Abstract
Metallurgical dust (MD) was used as raw material to prepare rare earth Ce-doped Fe-based catalysts. The results show that the Ce0.1/AMD-300 °C catalyst prepared from acid-modified diatomite (AMD) with mCe/mMD = 0.1 (mCe and mMD are the mass of Ce and MD, respectively) after being roasted at 300 °C can reach 99% NOx removal rate in the wide temperature range of 230–430 °C and exhibits excellent SO2 and H2O resistance. The MD effectively removes alkali metal elements by the modification process, increases the specific surface area and optimizes the pore structure of MD. The doping of Ce element makes Fe-based catalysts have more surface adsorbed oxygen Oα and a higher Ce3+/Ce4+ ratio. Through ammonia temperature-programmed desorption and hydrogen temperature-programmed reduction, it was found that the strong interaction between cerium and iron promotes the formation of more oxygen cavities in the catalyst, thereby generating more active and easily reducible oxygen species and promoting the transformation of Brønsted acid site to Lewis acid site. The research results provide a theoretical basis for the preparation of efficient and inexpensive Fe-based catalysts from MD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metallurgical dust (MD) is the general term for solid waste generated during sintering, ironmaking, steelmaking, and steel rolling. According to statistics, China’s crude steel production was 1.03 billion tons in 2021, accounting for 53% of the total global crude steel production, and a large amount of MD was produced simultaneously, accounting for about 10% of the total steel production. Most of the MD is returned to the blast furnace as raw materials for ironmaking, which is a crude utilization of MD; however, the harmful elements like Na, K, Zn, etc., are enriched in the blast furnace, resulting in blockage of the blast furnace and affecting the smooth operation of the blast furnace [1]. A small portion of MD is used in the construction field, such as road paving or bricks for construction, with low utilization efficiency. MD contains Fe, Al, Si, Zn, and other potential resources [2]. Rough treatment is not only easy to cause environmental pollution, but also a waste of a large number of potentially valuable resources. At present, “treating waste with waste” is one of the measures with significant energy saving and good effect on pollution control. It is a practical direction to prepare MD into adsorbent [3], flocculant [4], catalyst [5], etc., for environmental treatment, which can realize high value-added utilization of MD.
Emissions of NOx have led to a series of serious environmental problems, including the formation of photochemical smog, acidification of soil and freshwater, and impacts on biodiversity and vegetation ecosystems [6,7,8]. Ammonia selective catalytic reduction (SCR) is the current mainstream flue gas denitrification process, mainly due to its mature technology, high denitrification efficiency, system stability, and no secondary pollution [6]. In the past decades, various catalysts have been investigated for flue gas denitrification, including precious metal catalysts [9], metal oxide catalysts [10], molecular sieve catalysts [11], etc. As a commercial catalyst, V2O5–WO3/TiO2 catalyst can maintain a high NOx conversion rate in the range of 300–400 °C but has poor stability in the presence of SO2 and H2O [12]. Therefore, the development of other economical and environmentally friendly SCR catalysts with excellent water and sulfur resistance is still a hot research topic in this field [13, 14]. Metal catalysts are of great interest because of their better water and sulfur resistance and environmental friendliness.
Considering the composition and good plasticity of MD, the synthesis of iron-based catalysts using MD is a feasible research direction [15]. MD is rich in various active components like Fe2O3, Al2O3, TiO2, etc. Among them, Fe2O3 has excellent oxidation ability and surface acid sites, and study has shown that the temperature range of dust iron-based catalysts can be reduced to 200–300 °C and meet the requirements of denitration activity [16]. Huangfu et al. [17] prepared iron-based catalysts from red mud, which exhibited over 80% denitration activity and good H2O and SO2 tolerance above 350 °C. Li et al. [18] treated the red mud catalyst by SO2 activation method to form ferric sulfate species with more reducible Fe3+/Fe2+ active sites to further enhance the denitration activity. Chen et al. [19] prepared red mud catalysts loaded with cerium–tungsten oxides, which produced positive interactions between cerium, tungsten, and iron oxides in red mud to promote the formation of unsaturated chemical bonds and the activation of adsorbed NH3 species activation. It can be seen that the preparation of Fe-based SCR catalysts from MD can achieve high value-added utilization and expand the utilization pathway of metallurgical solid waste. However, due to the characteristics of high alkalinity and low specific surface area of MD, its direct use for NH3 SCR is not satisfactory, and its modification by certain doping can improve the physical and chemical structures of the catalyst, thus enhancing the catalytic performance.
