Abstract
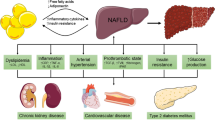
Nonalcoholic fatty liver disease (NAFLD) and its more aggressive form, nonalcoholic steatohepatitis (NASH), have become leading causes of chronic liver disease worldwide given the obesity epidemic. Unlike many other forms of chronic liver disease that have simple, highly accurate diagnostic algorithms, NAFLD and, in particular, NASH can be quite challenging to diagnose noninvasively. In this setting, identification of noninvasive biomarkers with excellent sensitivity and specificity for NASH has become a focal point within hepatology, given the disease prevalence and potential associated morbidity and mortality. Imaging diagnostic tests for NASH including ultrasound and MRI-based approaches have produced very promising data to accurately characterize hepatic steatosis and fibrosis. More recent data has also shown that radiological diagnostics have good accuracy to assess for NASH specifically. In this chapter, we will highlight the current data in support of imaging protocols for noninvasive diagnosis of NASH and outline areas in need of further investigation in this realm.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Imaging has taken on a critical role in the assessment of patients with chronic liver disease due to advancements that allow for more detailed staging and grading of disease. Whereas in the past imaging in patients with chronic liver disease had largely been utilized for assessment of underlying cirrhosis and complications thereof, advances in imaging-based techniques to include capability to provide surrogate quantitative assessments of steatosis and fibrosis have fundamentally changed clinical practice and care patterns in hepatology. These capabilities have become particularly relevant among individuals with nonalcoholic fatty liver disease (NAFLD) given the worldwide prevalence of NAFLD with the need to accurately and noninvasively diagnose and risk-stratify individuals. In particular, there is a critical need to identify and characterize individuals with nonalcoholic steatohepatitis (NASH), the more aggressive phenotype of NAFLD.
Over the past several years, imaging-based modalities to both screen for and quantify degree of hepatic steatosis and fibrosis have become more accurate and accessible. Development of noninvasive diagnostics for NASH poses ongoing challenges as many components of NASH have classically only been assessable via histology. There remains a need for further investigation into imaging techniques that can accurately capture presence and severity of NASH, though several modalities show promise. These imaging biomarkers for NASH would serve a critical role in risk stratification of large at-risk populations and help identify patients at most urgent need of therapy and monitoring. In this chapter, we will review the current state of imaging techniques for the assessment of hepatic steatosis, fibrosis, and NASH, and highlight areas of interest for future work (Table 14.1).
14.2 Radiological Diagnostics: Steatosis
14.2.1 Ultrasound
NAFLD by definition requires the presences of fat deposition in the liver, with a minimum of ≥5% of hepatocytes with steatosis [1]. Conventional ultrasound (CUS) is routinely obtained in clinical practice due to widespread availability, low cost, and tolerability. Assessment of steatosis is qualitatively inferred based on brightness of sonographic images of the liver compared to adjacent structures [2]. In addition to this dichotomous approach, overall degree of steatosis can also be categorically assessed (mild, moderate, or severe). The primary limitation of conventional US stems from the qualitative nature of these assessments that result in lower overall sensitivity, accuracy, and reproducibility [3]. In general, 20–33% steatosis is thought to be the level at which CUS can reliably detect steatosis for screening purposes with a sensitivity of approximately 80% and a specificity of 86% [4, 5]. Performance characteristics of CUS are particularly relevant in obese subjects where quality of images may be affected [6, 7]. Quantitative US (QUS) has been evaluated in a limited number of studies, and may offer superior diagnostic accuracy [8]. QUS uses additional acoustic parameters including backscatter coefficient to characterize tissue microstructure, and in comparison to MRI–proton density fat fraction (PDFF), it has had an AUROC of up to 0.98 [8].
The controlled attenuation parameter (CAP) measurement is an US-based method to assess degree of hepatic steatosis as part of vibration controlled transient elastography (VCTE) systems. During VCTE, a 3.5 MHz signal (M probe) or 2.5 MHz (XL probe) is emitted and a return wave is graded in dB/m. Similar to other US-based assessments of steatosis, there are concerns regarding reliability of CAP assessments. A meta-analysis of 2735 patients with various causes of liver disease noted an area under the receiver operating characteristic curve (AUROC) of 0.82 for presence of steatosis compared to liver biopsy [9]. There has been concern regarding impact of probe type (XL vs M probe) as it relates to CAP measurement, with XL probes potentially overestimating measurements [10, 11]. The optimal cutoffs for different degrees of steatosis are unclear, though a level of 288–302 db/s has been cited as a consideration for detection of 5% steatosis and 337 db/s for S ≥ 3 [12, 13].
