Abstract
Collar rot of betelvine (Piper betle L.) is an important disease in India, caused by Athelia rolfsii (Curzi) C.C. Tu and Kimbr. (syn. Sclerotium rolfsii Sacc.) Management of this soil borne pathogen is highly challenging in the shade house (boroj) condition, where betelvine is grown as a perennial climber. As betel leaves are consumed raw, application of chemical fungicides is highly restricted to safeguard human health. The present study compared various integrated disease management packages by suitable combination of biofumigation, biocontrol and soil solarization strategies and evaluated the best package at farmers’ field condition. The treatment combination of “biofumigation with 0.7 kg m−2 green biomass of Indian mustard cv. Pusa Mahak” + “curing of soil by resting for 5 months in the form of heap followed by soil solarization for 30 days” + “biocontrol with 10 g m−2 Trichoderma sp. T-Nam colonized whole rice grain” was found to be the most economical and effective disease management option with highest leaf yield in the experimental plot. This package resulted in 76.82% reduction in collar rot incidence, 29.94% increase in leaf yield and 41.45% increase in net income during March-June crop cycle, in farmers’ field condition, when compared to the Farmers’ Practice (soil drench with 4 L m−2 0.25% Blitox 50 W). Trichoderma was found to be highly tolerant to the biofumigation volatiles, which maintained a good soil population (32.78 × 103 CFU g−1 soil) in the farmers’ plots adopting integrated disease management. Biofumigation with Indian mustard and biocontrol with local isolate of Trichoderma offered an economical management of the collar rot disease in betelvine, without compromising the crop yield and the population of Trichoderma spp. in soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Betelvine (Piper betle L.) is a perennial, evergreen climber, grown for its heart shaped leaves that are widely used as masticatory and traditional medicinal preparations in South-East Asia. The coastal saline zone of West Bengal in India is famous for growing Mitha Pata cultivar of this crop, which is admired for its fennel-like fragrance, sweet taste and low fibre content. The shade loving vines are grown inside a specially constructed shade house, known as boroj. However, because of its proneness to several diseases, aggravated by the moist and humid microclimate inside the boroj, cultivation of betelvine is highly risky (Sengupta et al. 2011). Among various diseases, it is very difficult to manage and eradicate the collar rot disease, caused by Athelia rolfsii (Curzi) C.C. Tu and Kimbr. (syn. Sclerotium rolfsii Sacc.). During summer season (April – June), it infects the collar region of the vines, causing rapid wilting and resulting in 17–90% crop loss (Maiti and Sen 1982; Garain et al. 2020).
Inside the boroj, the vines are trailed over individual supports (jute stick), fixed in the ground at upright position. As the matured leaves are plucked periodically, the vines continue to grow to finally reach the roof of the boroj. Then, the vines are lowered on the ground and covered with a layer of soil, leaving the apical shoot with 2–3 young leaves to grow further. This “lowering of vines” and burying them with soil is a routine and unique cultural operation in betelvine cultivation that is performed three to four times in a year. The soil, used for this purpose, is collected from the surrounding agricultural fields and stored near the boroj. The soil borne pathogens, like Athelia rolfsii, often take entry into the boroj, along with this untreated soil.
Management of such a soil borne pathogen in the closed shade house condition (boroj) is highly challenging. Over the last few decades, soil fumigation with synthetic chemicals (metam-sodium, dazomet, methyl bromide, pentachloronitrobenzene) has been the most widely used method of soil borne disease control but certainly at the cost of several negative attributes like, high volatility, toxicity and carcinogenicity (Baker et al. 1996). Methyl bromide was found to contribute to stratospheric ozone depletion (Van den Berg et al. 1994) and hence was targeted for phase-out by 2005 from the industrialized countries and by 2015 from the developing countries, during the 9th meeting of Montreal Protocol in 1997 (Stapleton et al. 2000; Gullino et al. 2003). In addition to environmental safety, there is also a growing concern of fungicide residues. Since, green betel leaves are chewed raw, considerable emphasis is warranted for a less persistent and more eco-friendly means of managing betelvine diseases.
To reduce such toxic hazards to the environment and human beings, a bio-intensive integrated disease management approach would be an ideal option. Soil solarization has been found effective for managing A. rolfsii induced collar rot in betelvine (Deshpande and Tiwari 1991). The use of biocontrol agents like Trichoderma spp., is widely popularized in betelvine cultivation (Datta et al. 2011). But the use of native isolates of such fungal antagonists has been less explored in the problematic saline soils (17.5%) of the coastal saline zone of West Bengal.
