Abstract
The prevalence of nonalcoholic fatty liver disease has been continuously rising over the last three decades and is projected to become the most common indication for liver transplantation in the near future. Its pathophysiology and complex interplay with diabetes and the metabolic syndrome are not as yet fully understood despite growing scientific interest and research. Modern imaging techniques offer significant assistance in this field by enabling the study of the liver noninvasively and evaluation of the degree of both steatosis and fibrosis, and even in attempting to diagnose the presence of inflammation (steatohepatitis). The derived measurements are highly precise, accurate and reproducible, performing better than biopsy in terms of quantification. In this article, these imaging techniques are overviewed and their performance regarding diagnosis, stratification and monitoring are evaluated. Their expanding role both in the research arena and in clinical practice along with their limitations is also discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prevalence of nonalcoholic fatty liver disease has been continuously on the rise during the last three decades alongside the growing epidemics of obesity, diabetes and metabolic syndrome. It is currently the most common cause of abnormal liver tests and chronic liver disease in Western countries and is projected to become the most common indication for liver transplantation in the near future. Its rates are increasing worldwide, i.e. not only in the Western world but also in many less developed countries due to the adoption of an inactive, sedentary lifestyle combined with the consumption of cheap, processed, high-calorie foods that accompany industrialization and urbanization. In the USA, at least one third of the adult population is affected and the prevalence is comparable in Europe. In Greece, a high analogous rate of liver steatosis (approximately 31%) and steatohepatitis has also been reported [1] paralleling the global trend.
Nonalcoholic fatty liver disease (NAFLD) is defined as the presence of hepatic steatosis, documented either by imaging or by histology, in the absence of secondary causes of hepatic fat accumulation such as significant alcohol consumption (> 21 standard drinks in men and > 14 standard drinks in women on average per week, or a daily alcohol consumption ≥ 30 g for men and ≥ 20 g for women), long-term use of steatogenic medication, or monogenic hereditary disorders [2, 3]. It is subclassified into simple steatosis (nonalcoholic fatty liver, NAFL) and steatohepatitis (nonalcoholic steatohepatitis, NASH); the former generally runs a benign course, while the latter represents the progressive form of the disease with significant hepatic and extrahepatic morbidity and mortality. NASH is present in up to 25–30% of individuals with NAFLD. Nonalcoholic steatohepatitis may progress to cirrhosis, with the associated comorbidities (portal hypertension, hepatocellular carcinoma), and it also carries a substantial risk for cardiovascular disease and malignancy (accounting for half of deaths in patients with NASH) [4]. Approximately 20% of these patients progress to cirrhosis, in contrast to only 4% of those with simple steatosis [5]. Histologically, NAFL is characterized by simple, predominantly macrovesicular, steatosis in > 5% of hepatocytes, while NASH is differentiated by the additional features of ballooning degeneration of hepatocytes and inflammation, with or without fibrosis. The presence and degree of liver fibrosis on histology is the most important evidence-based predictor of adverse long-term outcomes [6, 7]. Therefore, differentiation between the two entities and assessment of fibrosis carries significant prognostic implications for risk stratification and patient management.
Nonalcoholic fatty liver disease and metabolic syndrome are closely linked in terms of both pathophysiology and epidemiology. Generally thought to represent the hepatic manifestation of metabolic syndrome, NAFLD may be more than this according to data suggesting that it could actually act as an independent risk factor for cardiovascular morbidity [8]. Although NAFLD may be seen in the absence of metabolic syndrome and vice versa, the presence of features of the metabolic syndrome (central obesity, hypertension, insulin resistance, increased serum triglycerides and decreased high-density lipoprotein) is associated with a higher risk of steatohepatitis (NASH). Even though it is still a matter of debate, it seems that cardiovascular risk is not the same across the entire NAFLD spectrum, patients with NASH showing a worse survival rate mainly due to cardiovascular disease [9]. Consequently, the incidental discovery of a fatty liver (e.g. during ultrasonography performed for another reason) should prompt a careful evaluation for all components of metabolic syndrome and identification of cardiovascular disease, provided that other secondary causes of steatosis have been excluded [3]. If risk factors for NASH are present or there is concern about advanced disease, then evaluation of fibrosis should follow, initially with noninvasive tests (imaging, serum markers, clinical scores) [4].
Despite the growing burden of NAFLD, the extensive research and the flood of scientific data, treatment remains an open question. No pharmacological treatment is currently approved for NAFLD, and therapy relies on lifestyle modifications through diet and physical exercise. Guidelines on how and when to follow up patients are also thus far undetermined. However, as new drugs enter clinical testing and more robust evidence from larger studies accumulates, the need for the optimal tool to establish the diagnosis, monitor therapy and follow up patients will become pressing.
Traditionally, the gold standard for the quantification of liver steatosis and diagnosis of NASH has been liver biopsy. With the wide application of ultrasound guidance and thinner needles, it is generally considered a safe method. However, despite its minimal invasiveness, complications may occur, the most important being bleeding which may even lead to death (≤ 0.01%) [10]. Additionally, due to its invasive nature, acceptance by patients is low, leading to poor compliance, especially if repeated biopsies are required during follow-up. A major limitation of liver biopsy is that it evaluates only a very small portion of the liver (approximately 1/50,000) leading to sampling errors, as distribution of fat and fibrosis is frequently nonuniform; this sampling variability may render longitudinal monitoring problematic due to staging inaccuracies. Furthermore, histological assessment is subject to nonnegligible intra- and inter-observer variation, although concordance for fibrosis and steatosis is generally higher than for inflammation [11, 12]. Liver biopsy is not feasible in screening studies of the general population, not only because of its invasiveness and associated risks but also on account of its high cost. Even in patients with NAFLD, biopsy should be considered only when clinical features, noninvasive markers and imaging suggest the possibility of NASH. For the reasons stated above, a noninvasive, accurate and safe method to detect and quantify hepatic steatosis, evaluate fibrosis and monitor disease is necessary. Imaging has the potential to meet these needs.
Scope
The aforementioned issues, briefly touched upon in the introduction, outline the purpose of this review: (a) to examine the role of imaging in the diagnosis of NAFLD and the performance of the different modalities, (b) to explore the potential of imaging in risk stratification and specifically in quantifying fibrosis and differentiating NASH from simple NAFL and (c) to discuss the role of imaging in the follow-up of these patients and the monitoring of treatment efficacy.