14.2.2 CT
CT is an infrequently used modality to assess for NAFLD in large part due to radiation associated with this modality, but individuals obtain CT scans for other indications as part of their medical care and thus CT imaging can be used to assess for steatosis. In general, CT is thought to be more specific to assess for hepatic steatosis than US. In unenhanced CT scans, attenuation values are used to evaluate hepatic triglyceride (TG) content as reduced attenuation has been correlated with amount of intrahepatic steatosis [14]. Using this approach, either to total attenuation value in Hounsfield units (HU), HU difference between the liver and spleen or ratio of liver to spleen HU is used. Depending on the HU cutoff chosen, non-contrast CT has reported sensitivity and specificity for hepatic steatosis of ≥30% between 73%–100% and 95%–100%, respectively [15]. The addition of contrast to CT evaluations can impact the sensitivity and specificity for hepatic steatosis evaluation, primarily due to variability in contrast protocols and alterations in perfusion.
14.2.3 MRI
There are several MRI-based methods to quantify hepatic steatosis and in general, MRI-based imaging is thought to have the highest diagnostic accuracy. The two primary MRI-based methods include MR spectroscopy (MRS) and the MRI-based proton density fat fraction (PDFF). MRS noninvasively measures proton signals as a function of their resonance frequency. These signal intensities correspond to specific frequencies of water or fat and can then be quantified into a fat signal fraction. MRS has excellent sensitivity, even for trace amounts of fat [16]. Despite this sensitivity, MRS has not taken on an important role clinically for the evaluation of hepatic steatosis due to several limitations. This includes restricted spatial coverage, potential for sampling error, need for additional equipment and special expertise for administration and interpretation, and the time-consuming nature of the exam [17].
MRI-PDFF assesses the ratio of MR-visible triglyceride (TG) protons to the sum of TG and water protons [18]. This method can correct for T1 decay and R2* and thus theoretically can account for impact from inflammation, edema, and iron overload. With MRI-PDFF, specific regions of interest (ROI) are determined and thus can account of heterogeneity of fat deposition and is more feasible for longitudinal assessments of changes in fat content. Given these benefits, MRI-PDFF is one of the most readily used methods for assessment of hepatic steatosis [19]. Prior studies have compared US and MRI-PDFF and have demonstrates higher performance characteristics of MRI-PDFF [20,21,22].
14.3 Radiological Diagnostics: Fibrosis
The majority of imaging-based tests to assess fibrosis have focused on assessment of liver stiffness as a surrogate for fibrosis due to the mechanical alterations in the hepatic parenchyma as a result of progressive fibrosis. Sheer wave elastography techniques apply the concept that the speed of a propagating mechanical sheer wave is mediated by the stiffness of that medium, and thus reflects the underlying degree of hepatic fibrosis [23]. The major caveat in applying this concept stems from the fact that several other factors including inflammation can also impact stiffness measurements and thus can affect reliability of this methodology, particularly among patients with NASH [24,25,26].
14.3.1 Ultrasound
There are several US-based methods that have been evaluated for the assessment of hepatic fibrosis. They include VCTE, acoustic radiation force impulse (ARFI), and sheer wave elastography (SWE). ARFI and SWE are of interest, given that it can be integrated into a CUS device. Among these three methods, the most data exist for VCTE in terms of performance characteristics within NAFLD and NASH. Overall, there is evidence in support of higher reliability of ARFI and SWE compared to VCTE to estimate fibrosis, but further studies are necessary to validate these findings. A single study of 172 patients with NAFLD assessed the AUROC for detection of advanced fibrosis using ARFI to be 0.90 [27]. In this study, BMI did not appear to impact ARFI assessments. This was further supported by results of a meta-analysis of ARFI to detect advanced fibrosis in NAFLD that found it to have a moderate degree of accuracy with a sensitivity of 80.2%, specificity of 85.2%, and an AUROC of 0.89 [28]. While there have been very limited studies comparing ARFI and SWE to VCTE, the existing data has suggested equivalent performance to detect advanced fibrosis with AUROC of 0.84, 0.89, and 0.86, respectively [29].
Overall, these US-based elastography methods have the highest performance for accurate assessment of advanced fibrosis or cirrhosis, with diminishing performance distinguishing across lower stages of fibrosis. Prior studies have reported the AUROC for VCTE to diagnose ≥F3 as 0.75–0.93. Overall, these US-based methods have several advantages for use including lower cost, time efficiency, portability, and comparatively lower cost. The primary disadvantage of this approach stems from concern about technical failure or unreliability of results among patients with very high BMIs, significant steatosis, ascites, and significant inflammation [30, 31]. In order to address some of the concerns related to performance among patients with higher BMI, two VCTE probes have been developed. The standard M probe examines wave propagation at 25–65 mm, whereas the XL probe examines wave propagation at 35–75 mm [32]. Prior studies have shown more reliable estimates of fibrosis among morbidly obese patients undergoing VCTE when the XL probe is used [33, 34]. There remains concern about severe steatosis affecting fibrosis assessments, though data has been conflicting [10, 35, 36]. The best performance characteristic for US elastography is the negative predictive value (NPV) with AUROCs of 0.77 (95% CI 0.72–0.82) for F ≥ F2, 0.80 (95% CI 0.75–0.84) for F ≥ F3, and 0.89 (95% CI 0.84–0.93) for F = F4 [13].