Biofumigation is another biological approach where plant materials are incorporated into the soil to control soil borne pathogens through the release of toxic volatiles (Kirkegaard and Sarwar 1998). Several cruciferous plants contain high quantities of glucosinolates, which in presence of an endogenous enzyme myrosinase, get hydrolysed to form isothiocyanates, thiocyanates, nitriles and oxa-zolidinethiones that are highly biocidal to many fungi, bacteria, nematodes and insects (Sarwar et al. 1998). Soil incorporation of chopped biomass of Indian mustard (Brassica juncea L.) has been found to produce allyl isothiocyanate upto a concentration of 100 nmol g−1 soil (Matthiessen and Kirkegaard 2006) that could effectively suppress the mycelial growth of A. rolfsii (Harvey et al. 2002).
In the present investigation, a comprehensive effort has been made to develop an integrated disease management package against the collar rot disease of betelvine, combining the use of biofumigation, native isolate of biocontrol agent and soil solarization.
Materials and methods
Experimental site and cultural conditions
To compare different integrated disease management treatments, an experiment was conducted during 2016 and 2017 at Sagar block, in the coastal saline zone of West Bengal, India. A six-year-old betelvine boroj (latitude 21°43′59.49"N, longitude 88°07′17.67"E), grown with Mitha Pata cultivar, was selected for the experiment, considering high collar rot disease incidence during the previous years (31% and 34% in 2014 and 2015, respectively). The vines were spaced at 60 cm (row-to-row) and 15 cm (plant-to-plant). Manuring, irrigation and other cultural operations were carried out as per the recommended package of practice. Based on the time of lowering the vines (March, July and October), the crop season was divided into three cycles (March to June, July to September and October to February). Considering the seasonal incidence of the collar rot disease (Garain et al. 2020), the experiment was planned to target the March-June crop cycle (Table 1).
Design of experiment and components of the integrated disease management treatments
To evaluate the effect of different levels of biofumigation, curing of soil and disease control, the treatments were arranged using split-split-plot design in three replications (supplementary data). The experimental plot (boroj) was divided into three blocks and each of them was used as one replication. Each block was divided into two main plots, each main plot into two sub-plots and each sub-plot into four sub-sub-plots. The sub-sub-plot was of 7.5 m × 1.2 m size, containing 100 vines in two rows. The main and sub-plots included two levels of biofumigation (‘without biofumigation’ and ‘biofumigation’) and two levels of curing of soil (‘without curing of soil’ and ‘curing of soil’), respectively. The sub-sub-plots were assigned with four levels of disease control (a. no treatment as ‘control’; b. application of 4 L m−2 0.25% Blitox 50 W as ‘chemical fungicide’; c. application of 10 g m−2 Trichoderma sp. T-Nam colonized whole rice grain as ‘biocontrol agent’ and d. combined application of ‘10 g m−2 Trichoderma sp. T-Nam colonized whole rice grain + 4 L m−2 0.25% Blitox 50 W’).
For biofumigation, Pusa Mahak cultivar of Indian mustard (B. juncea) was selected. The biofumigation potential and the effective dose of biofumigation of the cultivar were evaluated in a previous experiment (Garain et al. 2021). The seeds were sown (3 kg ha−1) on the biofumigation plots as an intercrop within the standing crop of betelvine, during January (Table 1). The mustard crop was harvested manually in March, at pre-flowering stage and the finely chopped green biomass was spread over the soil at the rate of 0.7 kg m−2. At the same time, the remaining matured betel leaves were harvested and the vines were lowered on the ground by coiling. The lowered vines and the mustard biomass were then covered with a 5-cm layer of soil.
The soil, used for covering the vines after lowering, was either untreated (for sub-plot ‘without curing of soil’) or subjected to special curing (for sub-plot ‘curing of soil’). The top soil was collected during October, from the nearby agricultural field (following a cropping system of “brinjal – beans – cucumber” for past two years) and was rested for five months as a heap (2 m height) outside the boroj. Then the soil was spread on the ground (10-cm layer), moistened and covered with transparent polythene sheet (25 μm) for 30 days, under the sun for soil solarization (Table 1).
The fungicide Blitox 50 W (manufactured by Tata Rallis India Ltd.), containing copper oxychloride 50% WP (w/w) as active ingredient, was selected due to its label claim for use in betelvine in India as per the recommendation of the Central Insecticide Board and Registration Committee (Anonymous 2020). The respective sub-sub-plots were drenched with 4 L m−2 0.25% Blitox 50 W, after lowering of vines, in the month of March (Table 1).
Trichoderma sp. T-Nam, previously isolated from the rhizosphere of betelvine on an improved Trichoderma Selective Medium (Elad and Chet 1983), was used as the biocontrol agent. Its antagonistic potential against A. rolfsii was confirmed (unpublished data). The Trichoderma culture was first mass multiplied on sterilized whole rice grains and stored at 4 °C. Then, 10 g of Trichoderma colonized whole rice grain was mixed with 1 kg vermicompost and applied m−2 area, in the respective sub-sub-plots, after lowering the vines in March (Table 1). The number of spores produced by Trichoderma colonized whole rice grain was counted with the help of a haemocytometer chamber and found to be in the range of 3.14 × 109 to 6.96 × 109 g−1 dry matter.
