Abstract
In this study, an approach based on seed treatment was undertaken to enhance resistance of Nicotiana benthamiana against wild fire disease caused by Pseudomonas syringae pv. tabaci. Seeds soaked in 10 μg ml−1 of three hemi-synthetic triterpenes (F3, F4 and F6) derived from the latex of Euphorbia officinarum and Euphorbia resinifera resulted in plants with reduced disease severity, which was correlated with a decrease of bacterial populations in planta. Analysis of plant defense markers revealed that H2O2 and guaiacol peroxidase were only slightly activated but were primed after pathogen infiltration. However, polyphenol oxidase, catalase and ascorbate peroxidase were directly induced by the triterpenic derivatives. These results underline the duality of the mode of action of the triterpenic compounds and suggest their use as plant defense inducers in crop protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triterpenes and their steroids derivatives belong to the group of isoprenoids or terpenoid compounds. They usually possess a tetracyclic or pentacyclic structure and are synthesized via the mevalonate pathway with 2,3-oxidosqualene as an intermediate precursor (Thimmappa et al. 2014). Sterols are considered as structural components of biological membranes and play an important role as hormones. For instance, brassinosteroid (BR) is involved in development but also in signalisation during plant defense against abiotic and biotic stress agents. In Nicotiana benthamiana BR acts as modulators between the growth and disease resistance against tobacco mosaic virus (Deng et al. 2016). Their exogenous application induced resistance in Arabidopsis thaliana against cucumber mosaic virus (Zhang et al. 2015) and in barley against Fusarium head blight (Ali et al. 2013). However, their roles in plant defense are sometimes controversial because in some pathosystems such as potato/Phytophthora infestans they enhance disease susceptibility (Bajguz and Hayat 2009).
In plants triterpenes often accumulate as conjugates with carbohydrates known as saponins. The combination of a hydrophobic aglycone backbone with hydrophilic glycoside makes them extremely amphipathic, contributing then to their foaming and emulsifying properties, which have been exploited for a wide range of commercial applications (Moses et al. 2014). They play significant roles in plants defense against pathogens, pests and herbivores because they possess antimicrobial, antiparasitic and insecticidal properties (Osbourn et al. 2011), and are induced when plants are subjected to biotic and abiotic attacks (De Costa et al. 2013).
Recently, we have hemi-synthetized several triterpene derivatives from the latex of Euphorbia officinarum and Euphorbia resinifera and showed that some of them were able to protect tomato plants against the fungal pathogen Verticillium dahliae (Smaili et al. 2017a). In this study, we have extended this work and tested three triterpenic derivatives against the wild fire disease of tobacco caused by the bacterial pathogen Pseudomonas syringae pv. tabaci (Guo et al. 2017). In addition, we have examined their effect on reactive oxygen species (ROS) accumulation and on the activity of enzymes involved in their detoxification and in plant defense responses.
Materials and methods
Chemicals, plant material and treatment
The hemi-synthetic triterpene 24-methylen-elemo-lanosta-8,24-dien-3-one (F3) was obtained from the oxidation of α-euphorbol as described in Smaili et al. (2017a). The triterpene derivatives 3β-acetoxy-norlup-20-one (F4) and 3-chloro-4α,14α-dimethyl-5α-cholest-8-ene (F6) were obtained from lupeol and 31-norlanostenol, respectively as described in Smaili et al. (2017b). The structures of the three products are shown in Fig. 1.
N. benthamiana seeds were surface sterilized for 10 min with 2% of sodium hypochlorite solution, followed by thorough washing in sterile distilled water Seeds were placed in Petri dishes holding filter paper imbibed with 5 ml of aqueous solution containing 10 μg ml−1 of F3, F4 or F6. Seeds treated with distilled water served as control. Seven days after germination seeds were transferred to pots containing a sterile mixture of peat and sand (3:1) in a greenhouse with temperatures of 26 °C/12 °C, a 12 h photoperiod and 60–70% relative humidity.
