Abstract
A small-scale granular-activated carbon (GAC) system was evaluated for removal of the plant growth regulator paclobutrazol [(2RS,3RS)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pentan-3-ol] from water. A solution with 50 µg L−1 of paclobutrazol was passed through canisters filled with 0.50–4.75 mm particle size (8 × 30 US mesh) granular-activated carbon at a flow rate of 6 L min−1. Paclobutrazol solution was exposed to varying amounts of contact time with GAC by increasing the number of filters in series. Analysis of samples using liquid chromatography–mass spectrometry (LC-MS/MS) found that paclobutrazol concentration decreased by 90 and 99% with a contact time of 12 and 59 s, respectively. In bioassay tests, broccoli hypocotyls at 14 days were 104% longer and begonia dry mass was 36% greater when treated with solutions that had a contact time of 59 s compared with the 0 s of GAC exposure. With the highest GAC contact time, begonia dry mass was the same as for plants treated with a zero paclobutrazol solution. Bituminous coal and coconut shell GAC sources were equally effective in reducing paclobutrazol concentration based on broccoli hypocotyl length, and paclobutrazol concentration measured using gas chromatography–mass spectrometry (GC-MS). Removal of paclobutrazol was not affected by solution pH from 4.0 to 10.0.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant grower operations are increasingly adopting capture and reuse of irrigation water runoff to improve water use efficiency, reduce water and fertilizer cost, and reduce discharge of contaminants into the environment (Uva et al. 2001; Beerling et al. 2014; Raudales et al. 2016). However, recirculated irrigation water may contain residual plant growth regulators (PGR), pesticides, herbicides, and other agrichemicals that can negatively affect overall crop quality (Adriansen 1989; Hwang et al. 2008; Agrawal et al. 2010; Smith and Santo 2011; Fisher et al. 2016).
A commonly used plant growth regulator for greenhouse floriculture crops is paclobutrazol [(2RS,3RS)-1-(4-chlorophenyl)-4,4-dimethyl-2-(1H-1,2,4-triazol-1-yl)pentan-3-ol] (Latimer 2015). Paclobutrazol is used on a wide range of plant species to reduce plant height, being taken up quickly (within 30 min) after application (Runkle 2012). Paclobutrazol has a half-life of around 6 months (164 days) in water and can suppress growth of sensitive crops such as begonia in the parts per billion (µg L−1) range (Million et al. 2002; MDEP 2012). Crop sensitivity and the degree of growth inhibition caused by paclobutrazol can vary between species and cultivars. For instance, a 10% reduction in overall plant growth was shown for begonia, impatiens, chrysanthemum, and petunia when applied at paclobutrazol concentrations of 2, 7, 10, and 130 µg L−1, respectively (Million et al. 1999b). Paclobutrazol is typically applied as a foliar spray at concentrations ranging from 1 to 200 mg L−1 or as a substrate drench application at 0.1–8.0 mg L−1 (Whipker 2015). Application via a substrate drench or subirrigation application is common because it results in uniform height control compared to a foliar spray (Million et al. 1999a, 2002). However, drenching and subirrigation increase both the applied volume and potential for residual chemical to contaminate recirculated irrigation water. For example, Hwang et al. (2008) reported that paclobutrazol treatment solution applied and recycled to kalanchoe through a subirrigation system required five irrigation events before the original paclobutrazol concentration decreased by 50%.
The discharge of water containing synthetic organic contaminants such as paclobutrazol can be a serious problem for the terrestrial and aquatic environments. Persistence of paclobutrazol in the soil has been shown to be as long as 3 years under field conditions (Jacyna and Dodds 1995). Paclobutrazol accumulation in the soil can negatively inhibit microbial growth (Maganhotto et al. 2002). The aquatic invertebrate Daphnia magna (water flea) exhibited physical deformities and death when exposed to a 240 µg L−1 paclobutrazol solution at the embryonic stage (Wang et al. 2011).