Disease incidence
Number of vines infected by A. rolfsii and showing collar rot symptoms (rapid wilting with water soaked lesion at collar region followed by visible presence of white ropy mycelium and sclerotia) were counted out of the 100 vines in each plot, during March to June crop cycle. The disease incidence (DI %) was then recorded as percentage of vines infected by A. rolfsii.
Yield parameters
As the vines began to grow, they were again trailed over the perpendicular support. Harvesting of matured leaves started from the middle of April and continued up to the end of June when the vines were lowered for the second time. Number of leaves harvested during March to June from each plot was recorded and extrapolated to express as “105 leaves ha−1”. To determine leaf thickness, 10 leaves were collected from each treatment. Circular discs were cut from each leaf by pressing a metal cap against the leaves. Then all the leaf discs were placed one over another and the total thickness was measured with a Vernier calliper scale. From this, average thickness of one leaf was calculated. For fresh weight calculation, 100 leaves were randomly collected from each treatment, weighed with the help of a digital balance and expressed as “fresh weight of 100 leaves”.
Economic analysis
The total cost of cultivation was calculated including the cost of manuring, inter-culture operations, irrigation, weeding, minor repairing, harvesting and the specific cost for a particular treatment. Gross and net incomes were calculated based on the average market price of betel leaves. Net income was calculated by subtracting the total cost of cultivation from gross income. Then the “cost of treatment” and “additional net income” were calculated for each individual treatment, by deducting the total cost and net income of control plot (“without biofumigation” + “without curing of soil” + “control” combination) from the total cost and net income of a particular treatment plot, respectively. All prices were extrapolated and expressed as “Indian Rupees per ha (₹ ha−1)”, according to the 2020 market rate. The “Benefit–Cost Ratio (BCR)” was calculated as a ratio between gross income and total cost and expressed as the amount of gross income for each rupee spent.
Validation of best performing integrated disease management package in farmers’ field
The best performing integrated disease management combination, selected on the basis of the two years experiment, was demonstrated in farmers’ field for two years (2018 and 2019) with 13 replications. The boroj were selected considering the high level of collar rot incidence (20% to 22%) over the past two years (2016 and 2017). Each of the selected farmers had 200 m2 boroj of similar age (five year old), growing same cultivar (Mitha Pata) and following uniform agronomical practices (nutrient management, irrigation schedule, etc.). Each boroj was divided into two halves of 100 m2 plot. In one half, Farmer’s Practice was followed where the soil was drenched by 0.25% Blitox 50 W at the rate of 4 L m−2, after lowering of vines during March. In the other half, the selected integrated disease management combination was followed. The disease incidence (DI %) and yield were recorded and the economic analysis was calculated for the March to June crop cycle, as discussed earlier.
Enumeration of Trichoderma population in the farmers’ field
The population of Trichoderma in soil was enumerated by serial dilution plating on the improved Trichoderma selective media (Elad and Chet 1983). Soil samples from both the plots (“farmer’s practice” and “integrated disease management”) were collected after the final treatment, at monthly intervals (15th of April, 15th of May and 15th of June) during 2018 and 2019. The samples were air dried under shade, ground into fine powder and mixed properly. A stock solution (10–1 dilution) was prepared by dissolving 100 g soil into 900 ml sterile distilled water. From this solution serial dilution of samples was prepared up to 10–5 dilution. One ml soil–water suspension from each diluted samples was then poured into sterilized Petri plate where 20 ml of the Trichoderma selective media was added. The plates were incubated in BOD incubator at 28 ± 10C for 4 days and the Trichoderma colonies were counted. The Trichoderma population was expressed as Colony Forming Unit (CFU) per gram of soil.
Statistical analysis
All statistical analysis was performed for the pooled data of the respective experiments with IBM’s SPSS Statistics 20. Shapiro–Wilk test was used for assessing the null hypothesis that the data of disease incidence, yield and economic parameters were normally distributed. Angular transformation was applied to normalise the data of disease incidence (values in percentage). After fulfilling the normality of data, the analysis of variance (ANOVA) and multiple comparisons were performed under split-split-plot design. The means of the treatments were compared based on LSD values and the significant differences were determined at p = 0.05.