Plant inoculation, disease assessment and determination of bacterial growth in planta
Plants were used for inoculation with P. syringae pv. tabaci as described by Faize et al. (2012) when 2-month-old. Bacteria grown overnight on liquid King’s B (KB) medium at 26 °C were centrifuged at 3000 g for 10 min and the pellet was suspended in sterile distilled water. Inoculum concentration was adjusted to 3 × 108 CFU ml−1 and the youngest leaf was infiltrated with 100 μl of the bacterial suspension using a syringe without a needle in four alternate mesophyll zones. The diameter of the lesions, i.e. the necrotic area and the surrounding chlorotic halo was measured at five days post-inoculation (dpi). Four plants were used for each inoculation trial.
To examine bacterial growth, leaf discs (1 cm diameter) previously infiltrated with 100 μl of bacterial suspension adjusted to 3 × 108 CFU/ml−1 were punched at 5 dpi, homogenized in 50 mM phosphate buffer, pH 7.2, then serially diluted and plated on KB medium agar plates. Colonies were counted after 48 h of incubation at 26 °C.
Diseases assessment and bacterial growth were analyzed using one-way ANOVA followed by Dunnett’s test (P < 0.05).
H2O2 and enzymes activities determination
Leaves infiltrated with bacterial suspension or distilled water (mock-inoculated) were collected at 0, 2, 4, 24, 48 and 72 h post inoculation (hpi) and used for H2O2 quantification as described by Alexieva et al. (2001). After extraction with 0.1% trichloroacetic acid (TCA) and centrifugation at 10000 g 1 ml of 1 mM potassium iodide and 10 mM phosphate buffer (pH 7) were added to the supernatant. The H2O2 concentration of the supernatant was evaluated spectrophotometrically by comparing its absorbance at 390 nm to a standard calibration curve.
Another set of leaves was used to determine the enzymatic activity by grinding 400 mg of leaf material in 2 ml of 50 mM of sodium phosphate buffet, pH 7.5 containing 1 mM diethylenetetraacetic acid (EDTA) and 1% polyvinylpyrrolidone. After centrifugation at 13000 g for 15 min the supernatant was recovered and immediately analyzed.
Guaiacol peroxidase (EC 1.11.1.7, GPX), catalase (EC 1.11.1.6, CAT) and ascorbate peroxidase (EC 1.11.1.11, APX) were determined as described in Faize et al. (2013). GPX activity was determined using guaiacol as substrate. The reaction mixture consisted of 50 μl crude enzyme extract and 1.8 ml of 50 mM acetate buffer, pH 5.5, containing 25 mM guaiacol. Reaction started after adding 25 mM of H2O2 and absorbance was recorded at 470 nm for 4 min. Activity was expressed as nmol min−1 mg protein−1. CAT measurement was carried out in a 3 ml reaction mixture consisting of 2.8 ml phosphate buffer (50 mM, pH 7), 20 mM H2O2 and 100 μl of supernatant. The absorbance at 240 nm was recorded for two min and results were presented as nmol min−1 mg protein−1. APX activity was determined in a 0.5 ml reaction mixture consisting of 50 mM phosphate buffer, pH 6, 50 mM of ascorbic acid, 2 mM H2O2 and 100 μl supernatant. Absorbance was recorded at 290 nm every 10 s during 3 min. Results were expressed as nmol min−1 mg protein−1.
Polyphenol oxidase (EC 1.10.3.1, PPO) activity was measured as described by Masia et al. (1998). Absorbance was recorded at 410 nm for 5 min after adding 100 μl of crude extract to 2.9 ml of phosphate buffer, pH 6.5, containing 25 mM of pyrocatechol and activity was expressed as ∆OD min−1 mg protein−1.