Technologies that are currently being used in greenhouse operations for remediating agrichemicals in irrigation water include ozone, hydrogen peroxide, ultra-violet radiation, electrochemical flocculation, and granular-activated carbon (GAC) (van Ruijven et al. 2014). There are numerous advantages of GAC compared with other treatment technologies, including low capital and operating cost, simplicity of use, ability to adsorb a wide range of organic compounds, and potential for GAC to be regenerated or re-activated (Brooks et al. 2000; Foo and Hameed 2009). Carbon adsorption is widely used for groundwater remediation, municipal water filtration, volatile organic compound removal, and cleanup of chemical spills (Ioannidou et al. 2010). Granular-activated carbon has also been proven to be a reliable technology in removing pesticides and herbicides such as 2,4-D, atrazine, alachlor, and carbofuran from waste water (Brooks et al. 2000). One important consideration when using GAC for adsorption of organic contaminants is the carbon source or raw material. Commonly used raw materials include wood, lignite, peat, coconut shell, and bituminous coal (Pollard et al. 1992), with coconut shell and bituminous coal often being favored because of their high carbon content and low cost (Babel and Kurniawan 2002; Jabit 2007). A range in particle sizes for GAC can be used depending on the application. However, 12 × 40 or 8 × 30 US mesh size ranges (1680–420 or 2380–595 µm) are commonly used for large-scale-, liquid phase filtration (Summers et al. 2014). Increasing concentration of suspended solids negatively affects adsorption by GAC, whereas decreasing solution pH and temperature normally improves carbon adsorption of organic contaminants (Brooks et al. 2000; DeSilva 2000).
Research has not been published investigating the remediation of paclobutrazol using GAC filtration, or how parameters such as solution pH and GAC source affect adsorption efficiency for paclobutrazol. Research objectives were to quantify the reduction of paclobutrazol from irrigation water with various contact times of GAC filtration, compare the effectiveness in removal of paclobutrazol between bituminous coal and coconut shell-based GAC, and determine if solution pH affected efficacy of paclobutrazol removal. Each experiment used a 50 µg L−1 initial paclobutrazol concentration, based on pilot data from nursery catchment ponds and collection tanks in the US that found paclobutrazol concentrations as high as 50 µg L−1 (Fisher et al. 2016). Procedures for the begonia and broccoli bioassays used in these experiments were modified from previous paclobutrazol research (Million et al. 1999a).
Materials and methods
Activated carbon system
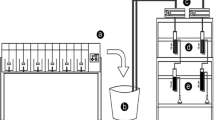
A small-scale GAC system consisting of filter canisters filled with 8 × 30 US mesh GAC was used for three separate experiments. For each experiment, a 50 µg L−1 of paclobutrazol solution was prepared using deionized water and paclobutrazol product, Piccolo (Fine Americas Inc., CA). A sump pump was used to feed the paclobutrazol solution through the GAC filtration system at a constant flow rate of 6 L min−1, maintained by a flow meter and pressure regulator. The filtration system was made up of three filter-housing compartments placed in series, with one small 12.7 cm × 27.9 cm (3.5 L), and two large 12.7 cm × 53.1 cm (6.5 L) housings. Filter housings contained a corresponding filter canister filled with 8 × 30 US mesh (0.50–4.75 mm) coconut shell GAC (Exp. 1) or bituminous coal GAC (Exps. 2 and 3) for the small 10.9 cm × 22.6 cm (1.9 L) and large 10.9 cm × 48.8 cm (4.1L) canisters, respectively. During Experiment 1, a foam pre-filter (386 cm3) was included in the large GAC canisters as recommended by the manufacturer. The addition of a pre-filter resulted in a decrease in total GAC volume and contact time. This pre-filter was not included in GAC canisters for Experiments 2 and 3, thus the calculated contact times were slightly greater compared to Experiment 1. Filters were connected using polyvinyl chloride tubing with 1.27 cm outside diameter. The GAC system was designed to allow paclobutrazol solution to feed through the GAC filters individually or in any combination in series. Desired contact times with GAC were achieved by manipulating the number of GAC filters which the paclobutrazol solution passed through (Table 1). Experiment 1 utilized all filters and Experiments 2 and 3 used one small filter. Each experiment included a non-filtered control treatment of 0 s of contact time where solution was exposed to zero GAC filters.
Contact times in seconds (CT) were calculated using the following equation:
where P represented the pore space of GAC (63%), V was the volume of the filter canisters in liters, and R was the flow rate of chemical solution (L min−1) passing through the filter.