Some studies have shown that rare earth elements have strong interactions with various metal oxides. Appropriate addition of rare earth components to metal-based catalysts can effectively adjust the acidity of the catalyst surface, optimize the structure of the active center, and improve the dispersion of catalyst active components. Among them, CeO2 has excellent redox performance and good oxygen storage and release ability and can significantly enhance the interaction between adsorbed species [20, 21]. Appropriate addition of CeO2 can effectively prevent the sulfation of active sites, reduce the thermal stability of (NH4)2SO4 and NH4HSO4, accelerate the decomposition of (NH4)2SO4, and improve the sulfur resistance of the catalyst [22]. At the same time, it has been reported that, compared with the impregnation method, the sol–gel method, and the hydrothermal method, the co-precipitation method has a stronger interaction between CeO2 and other metals oxides, which is more conducive to the high dispersion of active ingredients [23]. However, there is limited research on the preparation of iron-based catalysts using metallurgical dust containing iron as raw materials.
There have been many studies on the preparation of SCR catalysts from red mud, but there are few reports on the application of other metallurgical solid wastes in the field of SCR. In this study, the sintering machine tail ash was used as the source of active components of the catalyst, and the alkali metal elements in the MD were removed by acid hydrolysis modification to improve the physical and chemical properties of the MD. Then, the rare earth element Ce is doped by the co-precipitation method to exert the interaction between Ce and Fe, thereby improving the low-temperature denitration performance of the catalyst. To fully clarify the differences in the physicochemical properties of the catalysts, the bulk phase characteristics and physical structure of the catalysts were analyzed by X-ray diffraction (XRD), X-ray fluorescence spectroscopy (XRF), Brunauer–Emmett–Teller (BET), and scanning electron microscopy (SEM); and the surface species, surface acidity, and redox properties were analyzed by X-ray photoelectron spectroscopy (XPS), NH3 temperature-programmed desorption (TPD), and H2 temperature-programmed reduction (TPR). The research results can provide a preliminary theoretical basis for the preparation of novel high-efficiency low-temperature denitrification catalysts from MD containing iron, thereby achieving high value-added utilization of MD and expanding the utilization path of metallurgical iron-containing solid waste.
2 Experimental
2.1 Synthesis of catalysts
The original MD was modified by acid hydrolysis and alkali precipitation, and the rare earth element Ce was doped by co-precipitation. MD (20 g) was added into ultrapure water (500 mL), and then, a certain concentration of acid (HCl, HNO3 or H2SO4) was slowly added to adjust the pH to 0, followed by stirring and heating for 4 h. After the solution was cooled to room temperature, a precipitant (NaOH or NH3·H2O) was added to adjust the pH to 10. The obtained suspension was left to age for 24 h, then vacuum filtered, and washed alternately with ultrapure water and ethanol solution, and the filter cake was dried overnight. The dried sample was ground and put into a tube furnace for calcination at a heating rate of 10 °C/min to 500 °C for 4 h. The catalyst powder is compressed and crushed, and 423–635-μm particles are screened out for use. The acid-modified catalyst is named AMD, and according to the acid species, they are named as HNO3-AMD, HCl-AMD, and H2SO4-AMD. According to the type of precipitant, they are named as NaOH-AMD and NH3·H2O-AMD.
MD (20 g) was weighed and put into ultrapure water (500 mL). Then, H2SO4 was slowly added to adjust pH to 0, followed by stirring and heating for 3 h. After that, a certain amount of Ce(NO3)3·6H2O was weighed and dissolved in ultrapure water, and then the reaction solution was added. The solution continued to be stirred and heated for another 1 h. The steps remain unchanged after that. According to the different amounts of Ce doping, they are named Cey/AMD (y = mCe/mMD, where mCe and mMD are the mass of Ce and MD, respectively). According to different calcination temperatures, for example, when y = 0.1, they are named as Ce0.1/AMD-300 °C, Ce0.1/AMD-400 °C, Ce0.1/AMD-500 °C, and Ce0.1/AMD-600 °C.
2.2 Catalyst characterization
Sample elemental composition was detected using an XRF (ARL Advan’X IntellipowerTM 3600, Thermo Fisher, USA). The phase of the samples was detected using an XRD (Ultima IV, Rigaku, Japan), and the scanning angle 2θ ranged from 10° to 80°. The specific surface area, pore volume, pore size, and other information of the sample were detected by N2 adsorption and desorption (ASAP 2460, Mack Instruments, USA). The nitrogen adsorption–desorption isotherm was calculated by the BET equation to obtain the specific surface area, and the pore volume was calculated by the Barrett–Joyner–Halenda (BJH) model. With pore size, the samples were pretreated at 300 °C for 3 h. An SEM scanning electron microscope (NANO SEM430, FEI Corporation, USA) was used to study the surface microstructure of the samples. The elemental composition and chemical state information of the samples were detected with an XPS (K-alpha, Thermo Fisher, USA), and the charge correction was performed using the C 1s (284.80 eV) binding energy as the energy standard. The reduction ability of acid sites and reducible species on the sample surface were investigated by ammonia temperature-programmed desorption and hydrogen temperature-programmed reduction, and the test data were recorded with a thermal conductivity detector (TCD).
