Abstract
NO emissions from coal combustion are receiving significant attention in recent years. As a solid waste generated from metallurgical industry, metallurgical dust (MD) contains a large amount of metal oxides, such as Fe2O3, CaO, SiO2 and Al2O3, as well as other rare metal oxides. The influence of MD on the NO emissions and the mechanism of the coal combustion systems were analyzed. The results show that the peak values of NO emission decrease with the increase in MD mass percent, and the curve of NO emission can be divided into two stages including rapid generation (400−600 °C) and slow release (800−900 °C). The reduction of NO is significantly affected by temperature, volatile components, O2 and CO. CO has a significant catalytic action which can deoxidize NO to N2. The results obtained by X-ray diffraction and scanning electron microscopy indicate that multiple components in MD, such as Fe9TiO15, Fe2O3 and TiO2, can react with NO to produce TiN. Besides, the alkali metals in MD, such as Na, K and Ca, may catalyze NO precursor to inhibit NO emission. These results indicate that MD is cheap and highly efficient in controlling NO emissions during coal combustion processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Today, coal still serves as a key role in meeting the global energy needs, and air pollution caused by coal combustion has become an increasingly serious problem [1, 2]. NO x , as a series of pollutants produced in coal combustion, has brought great harm to human health and ecological environment, which has caused governments and organizations to strictly control the NO x emission [3].
The nitrogen oxides from coal combustion mainly contain NO and NO2 (approximately 95% NO and 5% NO2). In addition, there is a small amount of nitrous oxide (N2O) produced in coal combustion. However, low toxic NO emissions into the atmosphere can be oxidized to highly toxic NO2 [4, 5]. The main volatile nitrogen species are hydrogen cyanide, HCN, ammonia and NH3 [6, 7]. In recent years, the main methods for controlling NO x include flue gas NO x removal technologies and low NO x burning technologies [8]. Although flue gas NO x removal technologies are efficient in reducing NO x emissions, the operating and mounting costs are high [9,10,11,12]. In addition, pollution emissions of coal blended with metals or alkali have been widely investigated [13,14,15,16,17]. However, current technologies mainly focus on the metal catalysts and catalytic effects of mineral matter in the reduction in NO x emission into the atmosphere [18, 19]. Synthetic or natural agents have high costs, so that the use of solid wastes instead of synthetic agents during coal combustion to reduce NO x emissions has become the research hotspots. However, influences of the solid wastes from metallurgical industry on NO emissions are seldom reported.
As a kind of metallurgical iron-bearing solid waste from steel industry, metallurgical dust (MD) contains a large amount of Fe, Ca, Mg, Si and a variety of trace transition metals (e.g., Mn, Ti, V and Zn), which are the valuable metallurgical secondary resources [20]. The traditional MD utilization research is based on the idea of treatment in a large quantity, and expected to open up a way for utilizing MD in an extensive and low value-added mode. The research and practice mainly include the circulation of MD inside and outside metallurgical factories [21,22,23]. If MD is improperly applied, it will not only be a waste of secondary resources but also cause serious environmental pollutions.
It has been reported that Fe-based compounds, alkaline metal oxides, transition metal oxides and salts have catalytic effect on coal combustion [24]. Therefore, Fe, Na and Ca as catalysts are widely applied to examine the influence of minerals on NO emission from coal combustion [25]. In the present work, the feasibility for using metallurgical solid wastes to reduce NO emissions was investigated, and NO emission was analyzed by thermogravimetry-mass spectrometry (TG-MS), scanning electron microscopy (SEM) and X-ray diffraction (XRD) to better understand the effect of MD combustion on NO emission characteristics.
2 Experimental
2.1 Samples and instruments
The coal sample was provided by Yulin, Shanxi, China and MD came from Ma’anshan Iron and Steel Trade Co., Ltd. Table 1 gives the chemical compositions of MD. Table 2 shows the proximate, ultimate and ash analysis of coal. The particle size and characteristic diameters of MD and coal are shown in Table 3.