Paclobutrazol sample collection and analysis
Each experiment produced treated samples in blocks separated by time. All canisters were flushed with 50 µg L−1 of paclobutrazol solution for 2 min before beginning each block. For each exposure time run, paclobutrazol solution was then passed through the appropriate filters for 90 s (Exp. 1) and 45 s (Exps. 2 and 3) to ensure that filters completely cycled through with fresh paclobutrazol solution before a 3-L sample was collected. Solutions were collected in plastic containers and kept in a freezer below 20 °C to preserve treated paclobutrazol samples (Altland et al. 2015). Four 15-mL sub-samples from each sample were stored in Falcon collection tubes and later used in a bioassay study. A 500-mL sub-sample from each 3-L sample collected was also sent to a commercial pesticide analysis laboratory (Waters Agricultural Laboratories, GA), where residual paclobutrazol concentrations were determined by a LC-MS/MS (Exp. 1) and GC-MS (Exps. 2 and 3). The control samples that received 0 s contact time or no filters were also included in the analysis.
Bioassays
Broccoli and begonia were chosen as bioassay crops for this set of experiments based on previous trials showing that these species are sensitive to paclobutrazol at concentrations within the µg L−1 range (Million et al. 1999a, 2002). The sub-samples (15 mL) of treated paclobutrazol solution were applied to begonia seedlings [Begonia × semperflorens-cultorum ‘Super Olympia White’ (Knox Nursery, FL)] and broccoli seed [Brassica oleracea var. botrytis ‘Waltham 29’ (Bulk Seed Store, NC)]. Plants were grown in four-cell bedding plant trays (5.7 cm × 3.8 cm × 5.4 cm per cell, 117 cm3) filled with a blond sphagnum peat seedling mix (Pindstrup, Denmark) and grown for a total of 14 days in a growth chamber with minimum/maximum temperatures of 22 and 25 °C, respectively. Plants were placed under fluorescent lights at 100 µmol m−2 s−1 for 18 h resulting in 6.5 mol m−2 day−1.
Bioassays were arranged in a randomized block design with three blocks per species separated by space and crop species. Each four-cell tray represented one sample for each of the experimental blocks. There was one four-cell tray for each sample collected from every contact time treatment. In addition, each bioassay had a control treatment consisting of tap water (0 µg L−1 paclobutrazol). For broccoli, six seeds were planted to a 1 cm depth per cell in each four-cell tray and were thinned to two seedlings per cell 5 days after planting (leaving eight seedlings per four-cell tray). Using a similar procedure, one begonia plug was planted in each cell of a four-cell tray, producing a total of four plants per tray. All planted trays were lightly irrigated overhead to container capacity using tap water and left to sit for 1 h. Each cell of a four-cell tray was then irrigated with 15 mL of a corresponding paclobutrazol solution. Plants were subsequently irrigated as needed with 35 mL of fertilizer solution (20N-4.4P-16.6K, with micro-nutrients at 150 mg L−1) per cell. Broccoli seedlings were cut from the substrate surface and hypocotyl lengths were measured to the nearest mm 14 days after planting. Begonia plugs were separated from their root zones using a razor blade and weighed for dry mass after being oven dried (70 °C) for 48 h. Hypocotyl lengths of the eight broccoli seedlings within a given four-cell tray were averaged as one replicate for statistical analysis. Dry masses of the four begonia plugs per four-cell tray were averaged and analyzed in the same manner. Both bioassay crops were used in Exp. 1 and only broccoli was used in Exps. 2 and 3.
Exp. 1 Removal of paclobutrazol with varying GAC contact time
A 50 µg L−1 of paclobutrazol solution was passed through the small-scale GAC system filled with 8 × 30 US mesh coconut shell-based carbon source (Carbtrol Corporation, CT). A coconut shell GAC source was chosen because it has been reported to effectively remove organic pollutants, and is a globally used carbon source for water remediation (Martin 1980; Ali et al. 2012; Cobb et al. 2012). Filter housings contained a corresponding filter canister filled with 1.25 and 2.50 kg of 8 × 30 US mesh coconut shell GAC for the small 10.9 cm × 22.6 cm (1.9 L) and large 10.9 cm × 48.8 cm (4.1 L) canisters, respectively. This experiment used a randomized complete block design with one factor being GAC contact time with six levels (0, 12, 24, 36, 47, and 59 s) blocked over time. For each block, a freshly mixed paclobutrazol solution was randomly passed through GAC filters to generate three replicate solutions per contact time treatment, generating a total of 54 samples over three blocks (18 solutions per block). A one-way ANOVA was used to show the average reduction in paclobutrazol concentration, hypocotyl length, and begonia dry mass with each contact time treatment. The temperature, electrical conductivity, and pH of the three 50 µg L−1 of paclobutrazol solutions mixed during this experiment ranged from 24.8 to 26.0 °C, 0.99 to 1.53 μS cm−1, and 5.25 to 5.42, respectively.