Imaging methods for diagnosis and quantification of hepatic steatosis
Ultrasound
Ultrasound is the most widely available modality for initial evaluation of suspected hepatic steatosis, usually after detection of abnormal liver function tests. It is noninvasive, easy to perform and inexpensive and does not expose the patient to ionizing radiation. Moreover, incidental focal lesions (accounting for the abnormal serum tests) may be detected and the rest of the upper abdomen can be easily examined. However, it is operator- and equipment-dependent and difficult to perform in obese patients, while excessive intestinal gas may compromise the quality of the examination. Additionally, assessment of steatosis is subjective as there is no standard measure of liver echogenicity. The sensitivity for the detection of moderate and severe steatosis (defined as > 30% by histology) is reported at 84.8% and specificity at 93.6% [13], but they drop to 53.3–65 and 77–81.2%, respectively, if all degrees of steatosis are considered [14, 15] Specificity is also reduced in the presence of inflammation or fibrosis.
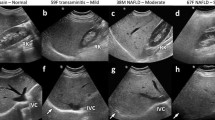
On ultrasound, fatty liver demonstrates increased echogenicity (“bright liver”) due to the increased interfaces created by fat droplets within hepatocytes, leading to more echoes returning to the transducer [16]. The decreased ability of ultrasound to penetrate the liver results in a hypoechoic distant field and inability to visualize the deepest parts of the liver and the diaphragm (Fig. 1). There is usually hepatomegaly and decreased conspicuity of the intrahepatic vessels (“featureless liver”). According to these imaging characteristics, liver steatosis is usually graded as mild (increased echogenicity but normal visualization of vessels and diaphragm), moderate (poor visualization of the intrahepatic vessels) and severe (diaphragm and deep parenchyma are not seen).
Doppler ultrasound has also been evaluated for assessment of NAFLD, suggesting alterations in portal vein hemodynamics, possibly due to reduced vascular compliance, especially in the setting of severe fatty infiltration [17,18,19]. However, it is not clear if the method offers any advantage over conventional greyscale ultrasound.
An ultrasound-based method to estimate liver fat, named controlled attenuation parameter (CAP), measures ultrasound attenuation in the liver, and the calculated value is expressed in decibels per meter (dB/m); steatosis grade is then assessed by comparing the measured value with validated cut-offs [20]. This measurement is performed simultaneously with transient elastography (see below).
Computed tomography
Computed tomography (CT) estimates hepatic steatosis quantitatively by measuring liver attenuation. Normally, liver CT density (attenuation) measures approximately 55 HU (Hounsfield units), about 10 HU higher than (normal) spleen. As fat has low attenuation (− 100 HU), a proportionate decrease in density is seen with increasing fat accumulation. Both the absolute and relative (to the spleen) values are used to characterize steatosis; a cut-off value of 40 HU has been suggested to predict fat content greater than 30% [21], a liver to spleen ratio less than 0.8–1.1 [22,23,24] and a liver minus spleen attenuation less than − 9 HU [23] (spleen is used as a reference value to minimize variations across different CT scanner models). Moreover, as a practical guide, the severely steatotic liver parenchyma appears much darker than the unenhanced hepatic vessels, giving the impression of a contrast-enhanced scan (Fig. 2). Cut-off values have also been calculated for post-contrast scans [23, 25] but are subject to variability as they depend on injection rate, patient’s circulation and phase of enhancement.
Unenhanced CT scan. In the posterior part of the right lobe, there is a geographically shaped hypodense area (arrows) due to irregular fat accumulation, giving a false impression of a mass. Attenuation in this area (28 HU) is markedly lower than that in the spleen (50 HU), while steatosis in the left lobe is much milder (47 HU). This example stresses the sampling error that may occur with biopsy, where only a minute part of the liver is examined
Although CT is, unlike ultrasound, a quantitative method, it still suffers from low sensitivity for mild steatosis [15, 26, 27]. Sensitivity and specificity of unenhanced CT for steatosis ≥ 30% are 72.7 and 91.3%, respectively, but—similarly to ultrasound—drop to 50 and 77.2% when milder degrees (steatosis > 5%) are also included [14]. CT density may be spuriously elevated due to increased iron, copper or glycogen content, thereby masking steatosis; likewise, low CT density values may also be caused by edema and inflammation. Exposure to radiation is also an important issue, which precludes use of this modality for screening.
Dual-energy CT, using different rapidly switched tube potentials, has the potential advantage of tissue discrimination. The main principle is that different materials (e.g. tissues) have different attenuations at different X-ray spectra (different tube voltage). Promising reports on measuring fat content have been published [28, 29]; however, contradictory results have also been reported [30, 31], perhaps due to the small differences in the attenuation energy curves of fat and water.
MRI
Magnetic resonance imaging (MRI) is presently the most accurate imaging modality for the evaluation of hepatic steatosis. The MR signal arises mainly from protons in water and fat molecules. By exploiting the different physical and chemical properties of water protons and fat protons, various MR techniques have been developed for identification and quantification of liver fat. For example, based on the different spin-lattice relaxation time (T1) of fat and water, the fat signal can be suppressed and the resulting images allow a gross visual assessment. However, more precise techniques are preferred for evaluation of liver fat.
Such a technique is the chemical shift, which is based on the different resonance frequencies between protons bound in a fat and water molecule. In its simplest implementation, the “in/opposed phase” sequence, images are acquired at specific echo times when water and fat signals are added and subtracted, producing “in-phase” and “opposed phase” images, respectively (two-point Dixon method). In the fatty liver, there is signal loss in the opposed phase images and the liver appears dark (Fig. 3). This sequence is available on every scanner and is part of all upper abdominal MR protocols. However, the assessment of hepatic steatosis is qualitative (or semiquantitative at best), and therefore, the technique cannot detect small changes in fat content, thus rendering it unsuitable for longitudinal follow-up. Still, sensitivity and specificity of the method are high (82–90 and 89.9–91%, respectively, for detecting all degrees of steatosis), and therefore, MRI is superior to US and CT for differentiating mild steatosis [15, 32, 33].