Results
Wild fire disease control using triterpene derivatives
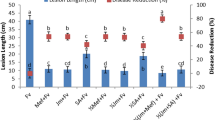
To determine the effect of F3, F4 and F6 on wild fire disease development the diameter of the lesions and bacterial growth were assessed on plants derived from seeds soaked in the triterpene derivatives or in distilled water at 5 dpi (Table 1). None of the three products affected germination (data not shown). However, they significantly reduced the diameter of the lesions in the inoculated leaves, when compared to the control. Reduction ranged from 35% with F6 to 54% with F3 (Table 1).
Bacterial growth was also significantly reduced in plants treated with the triterpenic products when compared to the control. Bacterial populations were reduced by at least 0.54 logarithmic units (Table 1).
ROS accumulation triggered by triterpene derivatives
H2O2 was quantified in leaves infiltrated with P. syringae pv. tabaci or with distilled water as an indicative of ROS (Fig. 2). When compared to the control, P. syringae pv. tabaci significantly enhanced the content of H2O2 only at 48 and 72 hpi. No significant increase was observed in mock-inoculated leaves from plants that germinated in the presence of F3 during the entire period of the experiment (Fig. 2a). However, H2O2 content increased earlier reaching its maximum at 2 hpi and it remained higher during the remaining time. A similar trend was also observed with F4 although significant increases were observed at 24 and 48 hpi (Fig. 2b). Inoculated plants derived from seeds soaked with F6 revealed higher levels of H2O2, which started to accumulate significantly from 2 hpi and remained elevated during the whole duration of the experiment (Fig. 2c). This result suggests that the three triterpenic derivatives were able to prime N. benthamiana for enhanced H2O2 accumulation.
Time course analysis of H2O2 accumulation in Nicotiana benthamiana derived from seeds soaked in triterpenic derivatives a) F3, b) F4 or c) F6. Plants derived from seeds soaked in 10 μg ml−1 of the product or in distilled water were inoculated 60 days after germination with 3 × 108 CFU/ml−1 of P. syringae pv. tabaci. Mock-inoculated plants were infiltrated with distilled watr. Data are means and confidence intervals (95%) from three replicates. Experiment was repeated twice and data of typical experiment is shown
Antioxidant enzymes activities induced by triterpene derivatives
To examine how treated plants are able to cope with generated H2O2 the activity of two antioxidant enzymes (APX and CAT) were analyzed.
A slight and significant induction of APX activity was triggered in the control by P. syringae pv. tabaci at 4, 24 and 72 hpi. In mock-inoculated leaves of plants that germinated in the presence of F3, the activity had a transient tenfold increase at 24 and 48 hpi. The same increase was observed in leaves inoculated with P. syringae pv. tabaci pre-treated with F3 at 24 and 48 hpi. However, the activity remained significantly elevated even at 72 hpi (Fig. 3a). A transient increase was also induced by F4 at 48 hpi in the mock inoculated plants and was eight times higher than the control, while in inoculated leaves the increase was earlier (24 hpi) and greater (10 times the control) (Fig. 3b). Plants derived from F6 showed elevated APX activity, which arose from 2 hpi reaching its maximum at 4 hpi, and decreased just after in either mock inoculated or inoculated leaves. However in the latter case the activity increased again at 48 hpi and was 20 times higher than in the control at 72 hpi (Fig. 3c).
Time course analysis of ascorbate peroxidase (APX) activity in Nicotiana benthamiana derived from seeds soaked in triterpenic derivatives a) F3, b) F4 or c) F6. Plants derived from seeds soaked in 10 μg ml−1 of the product or in distilled water were inoculated 60 days after germination with 3 × 108 CFU/ml−1 of P. syringae pv. tabaci. Mock-inoculated plants were infiltrated with distilled water. Data are means and confidence intervals (95%) from three replicates. The experiment was repeated twice and data of a typical experiment are shown
CAT activity was slightly enhanced in the inoculated control by P. syringae pv. tabaci from 2 hpi, picked at 4 hpi and decreased after although remained significantly elevated at, 24 and 48 hpi. In mock-inoculated as well as in inoculated leaves derived from plants that germinated in the presence of F3, activities were higher. They increased significantly from 2 hpi until 48 hpi (Fig. 4a). F4 promoted significantly CAT activity in mock-inoculated leaves from 2 to 24 hpi while in inoculated leaves higher and significant activities were recorded at 2, 24 and 48 hpi (Fig. 4b). Elevated activities were also obtained with F6 in both situations (Fig. 4c).