Results
Disease incidence
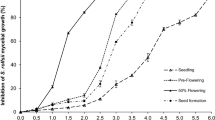
The impact of different levels of biofumigation, curing of soil and disease control on collar rot disease incidence, yield and economic parameters was evaluated based on the pooled data of 2016 and 2017. Variance analysis of disease incidence (Table 2) revealed a significant difference between the two levels of biofumigation (p = 0.008), two levels of curing of soil (p = 0.001)) and four levels of disease control (p < 0.001). Mean comparison revealed that disease incidence was significantly lower at “biofumigation” plots than at “without biofumigation” (Fig. 1). Similarly, the mean disease incidence in “curing of soil” was significantly lower than “without curing of soil”. Mean comparison of disease incidence divided different levels of disease control into three groups (a. “control”, b. “Blitox 50 W” and c. “Trichoderma sp. T-Nam” and “Trichoderma sp. T-Nam + Blitox 50 W”). The disease control levels “Trichoderma sp. T-Nam” and “Trichoderma sp. T-Nam + Blitox 50 W” recorded lowest disease incidences, which were statistically at par, but both differed from the “control” and “Blitox 50 W” (p = 0.05).
None of the interactions “biofumigation” × “curing of soil” or “biofumigation” × “disease control” or “curing of soil” × “disease control” had any significant effect on the disease incidence (p > 0.05). However, when all the three factors were considered together (Table 2), the interaction (“biofumigation” × “curing of soil” × “disease control”) was found to be significant (p = 0.027). As per the pooled data, the least disease incidence was recorded in the treatment combination of “biofumigation + curing of soil + Trichoderma sp. T-Nam and Blitox 50 W” (4.67 ± 0.73%), which was statistically at par with “biofumigation + curing of soil + Trichoderma sp. T-Nam” (4.83 ± 0.73%).
Yield parameters
Variance analysis of leaf yield (Table 2) showed a significant difference between the two levels of biofumigation (p < 0.001), two levels of curing of soil (p < 0.001)) and four levels of disease control (p < 0.001). Mean comparison revealed that yield was significantly higher with “biofumigation” than “without biofumigation” (Fig. 1). Similarly, the mean leaf yield in “curing of soil” was significantly higher than “without curing of soil”. The mean comparison of leaf yield divided the four levels of disease control into three groups (a. “control”, b. “Blitox 50 W” and c. “Trichoderma sp. T-Nam” and “Trichoderma sp. T-Nam + Blitox 50 W”). The disease control levels “Trichoderma sp. T-Nam” and “Trichoderma sp. T-Nam + Blitox 50 W” gave higher yield, which were statistically at par but both differed significantly (p = 0.05) from the “control” and “Blitox 50 W”.
Except the “biofumigation” × “curing of soil” (p > 0.05), all other treatment interactions, “biofumigation” × “disease control” (p < 0.001), “curing of soil” × “disease control” (p = 0.011) and “biofumigation” × “curing of soil” × “disease control” (p < 0.001) were found to be significant (Table 2). As per the pooled data, the highest yield (29.56 × 105 leaves ha−1) was obtained in the treatment combination of “biofumigation + curing of soil + Trichoderma sp. T-Nam”, which was followed by “biofumigation + curing of soil + Trichoderma sp. T-Nam and Blitox 50 W” (29.44 × 105 leaves ha−1). Lowest yield was recorded in “without biofumigation + without curing of soil + control” treatment combination (20.83 × 105 leaves ha−1).
None of the treatments with different levels of biofumigation, curing of soil and disease control or their interactions had any significant (p > 0.05) influence on thickness of betel leaves (Table 2). The biofumigation and curing of soil also had no significant (p > 0.05) influence on fresh weight of leaves. However, a significant difference was observed between the levels of disease control (p < 0.001), on fresh weight of leaves. The mean comparison of fresh leaf weight divided the four levels of disease control into two groups (a. “control” and “Blitox 50 W” and b. “Trichoderma sp. T-Nam” and “Trichoderma sp. T-Nam + Blitox 50 W”). The disease control levels “Trichoderma sp. T-Nam” and “Trichoderma sp. T-Nam + Blitox 50 W” resulted in significantly (p = 0.05) higher fresh weight of leaves than the other group.
Economic parameters
The average market price of betel leaf was ₹ 1.25 and ₹ 1.30 per leaf during 2016 and 2017, respectively. But the leaves from plots treated with Trichoderma sp. T-Nam fetched a better price (₹ 1.30 and ₹ 1.35 during 2016 and 2017, respectively) due to their superior quality in terms of colour, lustre, texture, shape and storability.
Both, the gross and net income varied significantly (p < 0.001) at different levels of “biofumigation”, “curing of soil” and “disease control” (Table 3). Mean comparison revealed that income was significantly higher with “biofumigation” than “without biofumigation” (Fig. 1). Similarly, the means of gross and net income in “curing of soil” were significantly higher than “without curing of soil”. The mean income was also significantly different at the four levels of disease control. Though gross income was highest in the disease control level with “Trichoderma sp. T-Nam + Blitox 50 W”, the net income was highest in “Trichoderma sp. T-Nam”. Except the “biofumigation” × “curing of soil” (p > 0.05) interaction, all other treatment interactions, i.e., “biofumigation” × “disease control” (p < 0.001), “curing of soil” × “disease control” (p < 0.01) and “biofumigation” × “curing of soil” × “disease control” (p < 0.001), had significant impact on gross and net income (Table 3). As per the pooled data, the highest gross income (₹ 3,917,130.00 ha−1) and net income (₹ 3,042,130.00 ha−1) was obtained in the treatment combination of “biofumigation + curing of soil + Trichoderma sp. T-Nam”, for the March-June crop cycle.