2.3 Catalytic performance tests
In this experiment, the fixed-bed catalytic reaction device was used for activity detection, as shown in Fig. 1. The parameters for simulating flue gas denitrification test are 500 × 10−4 vol.% NH3, 500 × 10−4 vol.% NO, 5 vol.% O2, and N2 balance, 5 vol.% H2O (water resistance), 50 × 10−4 vol.% SO2 (sulfur resistance), flue gas flow of 150 mL/min, and gas hourly space velocity (GHSV) of 30,000 h−1. Before the reaction, the impurities on the catalyst surface were removed by purging with N2 at 100 °C for 30 min. The catalyst activity test temperature range is 130–430 °C, and the heating rate is 10 °C/min. The test results were recorded every 50 °C, and the flue gas components were analyzed using a flue gas analyzer (Ecom J2KN, Ecom Instruments GmbH, Germany). The NOx removal rate of the catalyst is calculated according to Eq. (1).
where \({\eta }_{{\text{NO}}_{x}}\) represents the conversion rate of NOx; \({\varphi }_{{\left[{\text{NO}}_{x}\right]}_{\text{in}}}\) represents the volume fraction of NOx at the inlet; and \({\varphi }_{{\left[{\text{NO}}_{x}\right]}_{\text{out}}}\) represents the volume fraction of NOx at the outlet.
3 Results and discussion
3.1 MD raw material characteristics
The MD used in this experiment came from the sintering machine tail ash in Meishan Iron and Steel Group Co., Ltd., China, and its elemental composition was obtained by XRF detection. The results are shown in Table 1. The main element of MD is Fe, and the total iron (TFe) content reaches 55.64 wt.%. The MD contains a small amount of alkali metal elements including Ca, Mg, K, and other elements. Relevant studies have shown that alkali metal elements have no significant effect on the surface Lewis acid sites, but can inhibit the Brønsted acid sites, and inhibit the “fast SCR reaction” by promoting the competitive adsorption between NO and NH3 and weakening the surface O2 adsorption capacity. This leads to catalyst deactivation [24]. Therefore, it needs to be treated to enrich useful elements and remove harmful elements like alkali metals.
Phase analysis of MD was performed by XRD, and the results were obtained by comparing with Jade software and standard card, as shown in Fig. 2. The mineral composition in the original MD is very complex, including iron-containing phases such as magnetite (Fe3O4), hematite (Fe2O3), and siderite (FeCO3), as well as quartz/silica (SiO2), periclase (MgO), gibbsite (Al(OH)3) and other phases, and the test results are consistent with the XRF results in Table 1. In addition, there are some other components in the dust sludge as it is, but they cannot be displayed due to the low degree of crystallinity or the low content. Among them, magnetite has an inverse spinel structure, which is difficult to be effectively leached out by acid and cannot be effectively used; the rest of the quartz phase SiO2 can exist as a catalyst carrier structure, and gibbsite (Al(OH)3) can be present as the active component of the catalyst.
3.2 Catalytic performance
3.2.1 Catalytic activity
It can be seen from Fig. 3 that the NOx conversion rates of all catalysts increase first and then decrease. This is because, as the temperature increases, the reaction rate increases and the NOx conversion rate increases, but when the reaction temperature is too high, it will lead to oxidation and decomposition of NH3, which in turn reduces the NOx conversion [25].
Figure 3a shows the NOx conversion for AMD catalysts modified with different acids and for untreated MD catalysts. Among them, the denitration activity of the MD catalyst is poor, and the denitration rate only reaches 63% at 380 °C. After 380 °C, the denitration efficiency drops sharply to 5.8%. This is because the single iron-based catalyst has a strong oxidizing property, which makes NH3 prone to peroxidation. After modification by acid hydrolysis, the NOx removal rate of the MD catalyst increased significantly, with the highest point of NOx removal rate increased by 20%–30%, and the active range moved to the low-temperature range. For the acidolyzed MD catalysts with different acid types, the order of NOx removal rate is H2SO4-AMD > HNO3-AMD > HCl-AMD. Previous study [26] has shown that sulfuration modification of denitration catalysts can improve the ability of catalysts to resist alkali metal poisoning, and to a certain extent, enhance the acidity of catalysts and the number of chemically adsorbed oxygen, and improve the number of acid sites and redox properties. We speculate that this may be the reason why the H2SO4-AMD catalyst has the best denitration activity.