Time course analysis of catalase (CAT) activity in Nicotiana benthamiana derived from seeds soaked in triterpenic derivatives a) F3, b) F4 or c) F6. Plants derived from seeds soaked in 10 μg ml−1 of the product or in distilled water were inoculated 60 days after germination with 3 × 108 CFU/ml−1 of P. syringae pv. tabaci. Mock-inoculated plants were infiltrated with DW. Data are means and confidence intervals (95%) from three replicates. The experiment was repeated twice and data of a typical experiment are shown
These results suggest that the antioxidant enzymes APX and CAT are directly responsive to the triterpenic compounds.
Induction of plant defense by triterpenic derivatives
As marker of general plant defense responses the activity of GPX and PPO was studied.
A small increase of GPX activity was induced by P. syringae pv. tabaci in the control at 48 hpi. A slight activation was also observed at 24 hpi in mock inoculated leaves by F3. However, the most elevated activities were recorded from inoculated plants pre-treated with F3. In this case it reached its maximum at 48 hpi and was around 6 times higher than in other situations and decreased just after (Fig. 5a). Similar results were obtained with F4 (Fig. 5b) and F6 (Fig. 5c). However increases in GPX activity were earlier since they started from 2 hpi to reach their maximum at 24 hpi.
Time course analysis of guiaicol peroxidase (GPX) activity in Nicotiana benthamiana derived from seeds soaked in triterpenic derivatives a) F3, b) F4 or c) F6. Plants derived from seeds soaked in 10 μg ml−1 of the product or in distilled water were inoculated 60 days after germination with 3 × 108 CFU/ml−1 of P. syringae pv. tabaci. Mock-inoculated plants were infiltrated with distilled water. Data are means and confidence intervals (95%) from three replicates. The experiment was repeated twice and data of a typical experiment are shown
In the inoculated control P. syringae pv. tabaci slightly enhanced PPO activity from 2 hpi. This activity continues to rise until 24 hpi and decreased after that time. In plants pre-treated with F3 either mock inoculated or inoculated, activity was higher than that induced by P. syringae pv. tabaci in the control, although differences were not significant (Fig. 6a). However, activity was higher in plants pre-treated with F4 (Fig. 6b) and F6 (Fig. 6c) and activity were significantly higher in inoculated leaves followed by mock inoculated ones.
Time course analysis of polyphenol oxidase (PPO) activity in Nicotiana benthamiana derived from seeds soaked in triterpenic derivatives a) F3, b) F4 or c) F6. Plants derived from seeds soaked in10 μg ml−1 of the product or in distilled water were inoculated 60 days after germination with 3 × 108 CFU/ml−1 of P. syringae pv. tabaci. Mock-inoculated plants were infiltrated with DW. Data are means and confidence intervals (95%) from three replicates. The experiment was repeated twice and data of a typical experiment are shown
These results suggest that PPO is directly inducible by the triterpenic products while GPX is primed upon inoculation.