Exp. 2 GAC source material comparison for paclobutrazol removal
Two commonly used GAC sources, bituminous coal and coconut shell, were compared for paclobutrazol removal. This factorial experiment used a split-plot, randomized complete block design with two factors (two 8 × 30 US mesh GAC sources and two contact times) blocked over time. Samples were generated using one 12.7 cm × 27.9 cm (3.5 L) GAC filter housing and two 10.9 cm × 22.6 cm (1.9 L) filter canisters filled with 1.1 kg of COL-GL 60R bituminous coal-based GAC (Carbon Filtration Systems Inc., RI) and 1.0 kg of coconut shell-based GAC (Carbtrol Corporation, CT). There were three experimental blocks with two contact times (0 and 12 s). Within each block, four samples (two replicates per contact time) were randomly collected using one GAC source and then the canister was switched out with the alternative GAC source where four additional samples were then collected after randomly passing through one or no filters. The order of GAC source used was randomized within each block. The total porosity of both GAC sources was determined to be the same (63% v/v) and their calculated contact times were therefore equivalent.
The temperature, electrical conductivity, and pH of the paclobutrazol tank solution across the three experimental blocks ranged from 25.6 to 27.60 °C, 1.39 to 2.05 μS cm−1, and 5.17 to 5.41, respectively. Sub-samples were taken from each collected solution for a broccoli bioassay study. A two-way ANOVA was used to compare the change in paclobutrazol concentration and broccoli hypocotyl length with change in contact time treatment and GAC sources.
Exp. 3 Paclobutrazol removal using GAC with varying solution pH
Solution pH can influence the absorption rate of organic substances onto activated carbon. Studies have shown that removal of organic contaminants, including various pesticides, using GAC is more effective as solution pH decreases (Semmens et al. 1986; Hong 1998; Srivastava et al. 2009). The effect of solution pH on removal of paclobutrazol by bituminous coal GAC was tested using a randomized complete block design with two factors (four pH levels and two contact times) separated by time. There were four experimental blocks and each block was defined as one replicate solution taken from eight treatment combinations based on varying contact time with GAC (0 and 12 s) and solution pH (4.0, 6.0, 8.0, and 10.0). This experiment utilized one 12.7 cm × 27.9 cm (3.5 L) GAC filter housing and one 10.9 cm × 22.6 cm (1.9 L) filter canister filled with 1.1 kg of COL-GL 60R 8 × 30 bituminous coal-based GAC.
Each tank’s solution pH was adjusted to 4.0, 6.0, 8.0, or 10.0 using hydrochloric acid and sodium hydroxide. Temperature and electrical conductivity measurements across the pH levels and four experimental blocks ranged from 3.6 to 177.3 μS cm−1 and 27.9 to 29.4 °C, respectively. Sub-samples were taken from each collected solution to be used in a bioassay study. A two-way ANOVA was used to compare paclobutrazol concentration and broccoli hypocotyl length with GAC contact time between the four solution pH levels.
Results and discussion
Exp. 1 Removal of paclobutrazol with various GAC contact times
Paclobutrazol concentration decreased as contact time with GAC increased (Table 2). Analysis of paclobutrazol using LC-MS/MS found that the concentration decreased by 90 or 99% with a contact time of 12 or 59 s GAC, respectively, compared to samples that received 0 s GAC. Broccoli hypocotyls were 104% longer and begonia dry mass was 36% greater when treated with solutions that had a 59 s GAC contact time compared with the 0 s GAC treatment. With the highest GAC contact time, begonia dry mass was not significantly different compared with plants treated with the tap water control containing no paclobutrazol (Table 2). However, the broccoli hypocotyl length was 10% shorter for plants treated with samples that received 59 s GAC filtration compared with control plants.