Figure 3b shows the NOx conversion of AMD catalysts using different precipitants. Compared with the NH3·H2O-AMD catalyst, the denitration efficiency of the NaOH-AMD catalyst is lower, and the highest denitration efficiency is 47%, which is only obtained at 280 °C. This is mainly because the NaOH-AMD catalyst cannot be washed completely, leaving too many Na ions. The residual Na ions will lead to poisoning and deactivation of the catalyst, which greatly reduces the denitration efficiency. Compared with NaOH, using NH3·H2O as a precipitant can avoid the introduction of Na and K impurity ions; besides, the alkalinity of NH3·H2O is moderate, and the pH value range can be accurately controlled by adding slowly and repeatedly, which is conducive to the uniform precipitation of the catalyst.
Figure 3c shows the NOx conversion of Cey/AMD catalysts with different Ce doping amounts. As the Ce doping amount increased from 0.01 to 0.1, the catalysts could maintain nearly 99% activity in a certain temperature range, and the temperature window gradually widened with the doping amount, Ce0.1/AMD (200 °C range) > Ce0.07/AMD (150 °C range) > Ce0.04/AMD (100 °C range) > Ce0.01/AMD (50 °C range). Observing the range of 130–230 °C, it can be found that the low-temperature activity of the catalyst is improved at low doping levels (0.01, 0.04), but with the continuous increase in the doping amount (> 0.04), the low-temperature activity of the catalyst gradually decreased. Compared with Ce0.1/AMD, the NOx conversion rate of Ce0.13/AMD at high temperature was not significantly improved. Based on the consideration of economic and denitration activity, Ce0.1/AMD is considered as the optimal doping catalyst, which can reach nearly 99% denitration activity in the range of 230–430 °C.
Figure 3d shows the NOx conversion of Ce0.1/AMD catalysts at different calcination temperatures. It can be seen that the denitration activity of Ce0.1/AMD catalyst did not change much at 300 and 400 °C. With the further increase in the calcination temperature, the activity range of Ce0.1/AMD catalyst narrowed in the range of 130–330 °C. The denitration activity dropped sharply. Compared with the Ce0.1/AMD-300 °C catalyst, the denitration activity of Ce0.1/AMD-600 °C dropped from 99% to 25% at 230 °C. It can be concluded that the reasonable calcination temperature for the catalyst Ce0.1/AMD is 300–400 °C, and the optimal calcination temperature is 300 °C.
3.2.2 Catalyst water and sulfur resistance
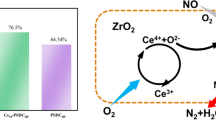
Figure 4 shows the test results of the water and sulfur resistance of Ce0.1/AMD catalyst. When SO2 was fed in, the denitration efficiency of Ce0.1/AMD catalyst dropped from 98% to about 93% within 70 min and quickly recovered to original value after disconnection, showing excellent SO2 tolerance, which was mainly due to Fe-based catalysts having good sulfur resistance and rare earth Ce doping. Relevant studies [27, 28] have shown that the addition of Ce makes SO2 stored in the catalyst in the form of Ce2(SO4)3 to prevent the active components of the catalyst from being deactivated by sulfurization. The principle is shown in Fig. 5. When SO2 and H2O were fed at the same time, the denitration efficiency of Ce0.1/AMD catalyst decreased from 98% to 90% within 60 min and recovered to 95% after disconnection. It can be seen that the coexistence of SO2 and H2O will aggravate the occurrence of catalyst poisoning, and the permanent deactivation of the catalyst is aggravated, mainly because H2O will promote the adsorption of SO2 on the catalyst surface and the deposition of ammonium sulfate on the catalyst surface, thereby affecting the denitration activity.
3.3 Catalyst characterization results
3.3.1 Phase composition
The Cey/AMD catalyst was characterized by XRD, as shown in Fig. 6a. Through the PDF card comparison, the diffraction peaks at 24.13°, 33.12°, 35.6°, 40.8°, 49.4°, 54.0°, 57.5°, 62.4°, and 64.0° correspond to the (012), (104), (110), (113), (024), (116), (122), (214), and (300) facets (PDF#89–0596). Besides, the diffraction peak at 26.6° is the (011) crystal plane of the quartz structure (PDF#85–1053). A series of Ce-doped catalysts prepared by co-precipitation all have good crystal structures without the appearance of impurity peaks. No Ce element-related diffraction peaks are found in Fig. 6, indicating that Ce exists in the catalyst in an amorphous or highly dispersed state. Compared with the undoped catalyst, the diffraction peak intensity of the Fe species in the Cey/AMD catalyst is reduced, mainly because Ce interaction with Fe can improve the dispersion of the active components of the catalyst. It can be seen from Fig. 6b that with the increase in the Ce doping amount, the diffraction peak corresponding to the (104) crystal plane shifts to a smaller 2θ angle, which means that the lattice constant becomes larger, due to the doped Ce ion radius (1.034 Å) larger than the Fe ion radius (0.645 Å).
