p. 673
Soffer, A., Koresh, J., Saggy, S., 1987. Separation devices. U.S. Patent 4,685,940
X. He, M.B. Hägg, Hybrid fixed–site–carrier membranes for CO2/CH4 separation. ProcEng 44, 118–119 (2012b)
J.E. Koresh, A. Soffer, The carbon molecular sieve membranes. General properties and the permeability of CH4/H2 Mixture. Sep Sci Technol 22(2–3), 973–982 (1987)
J.N. Barsema, N.F.A. van der Vegt, G.H. Koops, M. Wessling, Ag-functionalized carbon molecular-sieve membranes based on polyelectrolyte/polyimide blend precursors. Adv Funct Mater 15(1), 69–75 (2005). https://doi.org/10.1002/adfm.200305155
X. He, J.A. Lie, E. Sheridan, M.B. Hagg, Preparation and characterization of hollow fiber carbon membranes from cellulose acetate precursors. Ind Eng Chem Res 50(4), 2080–2087 (2011)
Kiyono, M., Williams, P.J., Koros, W.J., 2010. Effect of pyrolysis atmosphere on separation performance of carbon molecular sieve membranes. J Membr Sci 3(59(1–2)):2–10
J.A. Lie, M.B. Hagg, Carbon membranes from cellulose and metal loaded cellulose. Carbon 43(12), 2600–2607 (2005)
K.M. Steel, W.J. Koros, Investigation of porosity of carbon materials and related effects on gas separation properties. Carbon 41(2), 253–266 (2003)
H.H. Tseng, A.K. Itta, Modification of carbon molecular sieve membranestructure by self-assisted deposition carbon segment for gas separation. JMembrSci 389, 223–233 (2012)
M. Yoshimune, I. Fujiwara, K. Haraya, Carbon molecular sieve membranes derived from trimethylsilyl substituted poly(phenylene oxide)for gas separation. Carbon 45(3), 553–560 (2007)
L. Xu, M. Rungta, M.K. Brayden, M.V. Martinez, B.A. Stears, G.A. Barbay, W.J. Koros, Olefins-selective asymmetric carbon molecular sieve hollow fiber membranes for hybrid membrane-distillation processes for olefin/paraffin separations. J Membr Sci 423–424, 314–323 (2012b)
L. Xu, M. Rungta, W.J. Koros, Matrimid derived carbon molecular sieve hollow fiber membranes for ethylene/ethane separation. J Membr Sci 380(1–2), 138–147 (2011)
D.Y. Koh, B.A. McCool, H.W. Deckman, R.P. Lively, Reverse osmosis molecular differentiation of organic liquids using carbon molecular sieve membranes. Science 353(6301), 804–807 (2016)
H. Richter, H. Voss, N. Kaltenborn, S. Kämnitz, A. Wollbrink, A. Feldhoff, J. Caro, S. Roitsch, I. Voigt, High-flux carbon molecular sieve membranes for gas separation. Angew Chem Int Ed 56(27), 7760–7763 (2017)
IEA (2011) Technology roadmap carbon capture and storage in industrial applications 1–52
M.C. Romano, R. Anantharaman, A. Arasto, D.C. Ozcan, H. Ahn, J.W. Dijkstra, D. Boavida, Application of advanced technologies for CO2 capture from industrial sources. Energy Procedia 37, 7176–7185 (2013)
IPCC, in Prepared by Working Group III of the Intergovernmental Panel on Climate Change, ed. by B. Metz, O. Davidson, H. C. de Coninck, M. Loos, L. A. Meyer. IPCC special report on carbon dioxide capture and storage (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2005)
T. Brown, A. Gambhir, N. Florin, P. Fennell, Reducing CO2 emissions from heavy industry: a review of technologies and considerations for policy makers. Imperial College London, 1–32 (2012)
M. Naranjo, D.T. Brownlow, A. Garza, CO2 capture and sequestration in the cement industry. Energy Procedia 4, 2716–2723 (2011)
S. Joshi, P. Hadiya, M. Shah, A. Sircar, Techno-economical and experimental analysis of biodiesel production from used cooking oil. BioPhys Econ Resour Qual 4(1), 1–6 (2019). https://doi.org/10.1007/s41247-018-0050-7
J.A. Lie, T. Vassbotn, M.B. Hägg, D. Grainger, T.J. Kim, T. Mejdell, Optimization of a membrane process for CO2 capture in the steelmaking industry. IJGGC 1, 309–317 (2007)
S. Roussanaly, R. Anantharaman, Cost-optimal CO2 capture ratio for membrane-based capture from different CO2 sources. Chem Eng J 327, 618–628 (2017)
D.M. D’Alessandro, B. Smit, J.R. Long, Carbon dioxide capture: prospects for new materials. Angew Chem Int Ed 49(35), 6058–6082 (2010)
Acknowledgments
The authors are grateful to Indus University and School of Technology, Pandit Deendayal Petroleum University for the permission to publish this research.
Availability of data and material
All relevant data and material are presented in the main paper.
Author information
Authors and Affiliations
Contributions
All the authors make substantial contribution in this manuscript. SS, MS, AS, and MS participated in drafting the manuscript. SS, MS, and AS wrote the main manuscript; all the authors discussed the results and implication on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Rights and permissions
About this article
Cite this article
Shah, S., Shah, M., Shah, A. et al. Evolution in the membrane-based materials and comprehensive review on carbon capture and storage in industries. emergent mater. 3, 33–44 (2020). https://doi.org/10.1007/s42247-020-00069-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-020-00069-2