The contents of MgO, CaO, Al2O3, SiO2 and Ti were obtained from an inductively coupled plasma (ICP) emission spectrometer (Thermo Elemental-IRIS Intrepid). The mass percent of TFe in MD was measured by titrating dichromate. An X-ray diffractometer (Bruker D8 Advance) was used to determine mineral phases of samples, and the pattern was recorded from 20° to 80° (2θ) with a step size of 0.02° using a counting time of 0.4 s per step. The scanning electron microscope (Hitachi S-3400 N II) and laser particle size analyzer [LS-C (1), China] were used to analyze the morphology and particle size of the samples, respectively.
2.2 Emission characterization system
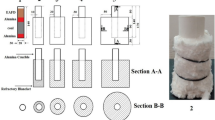
To investigate the influence of MD content on NO emissions, the samples containing 2, 6, 8 and 10% MD were mixed with coal by mass percent. The TG-MS system (France Dorset RAM) was employed to analyze the NO emissions under an air atmosphere with a flow rate of 50 mL/min. The parallel experiments were carried out in a 1500-kW tubular furnace (GSL-1700X, Hefei Branch Crystal), and the schematic diagram of the combustion system is shown in Fig. 1. The samples were placed in the tubular furnace connected by a Fourier transform infrared gas analyzer (GASMETTM DX4000, Temet Instruments, Finland), and then, the samples were heated to 150 °C at a heating rate of 15 °C/min. The same combustion experiments of a 2–10% blend of MD and coals are conducted in the combustion system shown in Fig. 1. Finally, the microstructure and mineral phase of the residues were analyzed by SEM and XRD, respectively.
3 Results and discussion
3.1 TG-MS analysis of samples
Figure 2 shows that the mass loss rate of the sample blended with MD is obviously reduced in combustion process. As shown in Fig. 3, the ignition temperature (the beginning temperature of combustion reaction) of fixed coal in sample decreased and the ignition temperature of raw coal was 452 °C, but the ignition temperature of sample decreased to 430 °C after adding 6% MD. When the mass percent of MD reached 10% in mixed sample, the ignition temperature of sample decreased to 420 °C. The components of MD, such as K, Ca, Na, Fe, Mg and some trace metal elements (Zn, Ni, Cr, Mn, Co, Cu), can be responsible for this phenomenon, owing to their catalytic effects on samples combustion [26].
MD has a significant effect on the sample combustion temperature as shown in Fig. 3. When the combustion reaction is over, the corresponding temperatures of peak values in the curve decrease with increasing the mass percent of MD. However, with the decrease in fixed carbon and organic minerals in mixed samples, organic minerals are difficult to decompose at low temperatures and high temperature is needed for organic minerals to take part in multiphase reactions that are limited from the inside to the outside, which can induce slow burning, prolonging the overall burning time and increasing burning temperature. The catalytic actions of metal ions in MD can promote the formation and strengthening of O–C bonds. The complex CO–M (M stands for metal ion) is formed by an O–C bond and a metal element, which increases the complex reaction as an active center. In addition, a large number of Fe2O3 particles and a few CaCO3 and Al2O3 particles as the oxygen carrier in MD can easily react with H2 and CO released from coal pyrolysis owing to their high activities in combustion processes [27].
3.2 Effect of MD on NO emission
Figure 4 displays that the NO emission can be divided into two stages which are rapid generation and slow release. The curves have bimodal structures with peak values of 400−600 °C and small peak values of 800−900 °C, and the latter is caused by the coke combustion. With the help of computer image processing, the NO peak of different samples was calculated, and NO drainage pattern with 0, 2, 6, 8 and 10% MD is 7185.3 dots, 38,852.4 dots, 29,845 dots, 22,146.5 dots and 15,756.3 dots, respectively, which indicate that the NO emissions decrease gradually.