To compare our results with large-scale installations, it is important to recognize that industrial flow rates and GAC filter volumes vary widely depending on the target contaminant, water volume treated, and numerous system design parameters. Activated carbon filtration systems are designed to remove a given contaminant below a target concentration by exposing it to a required contact time in the filter. The actual contact time depends on the volume of the filter bed, the porosity of the GAC within the filter bed, and the flow rate. For ease of filter system design, the contact time is usually described as an empty bed contact time (EBCT), which is calculated from the volume of the filter bed and flow rate, ignoring the porosity of the GAC. The EBCT for one GAC filtration unit, is therefore, calculated by dividing the total GAC tank or bed volume by the flow rate of solution passing through the system. In this experiment, the EBCT of the highest filtration level was 90 s. Industry-scale GAC systems for general water treatment are designed to have an EBCT ranging from 5 to 25 min (Miltner 2007), which was higher than in our study. Typical EBCTs for removal of agrichemicals such as atrazine and 2,4-dichlorophenoxyacetic acid (2,4-D) using GAC range from 10 to 20 min (Summers et al. 2010). However, in our small-scale trial system, a lower EBCT was highly effective presumably because of the low flow rate and lack of other contaminants such as suspended solids or dissolved ions in the water. Published data on GAC filtration efficacy are not available for the triazole chemical class, which contains various agrichemicals including paclobutrazol and uniconazole. However, the triazine chemical class (including atrazine, a commonly used herbicide) has a similar chemical structure to triazoles (Kamrin and Montgomery 2000). Orlandini (1999) reported that an EBCT of 20 min effectively removed atrazine using GAC in a pilot plant scenario for 17 months before becoming 30% saturated with contaminant, which increased to 23 months when solution was pre-treated with ozone prior to entering GAC filtration.
Exp. 2 GAC source material comparison for paclobutrazol removal
Both types of GAC (bituminous coal or coconut shell) were equally effective at reducing paclobutrazol based on both the measured concentrations and the bioassays. Average paclobutrazol concentration was reduced by 91% with 12 s of contact time (Table 3). Broccoli seedlings that received 50 µg L−1 of paclobutrazol without GAC treatment had 45% shorter hypocotyls compared to the control plants. The highest GAC contact time treatment reduced but did not completely eliminate the biological effect of paclobutrazol, resulting in a 15% decrease in hypocotyl length compared with the control plants that received zero paclobutrazol.
Activated carbon quality can be evaluated by several parameters such as iodine number and abrasion number. Iodine number is an indication of general porosity and surface area of GAC products (Miltner 2007). The abrasion or hardness number indicates the resistance to solid carbon breakdown overtime due to physical tumbling. A carbon source with a high abrasion number will be more resistant to breakdown and carbon loss during backwashing (Clements 2002). Coconut shell carbon products usually have higher iodine and abrasion numbers than bituminous coal sources. Iodine numbers for coconut shell and bituminous coal GAC sources in our study were 900 and 1100 mg g−1, and abrasion numbers were 80 and 90, respectively. However, GAC sources can range widely within a particular class of parent material, from 850 to 1300 mg g−1 iodine and 50–99 abrasion values depending on the carbon source and activation method (DeSilva 2000; Potwara 2012; Patil et al. 2013). Greenhouse and agriculture operations can require frequent backwashing of filters due to excessive buildup of suspended solids, dissolved organic compounds, and various salts that may be in irrigation water. Although there was no significant difference in paclobutrazol removal between the two carbon sources, coconut shell carbon could possibly be more suitable for a greenhouse operation based on its higher abrasion and iodine number. Research has shown that coal- and coconut-based GAC are both effective at removing pesticides that fall in the triazine and phenoxyalkanoic acid chemical classes (Hart and Chambers 1991; Cougnaud et al. 2005; Ignatowicz 2009). However, bituminous coal-based GAC products are most commonly used for filtering synthetic organic compounds such as pesticides because of the relatively low cost, product availability, and reliable performance (Chowdhury et al. 2013).