More complicated techniques, still based on the chemical shift effect, have been developed to accurately quantify liver fat. These techniques (multipoint Dixon) measure the proton density fat fraction (PDFF), which represents the concentration of fat within tissue. PDFF accurately and specifically reflects the concentration of triglycerides within hepatocytes (fat vacuoles) as other lipids within cell membranes and organelles are occult at MR imaging [34]. For a precise estimation, it is important to correct for many confounding factors like T1 bias, T2* bias (often due to the coexistence of iron), spectral complexity of fat, noise bias, and phase errors. PDFF measurements obtained from the whole liver can be graphically presented as a colour map, generated on a pixel-by-pixel basis, overcoming the sampling error issue which is inherent in liver biopsy (Fig. 4). Using this method, MRI is able to provide a standardized, reproducible and highly accurate measurement of hepatic fat, which is closely associated with histology [35, 36]. Sensitivity up to 96% and specificity up to 100% for detecting any degree of steatosis have been reported [37]. Various PDFF thresholds for stratifying steatosis grades have been proposed, each showing high specificity for the particular cohort studied though not validated in longer prospective studies. A fat-fraction threshold of 5.56% based on a large MR spectroscopy study [38] is commonly used to define steatosis; however, the optimal cut-off value still needs to be defined. The technique measuring PDFF is approved by the FDA [39] and is commercially available worldwide from most MR manufacturers.
Examples of MRI PDFF maps and corresponding MRS in three subjects with normal fat concentration, and mild and severe liver steatosis. Reprinted with permission from [49]
MRS
Magnetic resonance spectroscopy (MRS) is the most direct technique to separate the signal from fat and water using the chemical shift effect. It displays the signal amplitude of protons as a function of their resonance frequency. Water has a single resonance peak, while fat has multiple spectral peaks, as the multiple protons in fat have different chemical bonds, experience different magnetic fields and resonate at different frequencies (spectral complexity of fat). At clinical field strengths (1.5 and 3 T), six well-defined peaks are formed and should all be taken into account for accurate fat quantification. The area under the water peak and every fat peak is measured, the sum of the fat peak areas is calculated (=total fat signal)—or, alternatively, only the area under the main methylene peak is measured—and the total fat signal is added to the water signal. The signal fat fraction (FF) is given as the fat signal (F) divided by the sum of the water (W) and fat signals (FF = F/W + F). Spectroscopy is usually performed with a single cubic voxel, typically with a size of 2 x 2 x 2 or 3 x 3 x 3 cm3, which is placed manually in the liver parenchyma. Like the other MR methods outlined earlier, sensitivity and specificity for diagnosing any degree of steatosis (> 5%) are high and reported at 94.4 and 89.5%, respectively [40]. However, unlike other MRI methods, the whole liver is not evaluated and sampling errors may occur, similarly to liver biopsy. Both the acquisition and analysis of the collected spectra require expertise and specialized software, limiting the availability of the technique. Despite these practical limitations, MRS is considered to be the gold standard MR method for hepatic fat quantification [15, 40, 41]. The strengths and limitations of each imaging technique are summarized in Table 1.
PDFF as an imaging biomarker
The proton density fat fraction, measured by both MRI and MRS, provides an excellent measurement of hepatic fat content. It represents an inherent physical property of the tissue and is not confounded by inflammation or iron overload (which commonly coexist in liver disease) [37, 40]. It is highly correlated with tissue histological grades in patients with NAFLD [42, 43] and in diabetics [44] although this correlation may drop in the setting of advanced fibrosis [45]. It is a very precise, reliable and reproducible method across different scanner models and manufacturers [46,47,48] as well as among adults and children [45, 49]. It offers the advantage of measuring objectively the changes in fat content in longitudinal studies, which would not be possible by histology (obtaining multiple liver biopsies), and therefore the ability to assess response to therapy. Indeed, PDFF measurement has been successfully used as a primary endpoint in clinical trials, alternatively to biopsy, detecting smaller changes in liver fat compared to histology [50,51,52]. This wealth of validation data strongly and convincingly supports PDFF as a robust noninvasive biomarker of steatosis.
For a biomarker to establish its role, especially if it is to be used in large screening programs and clinical trials, it should be widely available and cost-effective. Although MRI scanners are abundant in developed countries, the PDFF pulse sequences may not be available in all imaging centres. Therefore, some time is needed until the technique is adopted and the necessary level of expertise is reached. The incremental cost of adding a fat quantifying sequence during an abdominal MRI performed for another reason is negligible. Moreover, a dedicated MRI examination for assessment of steatosis would require no more than 5 min and such a tailored “abbreviated” protocol could be offered at a cost comparable to that of a panel of serum tests or an abdominal ultrasound scan [53]. A recent UK study examined a hypothetical decision-tree model and concluded that use of multiparametric MRI as an adjunct or as a replacement of transient elastography resulted in a decrease in the number of biopsies required, with associated cost savings in both scenarios [54].
Despite the exciting prospect of MRI-derived virtual biopsy, there are some limitations that should be noted in addition to the limited availability of these complex techniques. PDFF sequences cannot evaluate other features of liver disease like fibrosis or inflammation, although other MRI techniques have been used for this purpose. Patients with pacemakers and implantable defibrillators are normally not allowed to undergo MR scanning, though this may not be an absolute contraindication in the future [55]. As wide-bore magnets are not yet widespread, large body habitus remains a barrier; however, the other available methods for liver evaluation (ultrasound, transient elastography) are also problematic in this patient group, which represents a considerable percentage of the NAFLD cohort. Finally, as mentioned above, the precise PDFF threshold between normal and abnormal liver fat has yet to be specified.
Imaging of fibrosis—risk stratification
The identification of liver steatosis, either as a chance finding or during a targeted examination following abnormal liver tests, establishes the diagnosis of NAFLD. Hepatic steatosis predicts future diabetes mellitus, cardiovascular events and arterial hypertension. It has been shown that the occurrence of steatosis, defined by imaging, was connected with aggravation of metabolic comorbidities associated with insulin resistance and that, conversely, resolution of steatosis coincided with their regression [56]. Although the degree of steatosis has been shown to be an independent factor for disease progression, patients with steatosis alone (without any inflammation or fibrosis) are at a very low risk of histological progression [57]. However, it has not yet been demonstrated that the quantification of steatosis is of clinical interest, except as a surrogate of treatment efficacy (e.g. after dietary or pharmacological interventions). Therefore, the diagnosis but not the quantification of liver fat is recommended [3]. Moreover, it is known that the relationship between liver fat and fibrosis is an inverse one and that decrease in hepatic fat may not indicate response to therapy but worsening of hepatic fibrosis. For example, in obese mice with NASH, inhibition of triglyceride synthesis improved steatosis but exacerbated liver damage and fibrosis [58]. In addition to this, it is known that in the late cirrhotic stage there is loss of fat droplets from hepatocytes leading to the so-called burnt-out NAFLD (thus explaining why NAFLD escaped detection as the cause of many cases of “cryptogenic” cirrhosis).