When 10% MD is added, only one NO peak is generated because the Fe content increases with the increase in MD in mixed samples. Fe can change the lattice structure of carbon, so that the binding force is weakened in structural unit. Therefore, when the coal is decomposed by heating, more bridge bonds are destroyed, which promotes the coal combustion. After the coal is fully burned, it will be beneficial for NO conversion to NO2, thus reducing the release of NO.
The coke is composed of carbon, minerals and a few O, H, S and N, and the nitrogen in coke reacts with O2 to produce NO. In addition, the catalytic effect of alkaline oxides in the coal ash can promote NO conversion to N2, and the melting of minerals in MD at high temperature leads to the closed pores and reduced reactive surface areas owing to the reduction in coke and the mixed alkaline oxide catalyst, inhibiting NO emissions. Regardless of the form of nitrogen in coal, in general, the nitrogen emission is higher in the temperature range of 400−600 °C, because the fuel-NO x can be produced not only through homogeneous reactions but also by multiphase reactions.
In addition, NO formation belongs to fast type at the low-temperature stage of 400−600 °C, and this type of NO is generated in high concentration of fuel in combustion process (excess air coefficient a = 0.7−0.8). Fennimore [28] suggested that the formation of NO was related to the hydrocarbon and nitrogen molecules. Firstly, the CH groups from the fuel reacted with N2 molecule to produce CN compound, and the reaction equation is as follows:
Most of nitrogen exists in the coke when the combustion temperature is relatively low, while 70–90% nitrogen is released through volatilization as temperature is very high. Zhang et al. [29] showed that more than 70% of NO x came from char combustion when the temperature reached 850 °C.
3.2.1 Reduction of NO by temperature
The effect of temperature on NO emission from mixtures is obvious in combustion process. With increasing the combustion temperature, the first peak got wider and preceded that of raw coal. Meanwhile, with the increase in the dosage of MD, the temperature of NO emission increased, but the peak value decreased and narrowed, and the distance between the first peak and the second peak became smaller, so that the NO release time in combustion process became shorter, as shown in Fig. 4. On the whole, the amount of NO emission reaches a peak value at 700−800 °C. Therefore, NO has the formation characteristics at medium temperature, and the amount of fuel nitrogen emission is enough large in this range. However, a rising temperature has little influence on NO emission. According to the TG curves and NO emission peak shown in Fig. 4, NO emission peak appeared after the maximum mass loss of sample; meanwhile, the temperature of NO emission is higher than the ignition temperature of the samples. The process of NO emission is over when the combustion is complete at 850−900 °C.
3.2.2 Reduction of NO by volatile components
The volatile content of samples influences NO emission mainly in two aspects. One is that the first peak value of NO increases with the increase in volatile content, reducing the peak temperature and shortening total emission time. The other is that the first peak area becomes wider and corresponding temperature increases with decreasing the volatile content, and the NO emission amounts decrease with the increase in mixing proportion of MD. When the high volatile sample burns at a high speed and volatile components are released from the coal, the surface of coal has more pores after the release of volatile. Furthermore, the more volatile matter and the more gaps lead to the increasing contact surface (the surface area) of air with coal, and the reductive atmosphere around the pulverized coal particles is conducive to the nitrogen intermediate product reducing N2 [30].
3.2.3 Reduction of NO by O2 concentration
Improving the oxygen concentration has obvious influence on emission characteristics in combustion process, which mainly predicates that with increasing the oxygen concentration, the first NO peak from raw coal exhibits a large emission value, and the oxygen concentration has an effect on the mixed double peak structure of NO emission from coal combustion. Thermal NO evolves from the recombination of N2 and O2, described by De Soete mechanism [31].
The component in the brackets of reaction (4) represents the active position of the sample.