14.3.2 MRI
Multiple MRI-based modalities have been investigated for the assessment of hepatic fibrosis [37, 38]. Magnetic resonance elastography (MRE) has been a primary modality of interest for quantitative assessment of hepatic fibrosis. In MRE, shear waves are generated using a vibrating plate placed against the body wall, and these shear waves are imaged using specific MRI sequences. These data are used to generate quantitative cross-sectional images of differential tissue stiffness. Using these cross-sectional images, regions of interest (ROIs) are then selected, and an overall stiffness measure is calculated. Prior meta-analyses have shown that MRE has higher accuracy with lower technical failure compared to US-based elastography with an AUROC of 0.93 to diagnose ≥F3 [39, 40]. MRE has similarly been shown to have superior accuracy to assess earlier stages of fibrosis compared to VCTE (AUROC 0.82 vs 0.67 for stage 1 or more and 0.89 vs 0.87 for stage 2) [21]. This represents the main advantage of MRE as a noninvasive modality for fibrosis assessment, particularly among those with severe obesity (BMI ≥35) [41]. There are presently several limitations precluding widespread use including high cost, need for specific programming and radiology expertise to perform the exam, and lack of portability.
3D MRE technology is thought to be even more promising with higher accuracy in individual stages of fibrosis. 3D MRE has the capacity to image shear wave fields in three dimensions of the entire liver as compared to assessing several ROIs in 2D MRE. Data is still emerging in application of this newer technology, but Loomba et al. had demonstrated higher AUROC to diagnosed advanced fibrosis using 3D MRE at 40 Hz vs 2D MRE at 60 Hz (0.98 vs 0.92) [42]. The main limitation of 3D MRE stems from the significant level of expertise to utilize and interpret this technology.
In addition to MRE, there are several other MRI-based methods to assess hepatic fibrosis. These include proton diffusion metrics, T1 relaxation time mapping, and corrected T1 decay using the multiscan platform [43]. The apparent diffusion coefficient (ADC) has been shown to have a significant relationship to fibrosis stage. The diagnostic performance of ADC has been inferior to elastography methods however [44]. Similarly, T1 relaxation time has also not been shown to be a reliable method to assess hepatic fibrosis.
14.4 Radiological Diagnostics: NASH
Developing imaging-based modalities to noninvasively assess for and grade NASH remains challenging. Prior studies have shown that patients with underlying NASH often have elevated liver stiffness measurements even in the absence of significant fibrosis due to the presence of necroinflammation. Studies have also shown a correlation between aminotransferase levels and liver stiffness measurements, suggesting a potential role for elastography to assess steatohepatitis [24]. This relationship is quite complex due to the variable, interdependent effects of different histologic components of NASH. The interaction between impact of inflammation on liver stiffness measurements based on degree of underlying fibrosis is a primary concern that can significantly impact diagnostic accuracy of imaging-based techniques for NASH [45]. Presently there is limited data based on small cohorts with variable study designs that impact interpretation and comparison across studies (Table 14.2). These variations include up to a 6-month time lag from imaging to biopsy and significant heterogeneity in patient characteristics, namely distribution of advanced fibrosis in each cohort and percentage of individuals with NASH vs simple steatosis.
14.4.1 VCTE and NASH Assessment
Studies evaluating VCTE to diagnose NASH have had highly variable results with AUROCs ranging from 0.35 to 0.80 [20, 21, 46]. In general, VCTE appears to be less accurate in distinguishing NASH from simple steatosis in the setting of NASH with minimal fibrosis. Park et al. evaluated 104 patients with biopsy-proven NAFLD, 73% with NASH, and 80% with stage 1–2 fibrosis. Using a cutoff of 5.60 kPa, the sensitivity was 61%, specificity was 59%, and AUROC was only 0.35 [21]. By contrast, Imajo and colleagues evaluated 142 patients with NAFLD, 76% with NASH and 68% with F0–2 fibrosis and found an AUROC of 0.80 for VCTE to detect NASH [20]. In order to optimize the predictive ability of VCTE data to assess for NASH, VCTE data has been combined with other biomarkers to create composite scores. Lee and coauthors designed a composite scoring system including CAP >250 dB/m, LSM >7 kPa, and ALT >60 IU/L. Using this score, the AUROC for NASH increased to 0.81, though the specificity was suboptimal with 21% of “high-risk” patients incorrectly categorized as having NASH [46]. In a similar fashion, addition of CAP score and CK-18 added modest improvement in the diagnostic accuracy of VCTE for NASH, with an AUROC of 0.82 in the study done by Imajo et al. [20]. Sasso and colleagues evaluated a combination of kPa, CAP, and AST to detect patients with NAS ≥4 and F ≥ 2. In the derivation cohort (N = 281), the AUROC was 0.83 and this high accuracy was maintained across three heterogenous external validation cohorts (0.84–0.92) [47].