Once diagnosis of NAFLD has been established, the next important step in patient management is to confirm or rule out the presence of steatohepatitis (NASH), taking into account the clinical risk factors; for instance, metabolic syndrome is a strong predictor of the presence of NASH in patients with hepatic steatosis [2]. The diagnosis of NASH provides important prognostic information and implies that more active follow-up or more aggressive therapy may be required. Numerous serum markers have been tested for the noninvasive diagnosis of steatohepatitis. Among these, cytokeratin-18 (CK-18) fragments that are created during cell death or apoptosis have been widely investigated and proposed as a promising biomarker, but its accuracy is modest. To date, there is no validated noninvasive procedure to replace biopsy and diagnosis can be made only by obtaining a tissue sample. Histologically, NASH is defined as the presence of macrovesicular steatosis, hepatocyte ballooning and lobular inflammation; fibrosis is not part of the diagnostic criteria.
Chronic inflammation and hepatocyte injury activate stellate cells, leading to fibrosis. Although fibrosis is not included in the histological criteria for NASH, it is frequently present and it is the most important determinant of outcome, both for cardiovascular and liver-related mortality [6, 7, 59, 60]. Progression of fibrosis in the fatty liver represents a complex procedure where the immune system, dietary factors, host genetics and the gut microflora play a role [61]. Diabetes, increased body mass index and aging have been shown to accelerate fibrosis in patients with NAFLD. Moreover, it seems that fibrosis progresses not only in patients with steatohepatitis but also in a subgroup of patients with simple NAFL (who demonstrate delicate fibrosis or mild inflammation but do not fulfil the histological criteria for NASH), albeit at a much slower pace [57, 62]. Hence, its identification is crucial for patient stratification and its monitoring important during follow-up.
Liver fibrosis can be evaluated using various noninvasive imaging tests. Conventional ultrasound, CT and MRI assess morphologic abnormalities of the liver, like the nodular contour and the increased caudate to right lobe ratio. These features suffer from low sensitivity and are not present at the initial stages of fibrosis, while many of them are entirely subjective. Moreover, it is known that a minority of patients with advanced fibrosis or even cirrhosis on liver biopsy may show normal morphologic features on conventional imaging [63]. Consequently, alternative, more quantitative and reliable methods were sought.
Ultrasound elastography is a technique that measures liver stiffness by measuring the velocity of mechanically induced shear waves propagating through the liver. Liver stiffness increases incrementally with histological stage of fibrosis. Transient elastography (TE), performed with FibroScan®, was the first technology of this kind to be extensively used and validated. The technique is quick and safe and it evaluates a volume of liver tissue, approximately 4 × 1 cm, which is at least 100 times bigger than liver biopsy [64]. TE has an excellent negative predictive value for cirrhosis, up to 96% [65], but performs moderately for lesser degrees of fibrosis and cannot accurately distinguish between intermediate fibrosis stages [66, 67]. Additionally, the technique is less reliable in obese patients; although an XL probe for these patients has been developed, obesity is still associated with less accurate and reliable measurements [68].
As mentioned earlier, it is possible to assess the liver for steatosis with the controlled attenuation parameter (CAP) technology while performing transient elastography. Therefore, using the same device, hepatic fat and stiffness can be concomitantly measured in a quick and inexpensive way. However, when directly compared with MRI, both CAP and TE proved less accurate for the quantification of steatosis and fibrosis, respectively [69].
It should be mentioned that TE is not literally an imaging technique as it does not display any images of the liver or the location where stiffness is measured. Alternative modalities like 2D shear wave elastography (or supersonic shear imaging, SSI) and acoustic radiation force impulse (ARFI) incorporate true imaging, and the site of measurement can be saved on the images and subsequently used for accurate comparisons during follow-up. Moreover, these imaging modalities allow hepatic parenchyma evaluation (e.g. screening for HCC) during the same session. They have shown promising results for the diagnosis and staging of fibrosis, though obesity is associated with an increased rate of measurement failure [70].
Magnetic resonance elastography (MRE) is the flagship of imaging techniques for the evaluation of hepatic fibrosis. MRE uses an acoustic wave generator, placed outside the scanner room, which is connected via a tube to a passive nonmetallic driver. The driver is placed in contact with the patient’s abdomen and transmits the vibrations into the body to generate propagating shear waves. Τhe microscopic displacements caused by the propagating waves are captured and processed to create quantitative cross-sectional maps (elastograms) of the whole liver. Liver stiffness is measured in a manually drawn region of interest or averaged from several slices and is reported in kilopascals (kPa) [71].
MRE can be performed in obese patients and in patients with ascites, showing a higher technical success rate and better accuracy compared with TE [69]. It can accurately diagnose early and mild fibrosis, and it is highly reproducible with excellent inter-observer agreement, higher than histological staging [72], partly due to the large volume of liver assessed. The MRE sequence lasts less than a minute and can be added to a standard upper abdominal protocol to provide a fully comprehensive evaluation of the liver. The main limitation of MRE is its limited availability, requiring added hardware (wave generator) and special software (for image acquisition and processing). Increased iron deposition in the liver may lead to technical failure as iron may decrease signal intensity to extremely low levels, prohibiting visualization of the propagating waves [73].
MR elastography has also been used to discriminate NASH from NAFL even before the onset of fibrosis. Researchers have found that liver stiffness was significantly higher in patients with inflammation (but without fibrosis) compared to simple steatosis, allowing differentiation between the two with reported sensitivity of 94% and specificity of 73% [74]. It is believed that early liver injury leads to changes in the extracellular matrix that increase the stiffness of hepatic tissue. Unfortunately, no further research on this issue was published and validation is still lacking.
Other imaging techniques
Apart from the aforementioned, numerous other imaging techniques have been devised in an effort to noninvasively diagnose liver fibrosis; some of them will be briefly presented in the following paragraphs.
Based on the idea that fibrous tissue accumulation increases tissue stiffness and may translate into increased arterial rigidity, researchers have found a positive correlation between degree of fibrosis and hepatic artery resistive index, measured with Doppler ultrasound [75]. Contrast-enhanced ultrasound (CEUS) of the liver is mainly used to characterize focal lesions, based on their specific hemodynamic properties. The differences in blood flow parameters, like time to peak enhancement, have been used to diagnose cirrhosis but do not seem effective for staging fibrosis [76].