3.3 Effect of CO on NO emission
Figure 5 shows that with the increasing amount of MD, CO emissions increase and the CO concentration reached the peak value at 500−600 °C. With the increase in combustion temperature, CO emission concentration decreased and especially for the 8 and 10% MD addition, CO emissions basically ended at 700 °C. Thus, the influence of MD on CO emission is obvious. The oxygen functional unity can react with Fe3+ to produce Fe(CO)3 on coal particles surface, which is connected with aromatic carbon in coal. Fe3 + with empty orbital can receive solitary electron, whose electronic effect can transfer to carbon ring by oxygen, then forming CO2 and CO from carbon ring rupture. A great deal of Fe particles in MD changes the carbon lattice structure, which weakens the unit structure bridge bond. When the coal is heated to decompose, more bridges are destroyed to promote coal combustion [32].
Comparing Figs. 4 and 5, it can be found that CO emission concentration increases with the increase in MD mass percent, but the change trend of the concentration of CO and NO is opposite at the beginning, and theoretical concentration of NO emission is often higher than the actual concentration. The reason is that parts of NO are reduced to N2 when the nitrogen in coal is oxidized to NO. However, CO has a significant catalytic reduction of NO, as shown in Eq. (5). With the decrease in CO emission concentration, the second peak appears in Fig. 4, and the effect of CO on NO is weakened. The reason may be that the effect of semi-coke increases and the catalytic effects of CO on NO reduction are not significant.
Because of the high concentration of CO and the precursor such as HCN, NH4 and NCO, a part of molecules containing nitrogen react with NO to produce N2, which not only delayed the formation of NO, but also reduced the concentration of NO emissions. In general, the higher concentration of NO in combustion process leads to the higher conversion of NO into N2, which is consistent with the conclusions in the literature [33, 34].
3.4 Mineral phase components and microstructures of residues
3.4.1 XRD analysis
Figure 6 shows the XRD results of the residues obtained from coal combustion at 900 °C. Figure 6a displays that Fe2O3, CaCO3, SiO2 and other amorphous phases are the main mineral phases. In Fig. 6b, Fe2O3, Fe9TiO15, SiO2 and other amorphous phases are the main mineral phases of the residues blended with 2% MD. Fe9TiO15 is not detected in the raw coal residue but is detected in the residue from sample blended with 2% MD.
The residues obtained from coal with 8% MD included Fe9TiO15, Fe2O3, TiO2 and other amorphous phases at 900 °C. TiN appears in the residues from coal with 10% MD. TiO2 is the precursor of TiN, and TiO2 is synthesized by the reaction of TiN with carbon thermal reduction reaction. The reaction mechanism is as follows [35]:
In the reaction process, C has two sources. One is from the free carbon in MD, and the other is from the raw coal. In the combustion process, the increase in the ratio of MD can promote TiN production and then depress the NO emission. Peelamedu [36] studied the transformation process of TiN powder using the high activity of TiO2, and taking C as raw materials, a reaction model was proposed, as shown in Fig. 7.
3.4.2 SEM analysis
Figure 8 displays the SEM images of residues from the mixed samples at a reaction temperature of 900 °C.
Figure 8 indicates that when 0, 2 and 6% MD are added to the raw coal, the surfaces of residues show loose and blocks structures with more microspores, but sintering phenomenon does not occur. However, when 10% MD is added to samples, the morphology of the residues changed significantly, in which the surface appeared as melting slag. Some particles segregation appeared to cause bump and overlap with fewer pores. Besides, some small particles are scattered on the surface, which exhibited a flocculent surface with microspores, and these particles are uniformly and densely covered.
With the increase in MD dosage, the mass percent of alkaline metal oxides and free SiO2 increased. Increasing the alkaline oxides percentage is beneficial for reducing NO. In addition, CaO·2SiO2·Al2O3 appears, whose reactions are shown in the following reactions [37]:
CaO·2SiO2·Al2O3 exhibited a flocculent surface structure; meanwhile, TiN appeared as a sphere as shown in Fig. 9. In addition, TiO2 particles have the catalytic effect on the reaction between SO2/NO and coal fly ash/CaO/CaSO4 sorbents [38, 39].