Highest benefit–cost ratio (4.48) was obtained under the treatment combinations “biofumigation + curing of soil + Trichoderma sp. T-Nam” and “biofumigation + without curing of soil + Trichoderma sp. T-Nam” (Table 3). The relative cost of individual disease management treatments from highest to lowest order was found as: “application of Blitox 50 W” (₹ 93,667.00 ha−1) > “curing of soil” (₹ 35,333.00 ha−1) > “biofumigation” (₹ 30,667.00 ha−1) > “application of Trichoderma sp. T-Nam” (₹ 23,667.00 ha−1)’. Highest additional yield (8.73 × 105 leaves ha−1) and additional net return (₹ 1,179,130.00 ha−1) was obtained in the “biofumigation + curing of soil + Trichoderma sp. T-Nam” treatment combination.
Performance of integrated disease management package in farmers’ field
Considering the lowest collar rot incidence, highest yield and maximum profit during the two years experiment, the best performing integrated disease management (IDM) package was found to be the combination of “biofumigation with 0.7 kg m−2 green biomass of Indian mustard cv. Pusa Mahak” + “curing of soil by resting for 5 months in the form of heap followed by soil solarization for 30 days” + “biocontrol with 10 g m−2 Trichoderma sp. T-Nam colonized whole rice grain”.
The data of disease incidence, yield and economic parameters showed significant (p = 0.05) difference between the two treatments (Table 4). The IDM package resulted in reduction in collar rot incidence by 76.82%, increase in leaf yield by 29.94% and increase in net income by 41.45%, over the “farmer’s practice”. The benefit–cost ratio was 5.53 in the IDM plots, compared to 4.52 in the farmer’s practice plots. The population of Trichoderma spp. in the soil was consistently and significantly (p = 0.05) higher in the plots following the IDM package than in the farmer’s practice plots, throughout the crop cycle.
Discussion
Biofumigation with Indian mustard significantly reduced collar rot disease incidence in betelvine. Soil incorporation of mustard biomass in the biofumigated plot may have negatively impacted the growth and development of A. rolfsii in the soil system. Stapleton and Duncan (1998) recorded 87–100% reduction in sclerotial germination in A. rolfsi through soil ammendment with fresh and dried crop residue of cruciferous plants. Indian mustard has also been reported to effectively suppress the mycelial growth of A. rolfsii (Harvey et al. 2002) and reduce stem rot disease in groundnut (Yella Goud et al. 2017) under field condition. Biofumigation with Brassica crops has been successfully demonstrated by many researchers to control other soil borne pathogens like, Rhizoctonia solani, Pythium spp., Fusarium spp., Sclerotinia sclerotium, etc. (Charron and Sams 1999; Relevante and Cumagun 2013; Baysal-Gurel et al. 2019).
The ‘curing of soil’ treatment consisted of two parts, firstly, resting the soil for five months outside the boroj in the form of a soil heap of 2 m height and secondly, exposing the soil to solar heat (soil solarization) before its application inside the boroj. Resting the soil in the form of a heap may have helped to decrease the viability of the sclerotia by limiting oxygen for respiration or through the effect of physical pressure on sclerotia at greater depths resulting in reduction of sclerotial germination (Punja and Jenkins 1984). Soil solarization has also been effectively utilized for managing A. rolfsii induced collar rot in betelvine (Deshpande and Tiwari 1991). Heating of sclerotia (A. rolfsii) in natural soil allows organic substances to leak from the sclerotia that apparently stimulate its extensive colonization by beneficial soil microorganisms (Lifshitz et al. 1983). All of these developments may have apparently weakened the sclerotia and finally reduced their inoculum potential and strongly promoted their colonization by soil microorganisms. Hence, pre-treatment of the soil before its application to the plant base eventually helped to restrict the entry of the soil borne inoculum of A. rolfsii, inside the boroj.