Similarly, the microcirculatory changes due to hepatic fibrinogenesis and the resulting increased hepatic resistance have been studied with perfusion CT (repeated scans of the liver to evaluate flow parameters after contrast administration). The perfusion changes occurring early during fibrosis have been successfully detected with this technique [77, 78]; however, the increased radiation burden is a significant barrier. The increased deposition of collagen into the extracellular space leads to its expansion; consequently, the amount of iodinated contrast that diffuses into this space, in the late or equilibrium phase of enhancement, should also be increased. Therefore, by using only two scans (unenhanced and equilibrium phase), the parenchymal enhancement can be measured and the extracellular volume calculated. The technique proved efficient in detecting cirrhosis and significant fibrosis, but not in distinguishing earlier stages [79, 80].
Apart from MR elastography, there have been other interesting attempts to use MRI to assess liver fibrosis and differentiate NASH from NAFL. Similarly to CT, MR perfusion has been used to detect fibrosis but showed lower accuracy than MRE [81]. Gadoxetic acid (Gd-EOB-DTPA) is a liver-specific contrast agent which is taken up and excreted by hepatocyte membrane transporters thus providing an estimation of liver function. The degree of enhancement has been used to stage liver fibrosis. Moreover, gadoxetic acid enhancement was significantly lower in NASH compared to simple steatosis [82], suggesting a potential ability to discriminate between these two forms of NAFLD. This decrease may be explained by the hepatocellular injuries caused by NASH and is proportional to the degree of liver fibrosis. However, the considerable overlap in the enhancement ratios and the low specificity argue against its use for differentiating simple steatosis from steatohepatitis [83]. Similarly to gadoxetic acid, ultrasmall superparamagnetic iron oxide particles (USPIOs) have been tested for the aforementioned differentiation. These particles are taken up by hepatic Kupffer cells resulting in drop of signal in the liver. This decrease was smaller in NASH compared to simple steatosis, probably due to dysfunction of Kupffer cells in the setting of steatohepatitis [84]. Post-contrast imaging is performed 72 h after administration of the particles, making the procedure somewhat cumbersome. Though the technique yielded promising results, further validation is needed. Research has also focused on myeloperoxidase, a proinflammatory and highly oxidizing inflammatory enzyme, which is abundant in livers with NASH. Using a specific MR imaging agent to target this enzyme, the inflammatory activity in the liver could be assessed, providing a potential biomarker for the direct noninvasive diagnosis of NASH [85]. Finally, a modified spectroscopy technique (MRS), focusing on phosphorus (31P) instead of protons (1H), has been exploited to study hepatocellular metabolism, but it performed only moderately in the discrimination between NASH and NAFLD [86].
Radiomics (texture analysis) is a new area of imaging research that aims to extract many quantitative features by applying sophisticated mathematical transformations and statistical analysis to the distribution of pixels in radiographic images (US, CT, MRI and even PET). A myriad of texture features are derived that can then be correlated with disease or genome. The method is most well developed in oncology; however, it has also been applied in the study of liver fibrosis [87, 88]. Currently, the problem with radiomics is the great variability in the features studied, the lack of standardization and poor validation; nevertheless, the method is a promising one and will likely play an important diagnostic and prognostic role in the future.
Imaging for monitoring and follow-up
Although NAFLD is common in the general population, it is generally not associated with significant mortality or morbidity. However, as stressed before, a considerably higher proportion of patients with NASH will progress to fibrosis and cirrhosis compared to patients with simple steatosis. This dogmatic division of NAFLD into a “good” and a “bad” form has become less rigid as our understanding and insight into the natural history of the disease expands; it is now accepted that both forms may progress—though at a different speed—to liver fibrosis [57, 62]. Since the best predictor is fibrosis severity, and not NASH, it is essential to be able to assess the amount of fibrosis present as advanced fibrosis portends the worst prognosis. Therefore, the ideal strategy would be to try to identify patients with liver fibrosis even in the absence of NASH.
Regarding steatosis, it is generally accepted that the degree of fatty change is not correlated with the advancement of fibrosis and therefore it should not be employed as an evaluation criterion for assessing NAFLD severity. Nonetheless, there are some studies suggesting that the degree of steatosis is positively associated with some (but not all) of the histological features of steatohepatitis both in adults and children [89, 90]. Although the prevalence of NAFLD is increased in patients with type 2 diabetes (up to 75%) and diabetics are more prone to develop NASH, there are data suggesting that NAFLD may actually be a predictor and determinant for diabetes (rather than being merely an epiphenomenon) [91, 92]. Therefore, quantification and monitoring of fat seems justified in addition to fibrosis assessment.
Despite the fact that no medical therapy is currently approved for NASH, research is intense and ongoing, and clinical trials would surely make full use of a reliable noninvasive marker that could replace biopsy (currently recommended in clinical trials by all guidelines but hampered by a considerable dropout rate of up to 25% [93]). Consequently, an accurate and reproducible noninvasive method for evaluation of liver steatosis and fibrosis is indispensable in the struggle to unravel and manage the disease.
Noninvasive imaging biomarkers for the diagnosis, staging and monitoring of NASH have been successfully used in clinical trials. MRI PDFF can reliably detect longitudinal changes as small as 2% (standard deviation of repeated PDFF measurements being < 1%), while histological grading of steatosis (percentage of hepatocytes with fat) into four grades (< 5, 5–33, 33–66 and > 66%) is much grosser. These small changes in liver fat appreciated by MRI PDFF, but not by histology, correlated well with MRS (the gold standard MR method for fat quantification) and were also associated with changes in body weight and serum markers [51]. MRI PDFF and MRE have been utilized to examine the efficacy of ezetimibe in lowering liver fat and correlated well with the histological results, proving that application of these MR techniques for assessing treatment response in NASH is feasible [94]. Moreover, it was shown that histological response was associated with a statistically significant decrease in MRI PDFF and that, on the other hand, a 29% reduction in MRI PDFF was required for steatosis to regress by one grade histologically. Although this decline was accompanied by equivalent improvement in hepatocellular ballooning, it was not associated with any significant change in lobular inflammation and fibrosis, this pointing to the necessity for caution before the 29% decrement on MRI PDFF is used as a cut-off of treatment response in NASH [95]. MRI PDFF and MRS detected slight changes in hepatic fat that escaped histological assessment in a trial evaluating colesevelam for NASH [52]. In the same trial, a decrease of 5% or greater in BMI correlated significantly with a decrease in MRI PDFF by 25% and with a decrease in histology-determined steatosis grade. Notably, no significant change in other components of the NAS (NAFLD Activity Score) score was observed for this range of BMI reduction. Consequently, further validation is needed before a specific percentage decrease in MRI PDFF can be suggested as a meaningful endpoint in trials evaluating weight loss—the backbone of treatment for NAFLD and NASH [96]. Other researchers employed MRI PDFF and MRE to screen for NAFLD and advanced fibrosis in patients with type 2 diabetes [97]. Assessing fibrosis simultaneously with steatosis provides a full estimate of the severity of the disease, as patients with NAFLD and diabetes have increased risk of cirrhosis and overall mortality. Despite the fact that screening is not advised for diabetics by the American Association for the Study of Liver Diseases, even in their recently revised guidelines [2], the authors of this study concluded that using MR techniques for screening is feasible and can be used to identify patients at risk.