The SEM morphology and EDS spectrum in Fig. 9 clearly show that the increasing MD percent can change the structures of the residues. Moreover, the increasing TiO2 content can promote the reaction of TiO2 with N to generate TiN. However, MD has more significant effect on inhibiting NO emission from the residues in combustion.
3.5 Influence of MD multiple components on NO emission
Existing research indicates that alkali metal has a catalytic role in the reduction reactions, so that the NO emission is further reduced. When coal incorporated with alkali metal and alkaline earth metal compounds, in the reaction process of metal ions in the embedded carbon lattice of internal combustion, carbon has microstructure changes whose electron transfers and becomes electron donor. Increase in the charge migration promoting carbon surface edge, angle and the defects in the active site can accelerate the speed of oxygen adsorption, reduce the activation and improve the reaction speed, which result in carbon burning fully [40]. Previous study [41] showed that the added sodium carbonate had catalytic reduction actions, whose reaction mechanism was that the products from rapid decomposition of sodium carbonate can react with water to produce sodium hydroxide, carbon dioxide and sodium atoms. These materials containing sodium can consume some active groups, and the reactions are as follows:
Obviously, the concentration of OH and H decreased after the addition of sodium, and the formation of thermal and fuel NO was related to the combustion environment. The reducing concentration of OH and H inhibited the formation of NO.
Wu et al. [42] found that several Fe-bearing compounds had some effects on coal-fired pollutants emissions, but different kinds of Fe-bearing compounds had different mechanisms of coal-fired pollution characteristics. The catalytic effect of FeCl3 is related to the existence of S in coal. The absorption of FeCl3 promotes FeCl3 itself to be able to participate in the absorption of SO2 to generate FeSO4 or Fe2(SO4)3. In addition, FeCl3 can also affect the emission characteristics of CO. The effect of FeCl2 on the emission characteristics of SO2, NO and CO is similar to that of FeCl3 in flue gas, but the effect is slightly worse than that of FeCl3. Fe2O3 is also able to reduce the volume concentration of SO2 and NO in flue gas; however, Fe2O3 is only absorbent, which has no catalytic effect on SO2 and NO generation process, and Fe2O3 has little effect on CO emission.
Ca is an alkaline earth metal element with high content of MD. The NO from coke reduction has obvious catalytic effect [43, 44]. In the CO2 atmosphere, the calcium-based absorbent is closely related to the NO emission peak value and temperature, and the peak value of NO emission decreases when the temperature is high. This is because the addition of the calcium-based absorbent is mainly affected by the following reaction:
At 700 °C, a large amount of N in raw coal is released and converted to NH3. At the same time, CaCO3 does not decompose in CO2 atmosphere, and it is a catalyst for reaction (16) to promote NO production.
In the process of thermal chemical conversion of coal, one part of alkaline substances in MD is volatilized into gas phase, and another part is kept in solid phase in the ash. Because volatilization of solid matter depends on its melting point, the alkaline substances and aluminosilicate compounds in MD have very high melting point, leading to eventual existence in ash. Meanwhile, Na, K and Ca are likely to appear in the inorganic lattice oxygen functional groups as exchanged metal cations. Therefore, in the gasification conditions, Na and K easily evaporate, and Mg, Ca and Al as divalent and trivalent metals in coke or ash have high retention rates and evaporate with increasing the gasification temperature. The basic material can promote NO reduction in the surface of coke in some extent. At the same time, the oxygen-containing functional groups can directly promote the NO reduction. Besides, alkaline substances have catalysis on NO precursor to produce NO. It can be seen that the NO emission is influenced by many factors.
In view of the catalytic action of alkaline minerals on the reduction of NO on the surface of coke, different reaction mechanisms have been put forward. For example, the catalytic reactions of CaO proposed by Illan-Gomez et al. [45] are shown as follows:
As shown in the above reactions, the catalytic reactions of minerals for NO on char surface appear during reduction reaction. First, in the low-valence metal oxide or metal elemental activity, NO adsorption is reduced to produce N2. At the same time, low-valence metal oxide is oxidized to high-valence metal oxides, and minerals of oxidation state around by coke are reduced to low-valence metal oxide or metal elements, then to complete the transmission process of oxygen from NO to coke.