Application of the native isolate, Trichoderma sp. T-Nam, was also superior in reducing collar rot incidence, either alone or in combination with Blitox 50 W fungicide, over the “control” and “Blitox 50 W”. Since betel leaves are consumed as raw, application of chemical fungicides to this crop is highly restricted. In India, only copper based contact fungicides (like copper oxychloride) are permitted for use in betelvine (Anonymous 2020). But, copper oxychloride (Blitox 50 W) alone could not provide satisfactory control against collar rot disease in betelvine. The presence of melanin might have imparted resistance to digestion of the sclerotia of A. rolfsii by chemical agents (Bloemofield and Alexander 1967). However, Trichoderma spp. has been proved to penetrate the highly melanized walls of sclerotia and degrade them completely (Elad and Mishagi 1985), which is not possible by using chemical fungicides. Application of Trichoderma harzianum has also been found to increase leaf yield in betelvine (Singh and Singh 2005), apart from successful control of collar rot disease (Datta et al. 2011). The increase in leaf yield and improvement of leaf quality, observed in the Trichoderma sp. T-Nam treated plots in our experiment corroborates with the previous findings. The Trichoderma can also be mixed with the cured soil directly before covering the lowered vines, for its uniform distribution in the boroj.
The combined application of “biofumigation + curing of soil + Trichoderma sp. T-Nam” as well as the “biofumigation + curing of soil + Trichoderma sp. T-Nam and Blitox 50 W” combination resulted in lowest disease incidence and highest leaf yield. However, the net income was significantly higher in “biofumigation + curing of soil + Trichoderma sp. T-Nam” due to the lower cost of treatment. Management of soil borne pathogens by soil drenching with chemical fungicides may be highly effective but a costly affair (Tripathi and Grover 1978), thus increasing overall cost of production. Application of Trichoderma has been reported to result in better economic return than chemical control of foot rot disease in betelvine (Dasgupta et al. 2011). Effective use of integrated disease management against collar rot in betelvine has been previously reported by several researchers (Anonymous 2015; Tripathi 2015), where biocontrol agents like the Trichoderma spp., organic manures like mustard oil cake and farm yard manure, soil drenching with chemical fungicides and balanced dose of fertilizers have been used in various combinations. There is also similar report of combined use of biofumigation along with T. harzianum and Pseudomonas fluorescens, which gave effective control against Rhizoctonia solani f sp. sasakii in Maize (Madhavi and Uma Devi 2018). However, the present study is the first of its kind in using biofumigation with Indian mustard in combination with soil solarization and native isolate of Trichoderma, in managing A. rolfsii induced collar rot disease of betelvine at field level.
Biofumigation did not show any negative effect on the population of Trichoderma in soil. Unlike A. rolfsii, the Trichoderma spp. is less sensitive to the biofumigation volatiles (Galletti et al. 2008; Garain et al. 2021). The extra biomass or food base, provided during the soil incorporation of a biofumigant crop, helps in multiplication of soil microbes (Omirou et al. 2010). The application of Trichoderma sp. T-Nam as well as restriction of chemical fungicides in the integrated disease management adopted plots, at farmers’ field condition, also aided in build-up of Trichoderma population in the soil. Similar observations were recorded by Vikram and Hamzehzarghani (2011) while working with sclerotial stem rot management in groundnut.
Conclusion
The present study opened up the scope of using local strains of biocontrol agents and biofumigation potentiality of Brassica crops for the eco-friendly management of soil borne pathogens in the coastal saline zone. Biofumigation with Indian mustard offered an economical management of the collar rot disease without compromising the crop yield and the population of Trichoderma spp. in soil. Integration of soil solarization, after a proper resting of the soil in the form of a heap, also reduced collar rot disease under field condition.
Availability of data and material
The data and material used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BCR:
-
Benefit-cost ratio
- CFU:
-
Colony forming unit
- DI:
-
Disease incidence
- FP:
-
Farmer’s Practice
- IDM:
-
Integrated Disease Management
- ₹:
-
Indian Rupee
- cv.:
-
Cultivar
- syn.:
-
Synonym
References
Anonymous (2015) Annual Report of Directorate of Medicinal and Aromatic Plants Research (DMAPR) - 2014–15, Boriavi, Anand, Gujarat, India, pp 73. https://dmapr.icar.gov.in//Publications/AnnualReport/E%20Annual%20Report%20%202014–15.pdf
Anonymous (2020) Major uses of Pesticides (Registered under Insecticides Act, 1968): Fungicides (upto 31/01/2020). Department of Agriculture Cooperation, Ministry of Agriculture and Farmers Welfare, Directorate of Plant Protection, Quarantine & Storage, Central Insecticide Board & Registration Committee, N.H.-IV, Faridabad-121 001 (Haryana), pp 7. http://ppqs.gov.in/divisions/cib-rc/major-uses-of-pesticides
Baker LW, Fitzell DL, Seiber JN, Parker TR, Shibamoto T, Poore MW, Longley KE, Tomlin RP, Propper R, Duncan DW (1996) Ambient air concentrations of pesticides in California. Environ Sci Technol 30:1365–1368. https://doi.org/10.1021/es950608l
Baysal-Gurel F, Liyanapathiranage P, Addesso KM (2019) Effect of Brassica crop-based biofumigation on soilborne disease suppression in woody ornamentals. Can J Plant Pathol. https://doi.org/10.1080/07060661.2019.1625444
Bloemofield B, Alexander M (1967) Melanins and resistance of fungi to lysis. J Bacteriol 93:1276. https://doi.org/10.1128/jb.93.4.1276-1280.1967
Charron CS, Sams CE (1999) Inhibition of Pythium ultimum and Rhizoctonia solani by shredded leaves of Brassica species. J Amer Soc Hort Sci 124: 462–467. https://doi.org/10.21273/JASHS.124.5.462
Dasgupta B, Dutta P, Das S (2011) Biological control of foot rot of betelvine (Piper betleL.) caused by Phytophthora parasitica Dastur. J Pl Prot Sci 3(1): 15–19. http://researcherslinks.com/current-issues/Biological/23/5/558
Datta P, Dasgupta B, Das S (2011) Efficacy of Trichoderma spp. against Sclerotium rolfsii. J Mycopathol Res 49(1): 31–38. https://www.cabdirect.org/cabdirect/abstract/20133277185
Deshpande AL, Tiwari RKS (1991) Effect of solarisation on sclerotia of Sclerotium rolfsii causing collar rot in betelvine. Indian Phytopathol 44(3): 353–355. https://www.researchgate.net/publication/333166230_Effect_of_solarisation_on_sclerotia_of_Sclerotium_rolfsii_causing_collar_rot_in_betelvine
Elad Y, Chet I (1983) Improved selective media for isolation of Trichoderma spp. or Fusarium spp. Phytoparasitica 11(1): 55–58. https://doi.org/10.1007/BF02980712
Elad Y, Mishagi IJ (1985) Biochemical aspects of plant-microbe and microbe-microbe interactions in soil. In: Copper - Driver GA, Swain T (eds), Chemically mediated interactions between plants and other organisms, Recent Adv Phytochem, New York: Plenum Press, pp 21. https://doi.org/10.1007/978-1-4757-9658-2_2
Galletti S, Sala E, Leoni O, Burzi PL, Cerato C (2008) Trichoderma spp. tolerance to Brassica carinata seed meal for a combined use in biofumigation. Biol Control 45:319–327. https://doi.org/10.1016/j.biocontrol.2008.01.014
Garain PK, Mondal B, Dutta S (2021) Effect of biofumigation by Indian mustard (Brassica juncea L.) on Sclerotium rolfsii Sacc., causing collar rot in betelvine (Piper betle L.). Indian Phytopathol 74:1015–1025. https://doi.org/10.1007/s42360-021-00407-2
Garain PK, Mondal B, Maji A, Dutta S (2020) Survey of major diseases in Mitha Pata variety of Betelvine (Piper betle L.) under Coastal Saline Zone of West Bengal, India. Int J Curr Microbiol App Sci 9(3):2490–2498. https://doi.org/10.20546/ijcmas.2020.903.285
Gullino ML, Camponogara A, Gasparrini G, Rizzo V, Clini C, Garibaldi A (2003) Replacing Methyl Bromide for Soil Disinfestation: The Italian Experience and Implications for Other Countries. Plant Dis 87(9):1012–1021. https://doi.org/10.1094/PDIS.2003.87.9.1012
Harvey SG, Hannahan HN, Sams CE (2002) Indian Mustard and Allyl Isothiocyanate inhibit Sclerotium rolfsii. J Amer Soc Hort Sci 127(1): 27–31. http://dx.doi.org/https://doi.org/10.21273/JASHS.127.1.27
Kirkegaard J, Sarwar M (1998) Biofumigation potential of brassicas. Plant Soil 201:71–89. https://doi.org/10.1023/A:1004364713152
Lifshitz R, Tabachnik M, Katan J, Chet I (1983) The effect of sublethal heating on sclerotia of Sclerotium rolfsii. Can J Microbiol 29:1607–1610. https://doi.org/10.1139/m83-246
Madhavi GB, Uma Devi G (2018) Effect of combined application of biofumigant, Trichoderma harzianum and Pseudomonas fluorescens on Rhizoctonia solani f. sp. sasakii. Indian Phytopathol 71:257–263. https://doi.org/10.1007/s42360-018-0039-6
Maiti S, Sen C (1982) Incidence of major diseases of betel vine in relation to weather. Indian Phytopathol 35(1): 14–17. https://www.cabi.org/isc/abstract/19851309785