In a recent paper, a novel multiparametric MR technique based on T1 and T2* mapping of the liver achieved high diagnostic accuracy for diagnosis of NASH, showing significant correlation with hepatocyte ballooning, lobular inflammation and fibrosis grade [98]. This technique, without the additional hardware needed for elastography or any injectable contrast material, can be performed quickly and sounds attractive as a noninvasive tool both for clinical practice and trials; further validation is required to confirm the findings.
From the examples presented herein, it is clear that MR imaging biomarkers are being increasingly used as surrogate endpoints in clinical trials. However, they are not—as yet—robust enough to diagnose NASH and assess the full effect of treatment. Further validation and larger studies are required to define the optimal use of these modalities in noninvasive screening and monitoring protocols. Additionally, these MR techniques have to be compared to ultrasound-based techniques which, although less accurate and precise, are more widely available and potentially more cost-effective.
Conclusions
As NAFLD becomes more prevalent in the general population and evolves into a major public health issue, awareness of its clinical significance is increasing within the scientific community in parallel with the intensification of research. New insights into the pathophysiology and course of the disease as well as its connection to the metabolic syndrome are becoming available, preparing the ground for future treatments. Our ability to study NAFLD has greatly expanded thanks to the impressive advances in modern imaging and the potential they offer to evaluate the liver noninvasively. As the sampling limitations of biopsy are increasingly recognized, its acceptance as a “gold standard” is gradually losing its golden shine; moreover, its complication rate, although low, could be an important issue when large cohorts are screened. Serum markers, used on their own or in complex prediction models, have not so far provided a reliable alternative for the accurate assessment of hepatic steatosis and fibrosis.
Imaging, on the other hand, has emerged as the most qualified candidate for the noninvasive evaluation of the liver. Importantly, the most promising and validated techniques are based on ultrasound imaging and magnetic resonance, neither of which involves ionizing radiation. Ultrasound-based techniques, namely transient elastography and controlled attenuation parameter, are inexpensive, quick and easy to perform; however, they carry a significant failure rate, especially in overweight patients. Magnetic resonance techniques allow more accurate, reproducible and precise quantification of hepatic steatosis and fibrosis. Magnetic resonance spectroscopy is considered to be the gold standard MR method; nevertheless, it is a complex technique and evaluates only a very small part of the parenchyma, with the concomitant sampling error. Measurement of proton density fat fraction can be performed throughout the whole liver on a pixel-by-pixel basis and is highly correlated with spectroscopy. It is more easily implemented, does not require significant expertise, is more widely available and is not limited by body habitus. Unlike fat, fibrosis does not have a unique molecular signature and can be assessed only indirectly. Of the various MR techniques suggested, magnetic resonance elastography is the most accurate, evaluating liver stiffness through the use of mechanical shear waves similarly to transient elastography. However, contrary to transient elastography, MR elastography is not influenced by patient demographics and allows evaluation of the entire liver.
Overall, MR techniques are the most robust and accurate methods for quantification of steatosis and fibrosis. A dedicated MR protocol combining proton density fat fraction measurement and elastography could be performed in a few minutes and at a reasonable cost; it could even prove cost-effective as an initial examination considering the expenses associated with false-positive or false-negative results obtained with less accurate biomarkers. Perhaps a more reasonable approach would be to scan patients with ultrasound-based techniques in the first place and then refer those with abnormal or inconclusive results for MRI. Despite the greater accuracy and inter-observer agreement of MRI compared to histology, the latter can detect features of inflammation that are still beyond contemporary imaging capability, although research may soon overcome this issue as well. Biopsy is not expected to be completely replaced in the immediate future; nonetheless, imaging techniques have already started to change the diagnostic algorithm for NAFLD, impact the design of clinical trials and shape the way patients will be monitored as soon as the new therapies enter clinical practice.
References
Zois CD, Baltayiannis GH, Bekiari A et al (2010) Steatosis and steatohepatitis in postmortem material from Northwestern Greece. World J Gastroenterol 16:3944–3949
Chalasani N, Younossi Z, Lavine JE et al (2017) The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67:328–357
EASL-EASD-EASO (2016) Clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 64:1388–1402
Rinella ME (2015) Nonalcoholic fatty liver disease: a systematic review. JAMA 313:2263–2273
Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ (1999) Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 116:1413–1419
Ekstedt M, Hagström H, Nasr P et al (2015) Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61:1547–1554
Younossi ZM, Stepanova M, Rafiq N et al (2011) Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology 53:1874–1882
Polyzos SA, Mantzoros CS (2016) Nonalcoholic fatty future disease. Metabolism 65:1007–1016
Athyros VG, Tziomalos K, Katsiki N, Doumas M, Karagiannis A, Mikhailidis DP (2015) Cardiovascular risk across the histological spectrum and the clinical manifestations of non-alcoholic fatty liver disease: an update. World J Gastroenterol 21:6820–6834
Rockey D, Caldwell S, Goodman Z et al (2009) Liver biopsy. Hepatology 49:1017–1044
Pournik O, Alavian SM, Ghalichi L et al (2014) Inter-observer and intra-observer agreement in pathological evaluation of non-alcoholic fatty liver disease suspected liver biopsies. Hepat Mon 14:e15167
Gawrieh S, Knoedler DM, Saeian K et al (2011) Effects of interventions on intra- and interobserver agreement on interpretation of nonalcoholic fatty liver disease histology. Ann Diagn Pathol 15:19–24
Hernaez R, Lazo M, Bonekamp S et al (2011) Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 54:1082–1090
Lee SS, Park SH, Kim HJ et al (2010) Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol 52:579–585