3.3.2 Microstructure
The microstructure images of different catalysts are shown in Fig. 7. It can be seen that the particle size distribution of the original MD is uneven, and there are many large particles in it. The surface of these large particles is smooth and there is no obvious pore structure, which is not conducive to the NH3 SCR reaction. After the original MD catalyst was treated by acid hydrolysis, the particle size was obviously refined, and a large number of pores were formed. The surface of the AMD catalyst doped with Ce is dominated by spherical particles, and the pore connectivity is good. The spherical particles can provide a larger specific surface area for the catalyst, which is beneficial to the adsorption of the reaction gas on its surface. With the increase in the calcination temperature, the small pores gradually collapsed, there was obvious adhesion and accumulation between particles, and the pores were blocked seriously. When the calcination temperature was raised to 600 °C, the surface of the particles was in a molten state, and no obvious inter-pore channels were observed.
3.3.3 Specific surface area and pores
As shown in Table 2, the specific surface area of the MD catalyst is small, only 3.18 m2/g. After acid hydrolysis treatment, most of the alkali metals in the dust can be removed, forming more pore structures, and the specific surface area is significantly increased to 13.42 m2/g. The developed pore structure helps the gas to diffuse quickly and reach the catalyst surface to participate in the reaction. The size of the surface area can affect the number of reactive sites, thereby affecting the progress of the NH3 SCR reaction [29]. Compared with the undoped Ce catalyst, the iron–cerium composite catalyst has a larger specific surface area, which reflects the interaction between cerium and iron, and can improve the catalyst dispersion and refine the catalyst particle size. Combining the denitration efficiency and NH3 TPD of AMD and Ce0.1/AMD catalysts, it can be seen that the larger specific surface area is conducive to the exposure of more acidic sites, thereby significantly improving the catalytic activity [30].
As the calcination temperature increased from 300 to 600 °C, the specific surface area of Ce0.1/AMD catalyst gradually decreased, which were 29.12, 24.58, 22.49, and 4.68 m2/g, respectively, and the pore size gradually expanded. The main reason is that with the increase in temperature, the pores gradually collapse, making the small pores connect large pores, and thereby reducing the specific surface area and increasing the pore size. When the temperature continued to rise to 600 °C, the specific surface area and pore volume decreased sharply, and the pore size increased significantly, mainly because the sintering phenomenon at high temperature destroyed the pore structure of the catalyst. The specific surface area of the Ce0.1/AMD catalyst at different calcination temperatures is consistent with the change in the NOx conversion rate of the Ce0.1/AMD catalyst, indicating that under the same conditions, the denitrification efficiency of catalyst at different calcination temperatures is mainly affected by specific surface areas.
The N2 adsorption and desorption curves of catalysts AMD and Ce0.1/AMD are shown in Fig. 8. According to the International Union of Pure and Applied Chemistry (IUPAC) classification system, AMD and Ce0.1/AMD catalysts show type II adsorption isotherms, and neither catalyst has a significant adsorption platform, indicating that the pore structure is relatively irregular, which is consistent with the SEM detection of the catalyst, where AMD is an H4-type hysteresis loop, and Ce0.1/AMD is an H3-type hysteresis loop. According to the pore size distribution diagram, the pore size of AMD catalyst is 2–10 nm, and the pore size distribution range of Ce0.1/AMD catalyst is 2–25 nm, which means that the doping of Ce changes the original pore structure, resulting in the increase in pore size. The main reason is that the doping amount of Ce is too large, and the Ce agglomerates on the surface of the catalyst to block the pores.
3.3.4 Surface properties
The surface elements and element valence states of AMD, Ce0.04/AMD, and Ce0.1/AMD catalysts were studied by XPS, and the energy scale was corrected by using C 1s with a binding energy of 284.8 eV as the standard.
The O 1s spectrum of the catalyst is shown in Fig. 9a. It is generally believed that the binding energy in the range of 529–530 eV corresponds to lattice oxygen (denoted as Oβ), and the range of 531–532 eV corresponds to surface adsorbed oxygen (denoted as Oα) [31, 32]. According to Oα/(Oα + Oβ), the Oα ratio was calculated, and the ratios in AMD, Ce0.04/AMD, and Ce0.1/AMD catalysts were 63.47%, 61.31%, and 65.28%, respectively. It can be seen from Table 3 that the doping of a small amount of Ce can increase the amount of lattice oxygen on the surface of the catalyst. When Ce continues to be doped, Oβ will be converted to Oα, which may be due to the interaction between Fe and Ce. As a result, the catalyst acquires additional surface oxygen vacancies. In the SCR reaction, Oα is more active in the oxidation process, which not only promotes the conversion of NO to NO2 but also greatly promotes the redox cycle of the active species [33].