Discussion
In this work we showed that seed soaking with the triterpenic products derived from E. officinarum and E. resinifera enhanced N. benthamiana resistance to P. syringae pv. tabaci by directly activating APX, CAT and PPO and priming GPX activity and H2O2 accumulation. To our knowledge the most similar compounds to the triterpenic products F3, F4 and F6 are brassinosteroids, which have been already reported to enhance plant disease resistance. For instance, their spray application induced resistance in Arabidopsis thaliana against cucumber mosaic virus (Zhang et al. 2015) and in barely against Fusarium head blight (Ali et al. 2013). Resistance induced by the triterpenic derivatives against P. syringae pv. tabaci seems to be mediated by H2O2 accumulation. This result could be compared with that of Xia et al. (2009), who showed that ROS are implicated in stress tolerance of cucumber induced by brassinosteroids. Interestingly, the three triterpenic derivatives were able to prime N. benthamiana for enhanced H2O2 accumulation after pathogen infection. Priming makes plants more tolerant to later stress or pathogen attack and is considered an important mechanism of induced resistance (Borges et al. 2014). H2O2 is known to act as a signalling molecule, and is widely regarded to play pivotal role in plant disease resistance (Petrov and Van Breusegem 2012). It is considered as one of the earliest plant response for pathogen detection and is required for downstream plant defences. However, to avoid ROS damage, antioxidant enzymes should be activated. It has been reported that a brassinosteroid treatment enhanced antioxidant enzymes in Arabidopsis inoculated with cucumber mosaic virus (Zhang et al. 2015). In this study we have shown that CAT and APX, which are involved in detoxification of H2O2, were directly activated by the triterpenic derivatives. In addition, GPX was primed while PPO activity was directly induced. GPX and PPO are involved in lignification, cross-linking of cell wall components and synthesis of phytoalexins. They also participate in ROS metabolism, allowing restriction of the pathogen to the infection site (Almagro et al. 2009). PPO is able to oxidize phenolic compounds generating quinones derivatives with bactericidal activities (Vaughn et al. 1988) In cucumber, GPX and PPO have been suggested to contribute to brassinosteroid-induced resistance (Khripach et al. 2000).
Our results emphasize the dual mode of action of these compounds when used for seed treatment. Treatment of seeds can provide a long-lasting protection without adverse effects on plant growth. Using triterpenic derivatives did not affect N. benthamiana growth at the concentration used here (data not shown) and the induced disease resistance lasted up to two months. Tomato seeds have been reported to be receptive to elicitors of plant defense responses, including jasmonic acid, methyl jasmonate and β-aminobutyric acid allowing protection of plants against a broad spectrum of pathogens and insects (Paudel et al. 2014). When applied to tomato seeds, methyl jasmonate improved disease resistance against Fusarium oxysporum f.sp. lycopersici (Krol et al. 2015). Moreover, soaking tomato seeds in jasmonic acid and in β-aminobutyric acid (BABA) resulted in plants with primed defences and increased resistance against fungal pathogens as well as insects (Worral et al. 2012).
Taken together these results revealed that triterpenes derived from E. officinarum and E. resinifera and seed soaking represent new environmentally friendly approach for disease control and can be extended to other crops of economic importance.
References
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Ali SS, Kumar GB, Khan M, Doohan FM (2013) Brassinosteroid enhances resistance to fusarium diseases of barley. Phytopathology 103:1260–1267
Almagro L, Gómez Ros LV, Belchi-Navarro S, Bru R, Ros Barceló A, Pedreño MA (2009) Class III peroxidases in plant defence reactions. J Exp Bot 60:377–390
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8