van Werven JR, Marsman HA, Nederveen AJ et al (2010) Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology 256:159–168
Ma X, Holalkere NS, Kambadakone RA et al (2009) Imaging-based quantification of hepatic fat: methods and clinical applications. Radiographics 29:1253–1280
Topal NB, Orcan S, Sığırlı D et al (2015) Effects of fat accumulation in the liver on hemodynamic variables assessed by Doppler ultrasonography. J Clin Ultrasound 43:26–33
Uzun H, Yazici B, Erdogmus B et al (2009) Doppler waveforms of the hepatic veins in children with diffuse fatty infiltration of the liver. Eur J Radiol 71:552–556
Balci A, Karazincir S, Sumbas H et al (2008) Effects of diffuse fatty infiltration of the liver on portal vein flow hemodynamics. J Clin Ultrasound 36:134–140
Sasso M, Beaugrand M, de Ledinghen V et al (2010) Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 36:1825–1835
Kodama Y, Ng CS, Wu TT et al (2007) Comparison of CT methods for determining the fat content of the liver. Am J Roentgenol 188:1307–1312
Iwasaki M, Takada Y, Hayashi M et al (2004) Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation 78:1501–1505
Park SH, Kim PN, Kim KW et al (2006) Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology 239:105–112
Zeb I, Li D, Nasir K, Katz R et al (2012) Computed tomography scans in the evaluation of fatty liver disease in a population based study: the multi-ethnic study of atherosclerosis. Acad Radiol 19:811–818
Johnston RJ, Stamm ER, Lewin JM et al (1998) Diagnosis of fatty infiltration of the liver on contrast enhanced CT: limitations of liver-minus-spleen attenuation difference measurements. Abdom Imaging 23:409–415
Qayyum A, Chen DM, Breiman RS et al (2009) Evaluation of diffuse liver steatosis by ultrasound, computed tomography, and magnetic resonance imaging: which modality is best? Clin Imaging 33:110–115
Limanond P, Raman SS, Lassman C et al (2004) Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology 230:276–280
Hyodo T, Yada N, Hori M et al (2017) Multimaterial decomposition algorithm for the quantification of liver fat content by using fast-kilovolt-peak switching dual-energy CT: clinical evaluation. Radiology 283:108–118
Hur BY, Lee JM, Hyunsik W et al (2014) Quantification of the fat fraction in the liver using dual-energy computed tomography and multimaterial decomposition. J Comput Assist Tomogr 38:845–852
Kramer H, Pickhardt PJ, Kliewer MA et al (2017) Accuracy of liver fat quantification with advanced CT, MRI, and ultrasound techniques: prospective comparison with MR spectroscopy. Am J Roentgenol 208:92–100
Artz NS, Hines CD, Brunner ST et al (2012) Quantification of hepatic steatosis with dual-energy computed tomography: comparison with tissue reference standards and quantitative magnetic resonance imaging in the ob/ob mouse. Investig Radiol 47:603–610
Yoshimitsu K, Kuroda Y, Nakamuta M et al (2008) Noninvasive estimation of hepatic steatosis using plain CT vs. chemical-shift MR imaging: significance for living donors. J Magn Reson Imaging 28:678–684
Bohte AE, van Werven JR, Bipat S et al (2011) The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol 21:87–97
Wells SA (2014) Quantification of hepatic fat and iron with magnetic resonance imaging. Magn Reson Imaging Clin N Am 22:397–416
Permutt Z, Le TA, Peterson MR et al (2012) Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease—MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 36:22–29
Idilman IS, Keskin O, Celik A et al (2016) A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiol 57:271–278
Tang A, Tan J, Sun M et al (2013) Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 267:422–431
Szczepaniak LS, Nurenberg P, Leonard D et al (2005) Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288:E462–E468
Kinner S, Reeder SB, Yokoo T (2016) Quantitative imaging biomarkers of NAFLD. Dig Dis Sci 61:1337–1347
Kang BK, Yu ES, Lee SS et al (2012) Hepatic fat quantification: a prospective comparison of magnetic resonance spectroscopy and analysis methods for chemical-shift gradient echo magnetic resonance imaging with histologic assessment as the reference standard. Investig Radiol 47:368–375
Wu CH, Ho MC, Jeng YM et al (2014) Quantification of hepatic steatosis: a comparison of the accuracy among multiple magnetic resonance techniques. J Gastroenterol Hepatol 29:807–813
Kukuk GM, Hittatiya K, Sprinkart AM et al (2015) Comparison between modified Dixon MRI techniques, MR spectroscopic relaxometry, and different histologic quantification methods in the assessment of hepatic steatosis. Eur Radiol 25:2869–2879
Idilman IS, Aniktar H, Idilman R et al (2013) Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology 267:767–775
Parente DB, Rodrigues RS, Paiva FF et al (2014) Is MR spectroscopy really the best MR-based method for the evaluation of fatty liver in diabetic patients in clinical practice? PLoS One 9:e112574
Schwimmer JB, Middleton MS, Behling C et al (2015) Magnetic resonance imaging and liver histology as biomarkers of hepatic steatosis in children with nonalcoholic fatty liver disease. Hepatology 61:1887–1895
Hines CD, Frydrychowicz A, Hamilton G et al (2011) T(1) independent, T(2) (*) corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging 33:873–881
Kang GH, Cruite I, Shiehmorteza M et al (2011) Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging 34:928–934
Reeder S, Hu H, Sirlin C (2012) Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging 36:1011–1014
Rehm JL, Wolfgram PM, Hernando D et al (2015) Proton density fat-fraction is an accurate biomarker of hepatic steatosis in adolescent girls and young women. Eur Radiol 25:2921–2930
Lin SC, Heba E, Bettencourt R et al (2017) Assessment of treatment response in non-alcoholic steatohepatitis using advanced magnetic resonance imaging. Aliment Pharmacol Ther 45:844–854
Noureddin M, Lam J, Peterson MR et al (2013) Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 58:1930–1940
Le TA, Chen J, Changchien C et al (2012) Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 56:922–932
Reeder SB (2013) Emerging quantitative magnetic resonance imaging biomarkers of hepatic steatosis. Hepatology 58:1877–1880
Blake L, Duarte RV, Cummins C (2016) Decision analytic model of the diagnostic pathways for patients with suspected non-alcoholic fatty liver disease using non-invasive transient elastography and multiparametric magnetic resonance imaging. BMJ Open 6:e010507
Nordbeck P, Ertl G, Ritter O (2015) Magnetic resonance imaging safety in pacemaker and implantable cardioverter defibrillator patients: how far have we come? Eur Heart J 36:1505–1511
Hamaguchi M, Kojima T, Takeda N et al (2005) The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 143:722–728