The Fe 2p spectrum of the catalyst is shown in Fig. 9b. Three peaks can be observed from Fig. 9b: the peak near 711, 719, and 724 eV is Fe 2p3/2 peak, satellite peak Fe 2p3/2.sat, and Fe 2p1/2 peak, respectively [34]. The Fe 2p peaks of the three catalysts were divided into four peaks, and it can be seen that the Fe element in the catalysts mainly coexisted in the form of Fe2+ (710.2 eV) and Fe3+ (712, 718, 723.9 eV). The study by Devadas et al. [35] showed that the hydroxyl-bonded Fe3+ can provide a certain weak acid site, which is beneficial to the low-temperature catalytic activity of the catalyst. It can be seen from Table 3 that with the increase in the Ce doping amount, the Fe3+ ratio shows a downward trend, and some electrons in Ce are transferred. Combined with Fig. 3c, this may be the reason why the Cey/AMD catalyst activity decreases with the increase in Ce doping amount in the low-temperature range of 130–230 °C.
The Ce 3d spectrum of the catalyst is shown in Fig. 9c. The Ce 3d characteristic peak intensity of the Ce0.04/AMD catalyst is weaker than Ce0.1/AMD, which is mainly because the Ce0.04/AMD catalyst is less Ce doping, resulting in a weak detection signal. Eight peaks are obtained by fitting the curve, v, v″, v″′, u, u″, and u″′ correspond to Ce4+ (882.0, 889.1, 898.1, 900.8, 906.8, 916.3 eV); while, v′ and u′ corresponds to Ce3+ (885.8, 903.9 eV), and it is generally believed that Ce3+ is in cerium–iron catalysts, because the formation of Ce3+ can increase oxygen vacancies and unsaturated chemical bonds, resulting in charge imbalance, which plays a crucial role in further promoting the surface adsorption of catalysts [36]. Compared with Ce0.04/AMD, the Ce3+ ratio of Ce0.1/AMD catalyst increased to 33.33%, which shifted the redox cycle Fe3+ + Ce3+ ↔ Fe2+ + Ce4+ to the left, inhibiting the formation of Fe3+, consistent with the XPS results of Fe 2p.
3.3.5 Surface acidity and redox
H2 TPR tests were performed on AMD, Ce0.01/AMD, Ce0.04/AMD, Ce0.07/AMD, and Ce0.1/AMD catalysts, and the spectra are shown in Fig. 10a. AMD catalyst has a weak reduction peak at 463 °C, which should be attributed to the reduction of Fe2O3 → Fe3O4, and a strong reduction peak at 608 °C, and is the continuous reduction of Fe3O4 → FeO → Fe [37]. When the Ce doping amount was 0.01 and 0.04, the high-temperature reduction peak remained unchanged while the low-temperature reduction peak intensity gradually increased, and the peak shifted to a lower temperature range, which was consistent with the improvement in the low-temperature activity of the catalyst in Fig. 3c. This phenomenon should be related to the formation of the iron–cerium solid solution by the catalyst. The doping of Ce makes the catalyst to form more oxygen vacancies, thereby generating more active and easily reducible oxygen species. It is worth noting that Ce0.01/AMD and Ce0.04/AMD did not show CeO2 reduction peaks, which should be related to the low doping amount of Ce. When the Ce doping amount continued to increase to 0.07 and 0.1, a strong reduction peak appeared around 490 °C. We believe that this reduction peak corresponds to the reduction of surface Ce4+ to Ce3+, and the reduction of Ce4+ and Fe3+ occurred at the same temperature. It is manifested as the reduction of CeO2, which is mainly caused by the strong interaction and co-reduction of the cerium–iron species in the catalyst [38]. For the Ce0.1/AMD catalyst, when the Ce element is heavily doped, the agglomeration is more serious, which is not conducive to the dispersion of surface active species, resulting in a slight decrease in the reduction peak area of the catalyst compared with Ce0.07/AMD and weakening the redox ability of the catalyst. The Ce0.1/AMD catalyst has the best activity in the case of weak redox ability, which is mainly related to the fact that the Ce0.1/AMD catalyst has more surface active oxygen and acid sites.
The surface acid intensity of the catalyst plays a very important role in the SCR reaction. NH3 TPD is often used to evaluate the surface acid site distribution of catalysts. The obtained NH3 TPD spectrum is shown in Fig. 10b. All catalysts are divided into three desorption peaks by fitting. NH4+ adsorbed on Brønsted acid sites is less thermally stable than NH3 adsorbed on Lewis acid sites, and NH4+ desorbs at low temperatures [39, 40]. In general, the peaks before 250 °C can be attributed to the desorption of NH3 bound to Brønsted acid and weak Lewis acid sites while the peaks after 250 °C correspond to the desorption of NH3 bound to strong Lewis acid sites [41]. The position and area of the desorption peak represent the intensity and number of acid sites, respectively. It can be seen from Table 4 that with the increase in doping amount, the acidity intensity and the number of the catalyst both increase, and the Ce0.1/AMD catalyst has the most strong acid sites and total acid sites. Combined with Fig. 3c, this is the main reason why the catalyst Ce0.1/AMD has the best catalytic activity.