4 Conclusions
-
1.
With increasing the mass percent of MD in coal combustion, the NO emission peak values decreased and the corresponding temperature of NO initial emission increased, but the time of NO emissions was significantly shortened and the peak value of NO emission decreased. The total amount of NO emissions decreased and the curves of NO emissions can be divided into two stages which are rapid generation (400−600 °C) and slow release (800–900 °C). The reduction of NO is obviously affected by temperature, volatile components, O2 and CO. CO has a significant catalytic action on the reduction of NO to N2.
-
2.
The multiple components in MD such as Fe9TiO15, Fe2O3 and TiO2 can react with NO to produce TiN. SEM images displayed that the MD dosage could change the structures of the residues. Moreover, with increasing the TiO2 content, a large number of TiO2 can promote the reaction of TiO2 with N to generate TiN. The alkali metals in MD such as Na, K and Ca can catalyze NO precursor to inhibit NO emission. In addition, some minerals of MD have the catalytic function for NO on char surface in the low-valence metal oxide or metal elemental activity, and then NO adsorption converts to produce N2. At the same time, low-valence metal oxide is oxidized to high-valence metal oxides and minerals in oxidation state around by coke are reduced to low-valence metal oxide or metal elements, then to completing the transmission process of oxygen from NO to coke. These results indicate that MD is cheap and highly efficient in controlling NO emissions during coal combustion processes.
References
E. Rokni, A. Panahi, X.H. Ren, Y.A. Levendis, Fuel 181 (2016) 772–784.
Z.C. Chen, Z.W. Wang, Z.Q. Li, Y.Q. Xie, S.G. Ti, Q.Y. Zhu, Energy 73 (2014) 844–855.
L.B. Duan, Y.Q. Duan, C.S. Zhao, E.J. Anthony, Fuel 150 (2015) 8–13.
D.S. Jin, B.R. Deshwal, Y.S. Park, H.K. Lee, J. Hazard. Mater. 135 (2006) 412–417.
W.D. Fan, Z.C. Lin, J.G. Kuang, Y.Y. Li, Fuel Process. Technol. 91 (2010) 625–634.
Y. Zhao, R.L. Hao, M. Qi, Chem. Eng. J. 269 (2015) 159–167.
A.C. Bose, K.M. Dannecker, J.L. Wendt, Energy Fuels 3 (1988) 301–308.
L. Jia, Y. Tan, E. J. Anthony, Energy Fuels 24 (2010) 910–915.
Y. Liu, F. Rehman, W.B. Zimmerman, Fuel 209 (2017) 117–126.
B.R. Deshwal, D.S. Jin, S.H. Lee, S.H. Moon, J.H. Jung, H.K. Lee, J. Hazard. Mater. 150 (2008) 649–655.
J. Riaza, M.V. Gil, L. Álvarez, C. Pevida, J.J. Pis, F. Rubiera, Energy 41 (2012) 429–435.
L. Dong, S.Q. Gao, G.G. Xu, Energy Fuels 24 (2010) 446–450.
Y. Ohtsuka, Z. Wu, F. Edward, Fuel 76 (1997) 1361–1367.
Z.H. Wu, Y. Sugimoto, H. Kawashima, Fuel 82 (2003) 2057–2064.
N. Tsubouchi, Y. Ohtsuka, Fuel 81 (2002) 1423–1431.
Z.B. Zhao, W. Li, J.S. Qiu, B.Q. Li, Fuel 81 (2002) 2343–2348.