Pais R, Charlotte F, Fedchuk L et al (2013) A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol 59:550–556
Yamaguchi K, Yang L, McCall S et al (2007) Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 45:1366–1374
Angulo P, Bugianesi E, Bjornsson ES et al (2013) Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 145:782–789.e4
Dulai PS, Singh S, Patel J et al (2017) Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 65:1557–1565
Wree A, Broderick L, Canbay A et al (2013) From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol 10:627–636
Singh S, Allen AM, Wang Z et al (2015) Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 13:643–54.e9
Rustogi R, Horowitz J, Harmath C et al (2012) Accuracy of MR elastography and anatomic MR imaging features in the diagnosis of severe hepatic fibrosis and cirrhosis. J Magn Reson Imaging 35:1356–1364
Kaswala DH, Lai M, Afdhal NH (2016) Fibrosis assessment in nonalcoholic fatty liver disease (NAFLD) in 2016. Dig Dis Sci 61:1356–1364
Ganne-Carrié N, Ziol M, de Ledinghen V et al (2006) Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology 44:1511–1517
Tsochatzis EA, Gurusamy KS, Ntaoula S et al (2011) Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol 54:650–659
Degos F, Perez P, Roche B et al (2010) Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 53:1013–1021
Wong VW, Vergniol J, Wong GL et al (2012) Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 107:1862–1871
Imajo K, Kessoku T, Honda Y et al (2016) Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 150:626–637.e7
Cassinotto C, Boursier J, de Lédinghen V et al (2016) Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 63:1817–1827
Venkatesh SK, Yin M, Ehman RL (2013) Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging 37:544–555
Runge JH, Bohte AE, Verheij J et al (2014) Comparison of interobserver agreement of magnetic resonance elastography with histopathological staging of liver fibrosis. Abdom Imaging 39:283–290
Horowitz JM, Venkatesh SK, Ehman RL et al (2017) Evaluation of hepatic fibrosis: a review from the society of abdominal radiology disease focus panel. Abdom Radiol 42:2037–2053
Chen J, Talwalkar JA, Yin M et al (2011) Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology 259:749–756
Tana C, Tana M, Rossi S et al (2016) Hepatic artery resistive index (HARI) and non-alcoholic fatty liver disease (NAFLD) fibrosis score in NAFLD patients: cut-off suggestive of non-alcoholic steatohepatitis (NASH) evolution. J Ultrasound 19:183–189
Ridolfi F, Abbattista T, Marini F et al (2007) Contrast-enhanced ultrasound to evaluate the severity of chronic hepatitis C. Dig Liver Dis 39:929–935
Ronot M, Asselah T, Paradis V et al (2010) Liver fibrosis in chronic hepatitis C virus infection: differentiating minimal from intermediate fibrosis with perfusion CT. Radiology 256:135–142
Wang L, Fan J, Ding X et al (2015) Assessment of liver fibrosis in the early stages with perfusion CT. Int J Clin Exp Med 8:15276–15282
Bandula S, Punwani S, Rosenberg WM et al (2015) Equilibrium contrast-enhanced CT imaging to evaluate hepatic fibrosis: initial validation by comparison with histopathologic sampling. Radiology 275:136–143
Guo SL, Su LN, Zhai YN et al (2017) The clinical value of hepatic extracellular volume fraction using routine multiphasic contrast-enhanced liver CT for staging liver fibrosis. Clin Radiol 72:242–246
Dyvorne HA, Jajamovich GH, Bane O et al (2016) Prospective comparison of magnetic resonance imaging to transient elastography and serum markers for liver fibrosis detection. Liver Int 36:659–666
Wu Z, Matsui O, Kitao A et al (2013) Usefulness of Gd-EOB-DTPA-enhanced MR imaging in the evaluation of simple steatosis and nonalcoholic steatohepatitis. J Magn Reson Imaging 37:1137–1143
Bastati N, Feier D, Wibmer A et al (2014) Noninvasive differentiation of simple steatosis and steatohepatitis by using gadoxetic acid-enhanced MR imaging in patients with nonalcoholic fatty liver disease: a proof-of-concept study. Radiology 271:739–747
Smits LP, Coolen BF, Panno MD et al (2016) Noninvasive differentiation between hepatic steatosis and steatohepatitis with MR imaging enhanced with USPIOs in patients with nonalcoholic fatty liver disease: a proof-of-concept study. Radiology 278:782–791
Pulli B, Wojtkiewicz G, Iwamoto Y et al (2017) Molecular MR imaging of myeloperoxidase distinguishes steatosis from steatohepatitis in nonalcoholic fatty liver disease. Radiology 284:390–400
Abrigo JM, Shen J, Wong VW et al (2014) Non-alcoholic fatty liver disease: spectral patterns observed from an in vivo phosphorus magnetic resonance spectroscopy study. J Hepatol 60:809–815
Zhang X, Gao X, Liu BJ et al (2015) Effective staging of fibrosis by the selected texture features of liver: which one is better, CT or MR imaging? Comput Med Imaging Graph 46(Pt 2):227–236
Daginawala N, Li B, Buch K et al (2016) Using texture analyses of contrast enhanced CT to assess hepatic fibrosis. Eur J Radiol 85:511–517
Chalasani N, Wilson L, Kleiner DE et al (2008) Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. J Hepatol 48:829–834
Carter-Kent C, Brunt EM, Yerian LM et al (2011) Relations of steatosis type, grade, and zonality to histological features in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr 52:190–197
Lonardo A, Ballestri S, Marchesini G et al (2015) Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis 47:181–190
Targher G, Marchesini G, Byrne CD (2016) Risk of type 2 diabetes in patients with non-alcoholic fatty liver disease: causal association or epiphenomenon? Diabetes Metab 42:142–156
Sanyal AJ, Abdelmalek MF, Suzuki A et al (2014) No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology 147:377–84.e1
Loomba R, Sirlin CB, Ang B et al (2015) Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 61:1239–1250
Patel J, Bettencourt R, Cui J et al (2016) Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol 9:692–701
Patel NS, Doycheva I, Peterson MR et al (2015) Effect of weight loss on magnetic resonance imaging estimation of liver fat and volume in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 13:561–568
Doycheva I, Cui J, Nguyen P et al (2016) Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther 43:83–95
Pavlides M, Banerjee R, Tunnicliffe EM et al (2017) Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver Int 37:1065–1073
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors have no conflicts of interests to report.
Rights and permissions
About this article
Cite this article
Chartampilas, E. Imaging of nonalcoholic fatty liver disease and its clinical utility. Hormones 17, 69–81 (2018). https://doi.org/10.1007/s42000-018-0012-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-018-0012-x