4 Conclusions
-
1.
Acid hydrolysis modification can remove alkali metal components in MD, enhance catalyst stability, improve tolerance to alkali metal poisoning, and optimize catalyst pore structure. Using H2SO4 acid hydrolysis, NH3·H2O precipitation can achieve the best acid hydrolysis modification effect. Sulfation treatment can enhance the acidity of the catalyst and the number of chemically adsorbed oxygen and improve the number of acid sites and redox properties. NH3·H2O has moderate alkalinity, which can avoid the introduction of impurity ions.
-
2.
Among Ce doping modified catalysts, Ce0.1/AMD-300 °C has the best NH3 SCR performance, can reach 99% denitrification rate in the range of 230–430 °C, and exhibits excellent resistance to SO2 and H2O. The strong interaction between Ce and Fe can effectively inhibit the crystallization process of Fe2O3, increase the specific surface area, and make the catalyst have more surface adsorbed oxygen Oα and a higher Ce3+/Ce4+ ratio. The formation of Ce3+ can increase the unsaturated chemical bonds, generate charge imbalance, and further promote the surface adsorption of the catalyst.
-
3.
The doping of a small amount of Ce promotes the formation of more oxygen cavities in the catalyst, thereby generating more active and easily reducible oxygen species. The doping of Ce will make Cey/AMD generate more acidic sites, effectively enhance the NH3 adsorption, and promote the conversion of Brønsted acid sites to Lewis acid sites. The Ce0.1/AMD catalyst has the strongest acid sites and total acid intensity, which is the main reason for its best catalytic activity.
References
S. Singh, A. Thakur, Mater. Today Proc. 46 (2021) 2955–2959.
S. He, L. Lin, Y.Q. Liu, Z.X. Hou, Y. Lv, W. Wu, J. Iron Steel Res. Int. 30 (2023) 697–707.
X.F. She, J.S. Wang, G. Wang, Q.G. Xue, X.X. Zhang, J. Iron Steel Res. Int. 21 (2014) 488–495.
X. Wang, N. Zhang, Y. Zhang, J. Liu, X. Xiao, K. Meng, B. Chu, C. Wang, P.K. Chu, J. Water Process. Eng. 34 (2020) 101173.
W. Shi, H. Ren, M. Li, K. Shu, Y. Xu, C. Yan, Y. Tang, Chem. Eng. J. 382 (2020) 122876.
Z. Zhang, J. Li, J. Tian, Y. Zhong, Z. Zou, R. Dong, S. Gao, W. Xu, D. Tan, Fuel Process. Technol. 230 (2022) 107213.
K. Xiao, R. Guan, J. Yang, H. Li, Z. Yu, S. Liang, W. Yu, J. Hu, H. Hou, B. Liu, Waste Manage. 85 (2019) 452–463.
Y. Xing, H. Zhang, W. Su, K. Li, J. Zhang, J. Shi, J. Tian, J. Wang, J. Clean. Prod. 289 (2021) 125756.
L. Yang, X. You, Z. Sheng, D. Ma, D. Yu, X. Xiao, S. Wang, New J. Chem. 42 (2018) 11673–11681.
S. Xie, L. Li, L. Jin, Y. Wu, H. Liu, Q. Qin, X. Wei, J. Liu, L. Dong, B. Li, Appl. Surf. Sci. 515 (2020) 146014.
B. Liu, N. Lv, C. Wang, H. Zhang, Y. Yue, J. Xu, X. Bi, X. Bao, Chin. J. Chem. Eng. 41 (2022) 329–341.
Y. Zhou, S. Ren, J. Yang, W. Liu, Z. Su, Z. Chen, M. Wang, L. Chen, J. Energy Inst. 98 (2021) 199–205.
Q. Yan, Y. Gao, Y. Li, M.A. Vasiliades, S. Chen, C. Zhang, R. Gui, Q. Wang, T. Zhu, A.M. Efstathiou, Appl. Catal. B: Environ. 255 (2019) 117749.
J. Liu, S. Zhou, H. Cheng, H. Li, W. Zhu, J. Liu, Fuel 318 (2022) 123607.
Z.F. Gao, H.M. Long, T.J. Chun, Z.J. Wu, Z.W. Yu, J. Iron Steel Res. Int. 25 (2018) 19–27.