Matthiessen JN, Kirkegaard JA (2006) Biofumigation and Enhanced Biodegradation: Opportunity and Challenge in Soilborne Pest and Disease Management. Crit Rev Plant Sci 25(3):235–265. https://doi.org/10.1080/07352680600611543
Omirou M, Rousidou C, Bekris F, Papadopoulou KK, Menkissoglou-Spiroudi U, EhaliotisC KDG (2010) The Impact of Biofumigation and Chemical Fumigation Methods on the Structure and Function of the Soil Microbial Community. Microb Ecol 61:201–213. https://doi.org/10.1007/s00248-010-9740-4
Punja ZK, Jenkins SF (1984) Influence of temperature, moisture, modified gaseous atmosphere and depth in soil of eruptive sclerotial germination of Sclerotium rolfsii. Phytopathology 74: 749–754. https://www.apsnet.org/publications/phytopathology/backissues/Documents/1984Articles/Phyto74n06_749.PDF
Relevante CA, Cumagun CJR (2013) Control of Fusarium wilt in bittergourd and bottlegourd by biofumigation using mustard var. Monteverde Arch Phytopathol Pflanzenschutz 46(6):747–753. https://doi.org/10.1080/03235408.2012.751285
Sarwar M, Kirkegaard JA, Wong PTW, Desmarchelier JM (1998) Biofumigation potential of Brassicas: III. In vitro toxicity of isothiocyanates to soilborne fungal pathogens. Plant Soil 201:103–112. https://doi.org/10.1023/A:1004381129991
Sengupta DK, Dasgupta B, Datta P (2011) Management of foot rot of betel vine (Piper betle L.) caused by Phytophthora parasitica Dastur. J Crop and Weed 7(2):179–183. https://www.cropandweed.com/vol7issue2/pdf/39.pdf
Singh A, Singh HB (2005) Trichoderma harzianum suppresses collar rot disease of Betelvine caused by Sclerotium rolfsii. Paper presented in Second Global Conference of Indian Society of Mycology and Plant Pathology at MPUAT, Udaipur, 25–29 November 2005, pp194.
Stapleton JJ, Duncan RA (1998) Soil disinfestations with Cruciferous amendments and subleathal heating effects on Meloidogyne incognita, Sclerotium rolfsii and Pythium ultimum. Plant Pathol 47: 737–742. https://www.researchgate.net/publication/259044054_Soil_disinfestation_with_cruciferous_amendments_and_sublethal_heating_Effects_on_Meloidogyne_incognita_Sclerotium_rolfsii_and_Pythium_ultimum
Stapleton JJ, Elmore CL, DeVay JE (2000) Solarization and biofumigation help disinfest soil. Calif Agri 54(6):42–45. https://doi.org/10.3733/ca.v054n06p42
Tripathi AK (2015) Management of Sclerotial wilt disease of betelvine in Madhya Pradesh. The Bioscan 10(4): 1683–1685. https://www.semanticscholar.org/paper/MANAGEMENT-OF-SCLEROTAIL-WILT-DISEASE-OF-BETELVINE-Tripathi/691f30f4daa823abb8124f2ee6b20267c8eae076
Tripathi NN, Grover RK (1978) Comparison of fungicides for the control of Pythium butleri causing damping off of tomato in nursery beds. Indian Phytopathol 30:489–496
Van den Berg F, Ross AH, Tuinstra LG, Leistra M (1994) Measured and computed concentrations of 1, 3-dichloropropene and methyl isothiocyanate in air in a region with intensive use of soil fumigants. Water Air Soil Pollut 78:247–264. https://doi.org/10.1007/bf00483035
Vikram A, Hamzehzarghani H (2011) Integrated Management of Sclerotium rolfsii (Sacc.) in Groundnut (Arachis hypogaea L.) under pot culture condition. Pest Technology 5(1): 33–38. http://www.globalsciencebooks.info/Online/GSBOnline/images/2011/PT_5(1)/PT_5(1)33-38o.pdf
Yella Goud T, Uma Devi G, Narayan Reddy P, Siva Sankar A (2017) Effect of Volatile Toxins of Brassica Residues on Groundnut Stem and Pod Rot Disease caused by S. rolfsii. Int J Curr Microbiol App Sci 6(11): 5466–5470. https://doi.org/10.20546/ijcmas.2017.611.524
Acknowledgements
The authors are thankful to Ramkrishna Ashram Krishi Vigyan Kendra for allowing to conduct the research work.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors designed the experiments. PKG and SD coordinated and performed the field experiments, analysis of results and manuscript drafting. PKG and BM performed the farmers’ field demonstration, data interpretation and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not required for this study, as the article does not contain any human and animal rights.
Consent to participate
Not required for this study, as the article does not contain any human and animal rights.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Garain, P., Mondal, B. & Dutta, S. Biofumigation based integrated disease management against Athelia rolfsii (syn. Sclerotium rolfsii Sacc.) induced collar rot disease of betelvine (Piper betle L.). J Plant Pathol 104, 1027–1038 (2022). https://doi.org/10.1007/s42161-022-01129-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-022-01129-8