J. Li, S. Wang, L. Zhou, G.H. Luo, F. Wei, Chem. Eng. J. 255 (2014) 126–133.
Z. Zhao, W. Li, J. Qiu, X. Wang, B. Li, Fuel 85 (2006) 601–606.
W. Yang, J. Zhou, Z. Zhou, Z. Lu, Z. Wang, J. Liu, K. Cen, Fuel Process. Technol. 89 (2008) 1317–1323.
B. Das, S. Prakash, P.S.R. Reddy, V.N. Misra, Resour. Conserv. Recycl. 50 (2007) 5040–5057.
T. Kuroki, Y. Uchida, H. Takizawa, K. Morita, ISIJ Int. 47 (2007) 592–595.
X.F. She, J.S. Wang, Q.G. Xue, Y.G. Ding, S.S. Zhang, J.J. Dong, H. Zeng, Int. J. Miner. Metall. Mater. 18 (2011) 277–284.
L.Z. Shen, Y.S. Qiao, Y. Guo, J.R. Tan, J. Hazard. Mater. 177 (2010) 495–500.
Z.F. Gao, Z.J. Wu, M.D. Zheng, Energy Fuels 30 (2016) 3320–3330.
L. Deng, X. Jin, Y. Zhang, D.F. Che, Fuel 175 (2016) 217–224.
L.H. Wei, D. Qi, R.D. Li, Journal of China Coal Society 35 (2010) 1706–1710.
F. He, H. Wang, Y.N. Dai, Natural Gas Chem. 16 (2007) 155–161.
C.P. Finimore, Combust. Flame 26 (1976) 249–260.
Y.C. Zhang, J. Zhang, C.D. Sheng, J. Chen, Y.X. Liu, L. Zhao, F. Xie, Energy Fuels 25 (2011) 240–245.
F. Normann, K. Andersson, B. Leckner, F. Johnsson, Energy Fuels 24 (2010) 910–915.
G.G. De Soete, E. Croiset, J.R. Richard, Combust. Flame 117 (1999) 140–154.
S.J. Wang, C. J. Huang, F. Wu, J. Energy Inst. 86 (2013) 167–170.
T.C. Brown, B.S. Haynes, Energy Fuels 6 (1992) 154–159.
A. Molina, E.G. Eddings, D.W. Pershing, A.F. Sarofim, Combust. Flame 136 (2004) 303–312.
B. Guo, Z. Liu, L. Hong, H. Jiang, Surf. Coat. Technol. 198 (2005) 24–29.
R.D. Peelamedu, M. Fleming, D.K. Agrawal, R. Roy, J. Am. Ceram. Soc. 85 (2010) 117–122.
L.J. Liu, H.J. Liu, M.Q. Cui, Fuel 112 (2013) 687–694.
S.Q. Wang, Y. Zhao, P.P. Zhang, Chem. Eng. Res. Des. 89 (2011) 1061–1066.
K.T. Lee, K.C. Tan, I. Dahlan, A.R. Mohamed, Fuel 87 (2008) 2223–2228.
Z. Zhao, W. Li, J. Qiu, B. Li, Fuel 81 (2002) 2343–2348.
O. Yasuo, Z.H. Wu, F. Edward, Fuel 76 (1997) 1361–1367.
F. Wu, S.J. Wang, G. Zhang, P. Zhu, Z.Y. Wang, J. Energy Inst. 87 (2014) 134–139.
Z. Zhao, W. Li, J. Qiu, B. Li, Fuel 82 (2003) 1839–1844.
F. Okasha, Fuel. Process. Technol. 88 (2007) 401–408.
M. Illan-Gomez, A. Linares-Solano, L.R. Radovic, C. Salinas-Martínez de Lecea, Energy Fuels 58 (1996) 58–168.
Acknowledgements
This work was financially supported by the Joint Fund of the National Natural Science Foundation of China and the Baosteel Group Corporation (No. U1660106).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, Zf., Long, Hm., Chun, Tj. et al. Effect of metallurgical dust on NO emissions during coal combustion process. J. Iron Steel Res. Int. 25, 19–27 (2018). https://doi.org/10.1007/s42243-017-0007-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-017-0007-x