Exp. 3 Paclobutrazol removal using GAC with varying solution pH
There were no differences in reduction of paclobutrazol concentrations between the four pH levels ranging from 4 to 10 (Fig. 1a). The average reduction with 12 s of GAC contact time across all pH levels was 94%. Plants from all pH levels that received 12 s of GAC contact time were 22% shorter than the control plants (Fig. 1b), but showed more growth than plants receiving 50 µg L−1 of paclobutrazol solution with no GAC filtration. Although carbon adsorption of organic compounds has been reported to decrease at high solution pH (Brooks et al. 2000; DeSilva 2000), the lack of pH effect on removal of paclobutrazol in our study is a positive result for GAC use in horticulture, because irrigation water pH can vary widely (Argo et al. 1997).
In a commercial installation of GAC to remove paclobutrazol, several other water quality parameters may influence removal efficacy. Influent concentration of the target contaminant and abundance of competing adsorbates in solution can influence the adsorption efficacy, service life, and backwashing frequency needed for a GAC filtration system (DeSilva 2000; Kennedy and Summers 2015). In general, increasing the influent concentration of a contaminant will increase the percentage of contaminant absorbed onto GAC when filtered (Chowdhury et al. 2013). However, high contaminant concentration also reduces time required for GAC adsorption sites to become saturated with the target contaminant, thereby decreasing the service life of the filter. High amounts of dissolved organic matter or suspended solids (concentrations of 1000 or 50 mg L−1, respectively) can negatively influence adsorption and capacity for removing other target contaminants by competing for adsorption sites. Minimal pre-filtration will also increase backwashing and GAC replacement frequencies. (Freeman and Harris 1995; Corwin and Summers 2012; Kennedy and Summers 2015; Sounthararajah et al. 2015). Fouling of GAC can also occur from biological growth, calcium carbonate, iron, and manganese precipitates (Environmental Protection Agency 1999). Determining the range of influent concentration of a target contaminant and reducing competing compounds such as suspended solids should be prioritized when designing a GAC system for greenhouse production. This can be accomplished through water testing and pre-filtering irrigation water prior to contact with GAC filters.
It is possible that GAC may remediate various paclobutrazol or other agrichemical products differently depending on chemical formulation. GAC could also be effective at removing agrichemicals related to paclobutrazol such as uniconazole and various fungicides categorized in the triazole chemical group. Future research efforts are needed to explore the removal of other common agrichemicals using GAC and the development of best management practices for growers wanting to use this technology. The results from this study have also supported previous studies using broccoli or begonia bioassays as an effective strategy for detecting biologically active concentrations of paclobutrazol without the use of a chemical water analysis (Barrett 2006).
References
Adriansen E (1989) Growth and flowering in pot plants soaked with plant growth regulators solutions in ebb and flood benches. Acta Hortic 251:319–327
Agrawal A, Pandey RS, Sharma B (2010) Water pollution with special reference to pesticide contamination in India. J Water Res Prot 2:432–448. https://doi.org/10.4236/jwarp.2010.25050
Ali I, Asim M, Khan TA (2012) Low cost adsorbents for the removal of organic pollutants from wastewater. J Environ Manag 113:170–183
Altland JE, Morris L, Boldt J, Fisher PR, Raudales RE (2015) Sample container and storage for paclobutrazol monitoring in irrigation water. HortTechnology 25:769–773
Argo WR, Biernbaum JA, Warncke DD (1997) Geographical characterization of greenhouse irrigation water. HortTechnology 7:49–55
Babel S, Kurniawan TA (2002) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater B 97:219–243
Barrett JE (2006) Detecting growth regulator residues. Greenh Product News 13:40