C.G. Zhang, T.H. Chen, H.B. Liu, D. Chen, B. Xu, C.S. Qing, Appl. Surf. Sci. 457 (2018) 1116–1125.
L. Huangfu, A. Abubakar, C. Li, Y. Li, C. Wang, J. Yu, S. Gao, R. Soc. Open Sci. 6 (2019) 191183.
C. Li, H. Zeng, P. Liu, J. Yu, F. Guo, G. Xu, Z. Zhang, RSC Adv. 7 (2017) 53622–53630.
Q.Z. Chen, D. Wang, C. Gao, B. Wang, S.L. Niu, G.J. Zhao, Y. Peng, J.H. Li, C.M. Lu, J. Crittenden, Green Energy Environ. 8 (2023) 173–182.
S. Wang, Z. Chen, B. He, Z. Yan, H. Wang, L. Liu, X. Wang, Catalysts 9 (2019) 496.
Z. Xiong, Z. Li, Y. Du, C. Li, W. Lu, S. Tian, Environ. Sci. Pollut. Res. 28 (2021) 5914–5926.
N.T. Tinh, N.T.T. Van, N.P. Anh, H.K.P. Ha, N. Tri, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng. 54 (2019) 352–358.
J. Xu, H. Yu, C. Zhang, F. Guo, J. Xie, New J. Chem. 43 (2019) 3996–4007.
P. Raghunath, M.C. Lin, J. Phys. Chem. C 112 (2008) 8276-8287.
J. Liu, X. Li, Q. Zhao, J. Ke, H. Xiao, X. Lv, S. Liu, M. Tadé, S. Wang, Appl. Catal. B: Environ. 200 (2017) 297–308.
J. Zhao, Y. Yue, W. Hua, Z. Gao, Catal. Lett. 116 (2007) 27–34.
M. Peralta, V. Milt, L. Cornaglia, C. Querini, J. Catal. 242 (2006) 118–130.
R. Jin, Y. Liu, Y. Wang, W. Cen, Z. Wu, H. Wang, X. Weng, Appl. Catal. B: Environ.148–149 (2014) 582–588.
F. Gao, X. Tang, H. Yi, J. Li, S. Zhao, J. Wang, C. Chu, C. Li, Chem. Eng. J. 317 (2017) 20–31.
J. Han, J. Meeprasert, P. Maitarad, S. Nammuangruk, L. Shi, D. Zhang, J. Phys. Chem. C 120 (2016) 1523–1533.
M.N. Khan, L. Han, P. Wang, D. Zhang, iScience 23 (2020) 101173.
T. Zhang, S. Ma, L. Chen, R. Li, X. Leng, Y. Li, F. Yuan, X. Niu, Y. Zhu, Appl. Catal. A Gen. 570 (2019) 251–261.
K. Kang, X. Yao, Y. Huang, J. Cao, J. Rong, W. Zhao, W. Luo, Y. Chen, J. Hazard. Mater. 416 (2021) 125821.
F. Liu, H. He, J. Phys. Chem. C 114 (2010) 16929–16936.
M. Devadas, O. Kröcher, M. Elsener, A. Wokaun, G. Mitrikas, N. Söger, M. Pfeifer, Y. Demel, L. Mussmann, Catal. Today 119 (2007) 137–144.
L. Yan, Y. Ji, P. Wang, C. Feng, L. Han, H. Li, T. Yan, L. Shi, D. Zhang, Environ. Sci. Technol. 54 (2020) 9132–9141.
X. Li, J. Chen, C. Lu, G. Luo, H. Yao, Fuel 294 (2021) 120552.
C. Bigey, L. Hilaire, G. Maire, J. Catal. 198 (2001) 208–222.
L. Zhang, L. Shi, L. Huang, J. Zhang, R. Gao, D. Zhang, ACS Catal. 4 (2014) 1753–1763.
R. Jin, Y. Liu, Z. Wu, H. Wang, T. Gu, Chemosphere 78 (2010) 1160–1166.
S. Chen, Q. Yan, C. Zhang, Q. Wang, Catal. Today 327 (2019) 81–89.
Acknowledgements
The research was financially sponsored by the Major Scientific Research Planning Project of Colleges and Universities in Anhui Province (Grant No. 2023AH040147), the National Natural Science Foundation of China (Grant No. 52074093), and the Outstanding Youth Scientific Research Project of Colleges and Universities in Anhui Province (Grant No. 2022AH030044).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Hong-ming Long is an editorial board member for Journal of Iron and Steel Research International and was not involved in the editorial review or the decision to publish this article. The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Zf., Long, Hm., Gao, Xp. et al. Denitrification characteristics and reaction mechanism of Ce-doped Fe-based catalysts from modified metallurgical dust containing iron. J. Iron Steel Res. Int. (2024). https://doi.org/10.1007/s42243-024-01293-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42243-024-01293-4