Borges AA, Jiménez-Arias D, Expósito-Rodríguez M, Sandalio LM, Pérez JA (2014) Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front Plant Sci 5:642. https://doi.org/10.3389/fpls.2014.00642
De Costa F, Yendo AC, Fleck JD et al (2013) Accumulation of a bioactive triterpene saponin fraction of Quillaja brasiliensis leaves is associated with abiotic and biotic stresses. Plant Physiol Biochem 66:56–62
Deng XG, Zhu T, Peng XJ, Xi DH, Guo HQ, Yin Y, Zhang DW, Lin HH (2016) Role of brassinosteroid signaling in modulating tobacco mosaic virus resistance in Nicotiana benthamiana. Sci Rep 6:20579. https://doi.org/10.1038/srep20579
Faize M, Burgos L, Faize L, Petri C, Barba-Espin G, Díaz-Vivancos P, Clemente-Moreno MJ, Alburquerque N, Hernandez JA (2012) Modulation of tobacco bacterial disease resistance using cytosolic ascorbate peroxidase and Cu,Zn-superoxide dismutase. Plant Pathol (5):858–866
Faize M, Faize L, Petri C, Barba-Espin G, Diaz-Vivancos P, Clemente Moreno MJ, Koussa T, Rifai LA, Burgos L, Hernandez JA (2013) Cu/Zn superoxide dismutase and ascorbate peroxidase enhance in vitro shoot multiplication in transgenic plum. J Plant Physiol 170:625–632
Guo YS, Su XY, Cai LT, Wang HC (2017) Phenotypic characterisation of Pseudomonas syringae pv. tabaci, the causal agent of tobacco wildfire. J Plant Pathol 99:499–504
Khripach V, Zhabinskii VN, de Groot AE (2000) Twenty years of brassinosteroids: steroidal plant hormones warrant better crops for the XXI century. Ann Bot 86:441–447
Krol P, Igielski R, Pollmann S, Kepczynska E (2015) Priming of seeds with methyl jasmonate induced resistance to hemibiotroph Fusarium oxysporum f.Sp. lycopersici in tomato via 12-oxo-phytodienoic acid, salicylic acid and flavonol accumulation. J Plant Physiol 179:122–132
Masia A, Ventura M, Gemma H, Sansavini S (1998) Effect of some plant growth regulator treatments on apple fruit ripening. Plant Growth Regul 25:127–134
Moses T, Papadopoulou KK, Osbourn A (2014) Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit Rev Biochem Mol Biol 49:439–462
Osbourn A, Goss RJ, Field RA (2011) The saponins: polar isoprenoids with important and diverse biological activities. Nat Prod Rep 28:1261–1268
Paudel S, Rajotte EG, Felton GW (2014) Benefits and costs of tomato seed treatment with plant defense elicitors for insect resistance. Arthropod Plant Interact 8:539–545
Petrov V.D., Van Breusegem F. (2012). Hydrogen peroxide-a central hub for information flow in plant cells. AoB Plants. pls014 https://doi.org/10.1093/aobpla/pls014
Smaili A, Mazoir N, Rifai LA, Koussa T, Makroum K, Kabil EM, Benharref A, Faize M (2017a) Triterpene derivatives from Euphorbia enhance resistance against Verticillium wilt of tomato. Phytochemistry 135:2662–2667
Smaili A, Mazoir N, Rifai LA, Koussa T, Makroum K, Benharref A, Faize L, Alburquerque N, Burgos L, Belfaiza M, Faize M (2017b) Antimicrobial activity of two semisynthetic triterpenic derivatives from Euphorbia officinarum latex against fungal and bacterial phytopathogens. Nat Prod Commun 12:331–336
Thimmappa R, Geisler K, Louveau T, O’Maille P, Osbourn A (2014) Triterpene biosynthesis in plants. Annu Rev Plant Biol 65:225–257
Vaughn KC, Lax AR, Duke SO (1988) Polyphenol oxidase-the chloroplast oxidase with no established function. Physiol Plant 72:659–665
Worral D, Holroyd GH, Moore JP, Glowacz M, Croft P, Taylor JE, Paul ND, Roberts MR (2012) Treating seeds with activators of plant defence generates long-lasting priming of resistance to pests and pathogens. New Phytol 193:770–778
Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen Z, Yu JQ (2009) Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol 150:801–814
Zhang DW, Deng XG, Fu FQ, Lin HH (2015) Induction of plant virus defense response by brassinosteroids and brassinosteroid signaling in Arabidopsis thaliana. Planta 241:875–885
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smaili, A., Mazoir, N., Aicha Rifai, L. et al. Induced resistance to wild fire disease of Nicotiana benthamiana using seed treated with triterpene derivatives from Euphorbia. J Plant Pathol 100, 75–83 (2018). https://doi.org/10.1007/s42161-018-0031-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-018-0031-0