Beerling EAM, Blok C, van der Maas AA, van Os EA (2014) Closing the water and nutrient cycles in soiless cultivation systems. Acta Hortic 1034:49–55
Brooks D, Roll RR, Naylor W (2000) Wastewater technology fact sheet granular activated carbon adsorption and regeneration. Environmental Protection Agency, USA, (832-F-00-017)
Chowdhury ZK, Summers RS, Westerhoff GP, Leto BJ, Nowack KO, Corwin CJ (2013) Activated carbon: solutions for improving water quality. Denver, Colorado
Clements M (2002) Granular activated carbon management at a water treatment plant. Dissertation. Rand Afrikaans University
Cobb A, Warms M, Maurer EP, Chiesa S (2012) Low-tech coconut shell activated charcoal production. Int J Serv Learning Eng 7(1):99–104
Corwin CJ, Summers RS (2012) Controlling organic contaminants with GAC adsorption. Am Water Assoc 104(1):43–44
Cougnaud A, Faur C, Cloirec PL (2005) Removal of pesticides from aqueous solution: quantitative relationship between activated carbon characteristics and adsorption properties. Environ Technol 26:857–866
DeSilva F (2000) Activated carbon filtration. Water Qual Products Mag. https://www.watertreatmentguide.com/activated_carbon_filtration.htm. Accessed 29 Jan 2018
Environmental Protection Agency (1999) Enhanced coagulation and enhanced precipitative softening guidance manual. Environmental Protection Agency, USA, (815-R-99-012)
Fisher P, Grant G, Zayas V, Raudales R, Altland J, Boldt J (2016) Granular activated carbon to remove agrichemicals from water. Greenh Grow Mag, 20–22
Foo KY, Hameed BH (2009) Detoxification of pesticide waste via activated carbon adsorption process. J Hazard Mater 175:1–11
Freeman H, Harris E (1995) Hazardous waste remediation: innovative treatment technologies. Lancaster, Pennsylvania
Hart J, Chambers VK (1991) Removal of pesticides by GAC and GAC/oxidants. Department of the Environment, USA, 2922 https://dwi.defra.gov.uk/research/completed-research/reports/dwi0256.pdf. Accessed 29 Jan 2018
Hong S (1998) The role of pH and initial concentration on GAC adsorption for removal of natural organic matter. Korean Soc Environ Eng 3(4):183–190
Hwang SJ, Lee MY, Sivanesan I, Jeong R (2008) Afr J Biotech 7(10):1487–1493
Ignatowicz K (2009) Selection of sorbent for removing pesticides during water treatment. J Hazard Mater 169:953–957
Ioannidou OA, Zabaniotou AA, Stavropoulos GG, Azharul Islam M, Albanis TA (2010) Preparation of activated carbons from agricultural residues for pesticide adsorption. Chemosphere 80:1328–1336
Jabit NB (2007) The production and characterization of activated carbon using local agricultural waste through chemical activation process. Thesis, Zonguldak Karaelmas University
Jacyna T, Dodds KG (1995) Some effects of soil-applied paclobutrazol on performance of ‘Sundrop’ apricot (Prunus armeniaca L.) trees and on residue in the soil. N Z J Crop Hortic Sci 23(3):323–329
Kamrin MA, Montgomery JH (2000) Agrochemical and pesticide desk reference. CRC Press, Boca Raton, FL
Kennedy AM, Summers RS (2015) Effect of DOM size on organic micropollutant adsorption by GAC. Environ Sci Technol 49:6617–6624
Latimer JG (2015) Growth regulators for containerized herbaceous perennial plants, 2014-15 Guide to Growing Top-Quality Perennials. Grow Talks Mag Ball Publishing, Batavia, IL
Maganhotto CM, Silva S, Vieira RF, Nicolella G (2002) Paclobutrazol effects on soil microorganisms. Appl Soil Ecol 22:79–86
Martin R (1980) Activated carbon product selection for water and wastewater treatment. Ind Eng Chem Product Res Dev 19(3):439
Massachusetts Department of Environmental Protection (2012) Paclobutrazol. The Commonwealth of Massachusetts. Massachusetts Department of Agricultural Resources. https://www.mass.gov/eea/docs/agr/pesticides/rightofway/docs/paclobutrazol-review-jan-2012.pdf. Accessed 29 Jan 2018
Million JB, Barrett JE, Nell TA, Clark DG (1999a) Paclobutrazol distribution following application to two media as determined by bioassay. HortScience 34:1099–1102
Million JB, Barrett JE, Nell TA, Clark DG (1999b) Inhibiting growth of flowering crops with ancymidol and paclobutrazol on subirrigation water. HortScience 34:1103–1105
Million JB, Barrett JE, Nell TA, Clark DG (2002) One-time vs. continuous application of paclobutrazol in subirrigation water for the production of bedding plants. HortScience 37:345–347
Miltner D (2007) Granular activated carbon. Environmental Protection Agency Water Treatment Database, USA. https://iaspub.epa.gov/tdb/pages/treatment/treatmentOverview.do. Accessed 29 Jan 2018
Orlandini E (1999) Pesticide removal by combined ozonation and granular activated carbon. Dissertation. Wageningen University
Patil A, Hatch G, Michaud C, Brotman M, Regunathan P, Tallon R, Andrew R, Murphy S, Strat SV, Kim M, Kappel B, Battenberg G (2013) Granular activated carbon (GAC) fact sheet. Water Quality Association. https://www.wqa.org/Portals/0/Technical/Technical%20Fact%20Sheets/2016_GAC.pdf. Accessed 29 Jan 2018
Pollard SJT, Fowler GD, Sollars CJ, Perry R (1992) Low-cost adsorbents for waste and wastewater treatment: a review. Sci Total Environ 116:31–52
Potwara R (2012) The ABCs of activated carbon. Water Quality Products Magazine. https://www.wqpmag.com/abcs-activated-carbon. Accessed 29 Jan 2018
Raudales RE, Fisher PR, Hall CR (2016) The cost of irrigation sources and water treatment in greenhouse production. Irrig Sci 35:43–54
Runkle ES (2012) Successful use of paclobutrazol. Greenh Product News 22(4):62
Semmens MJ, Norgaard GE, Hohenstein G, Staples AB (1986) Influence of pH on the removal of organics by granular activated carbon. Am Water Works Assoc 78(5):89–93
Smith G, Santo PD (2011) Neutralising pesticides in recirculating water systems within a protected cropping system. Horticulture Australia Ltd., Australia, (Project Number: VG09121)
Sounthararajah DP, Loganathan P, Kandasamy J, Vigneswaran S (2015) Effects of humic acid and suspended solids on the removal of heavy metals from water by adsorption onto granular activated carbon. Int J Environ Res Public Health 12(9):10475–10489
Srivastava B, Jhelum V, Basu DD, Patanjali PK (2009) Adsorbents for pesticide uptake from contaminated water: a review. J Sci Ind Res 68:839–850
Summers RS, Nappe DRU, Snoeyink VL (2010) Quality and treatment: a handbook of community water supplies. Mcgraw, New York
Summers RS, Kennedy AM, Knappe DRU, Reinert AM, Fotta ME, Mastropole AJ, Corwin CJ, Roccaro J (2014) Evaluations of available scale-up approaches for the design of GAC contactors. Water Research Foundation, Environmental Protection Agency, USA (Web Report #4235)
Uva WL, Weiler CT, Milligan RA (2001) Economic analysis of adopting zero runoff subirrigation systems in greenhouse operations in the northeast and north central United States. HortScience 36(1):167–173
van Ruijven JPM, van Os EA, van Der Staaij M, Beerling EAM (2014) Evaluation of technologies for purification of greenhouse horticultural discharge water. Acta Hortic 1034:133–140
Wang KS, Lu CY, Chang SH (2011) Evaluation of acute toxicity and teratogenic effects of plant growth regulators by Daphnia magna embryo assay. J Hazard Mater 190:520–528
Whipker BE (2015) Plant growth regulators for annuals. grower talks magazine. Ball Publishing, 2015 Plant Growth Regulator Guide. http://e-gro.org/pdf/resources/2015-16%20%20PGR%20Guide%20Annuals%20low-res.pdf. Accessed 29 Jan 2018
Acknowledgements
We thank the USDA-ARS Floriculture and Nursery Research Initiative award 58-3607-8-725, the National Institute of Food and Agriculture, USDA, Award 2014-51181-22372, Gene and Barbara Batson Endowed Nursery Fund, and industry partners of the Floriculture Research Alliance (https://www.floriculturealliance.org) for supporting this research.
Funding
This study was funded primarily by the NIFA Grant Clean Water3—reduce, remediate, recycle: informed decision-making to facilitate use of alternative water resources (Grant Number: 2014-51181-22372).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by E. Fereres.
Rights and permissions
About this article
Cite this article
Grant, G.A., Fisher, P.R., Barrett, J.E. et al. Removal of paclobutrazol from irrigation water using granular-activated carbon. Irrig Sci 36, 159–166 (2018). https://doi.org/10.1007/s00271-018-0572-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00271-018-0572-1