Other US-based elastography methods such as ARFI and SWE have not been extensively evaluated for accuracy to diagnose NASH. Palmeri et al. did find in their study of 172 patients with NAFLD that there was no clear association with ARFI results and histologic inflammation or hepatocyte ballooning [27]. To the contrary, Braticevici et al. assessed ARFI in 71 patients with biopsy-proven NASFL and noted an AUROC of 0.87 (0.78 = 0.95), sensitivity of 76%, and specificity of 83% to detect NASH [48].
14.4.2 MRE and NASH Assessment
Both VCTE and MRE have been evaluated to differentiate NASH from isolated hepatic steatosis. Several studies have evaluated the diagnostic accuracy of 2D MRE for NASH. The associated AUROCs of these studies have been highly variable, ranging from 0.70 to 0.93 [20, 21, 42, 45, 49]. The highest AUROC of 0.93 with a sensitivity of 94% and specificity of 73% when using a threshold of 2.74 kPa was reported by Chen et al. in their retrospective study of 58 patients [45]. Main limitations of that study that potentially may account for discrepancy in other reported AUROCs was the lack standardized histologic diagnosis of NASH and a period of up to 90 days between MRE and liver biopsy. Imajo et al. reported the next highest AUROC of 2D MRE to assess for the presence of NASH at 0.81. In this study, 142 NAFLD patients, 32% of whom had stage 3–4 fibrosis and 76% of whom had NASH histology underwent 2D MRE [20]. Addition of PDFF results and CK-18 levels to the 2D MRE data increased the AUROC minimally to 0.82. The remaining studies using 2D MRE reported AUROC between 0.70 and 0.75 to diagnose NASH. One study evaluated application of 3D MRE at both 40 Hz and 60 Hz to evaluate for NASH with AUROCs of 0.74 and 0.76, respectively, comparable to results reported for use of 2D MRE in that study (AUROC 0.75) [42].
14.4.3 Multiparametric MRI and NASH Assessment
Multiparametric MRI/MRE has been perhaps the most promising methodology being investigated to assess for presence and severity of NASH [38, 43, 50,51,52,53]. Multiparametric MRI is a non-elastography-based approach that can quantitatively assess degree of hepatic inflammation and fibrosis through application of T1 mapping and damping ratios [53]. A study of 71 patients with biopsy-proven NAFLD underwent MRI multiscan resulted in an AUROC of 0.8 to diagnose NASH [52]. However, it is important to highlight that in this study, both inflammation and fibrosis increased the corrected T1 measurement and thus caused the differentiation between NASH from fibrosis challenging. The Liver Inflammation and Fibrosis (LIF) score, an MRI-derived quantitative assessment, has been shown to be correlated with hepatic fibrosis and inflammation. The LIF score was used in combination with MRI-PDFF assessment among individuals in the UK Biobank cohort and a US-cohort with liver biopsies and was shown to accurately distinguish between individuals with and without NAFLD/NASH and among those with F ≥2 and PDFF ≥5% [54]. Another study of 77 patients with biopsy-proven NAFLD combined findings of liver MultiScan with fasting glucose to identify patients with NASH with a NAS ≥4 and high-risk NASH (NAS ≥4 and F ≥ 2). Using PDFF alone, the AUROC for NASH was 0.85 (0.77–0.94) and when using cT1 alone, the AUROC for NASH was 0.81 (0.70–0.91). When PDFF, cT1, and fasting glucose were combined, the AUROC was 0.89 (0.81–0.96) for NASH [55].
Multiparametric MRE applies additional mechanical parameters including use of multiple frequencies to help better differentiate inflammation from fibrosis. Gallego-Duran and colleagues evaluated a “NASHMRI” protocol using optical processing methods (E3 harmonic mean, E57 second-order contrast, and E73 averaged mean curvature) applied to conventional non-enhanced MRI to predict NASH [56]. In the estimation cohort, the AUROC was 0.88 and in the validation cohort (N = 87), the AUROC was 0.83 with a sensitivity of 87%, specificity of 60%, PPV 71%, and NPV 81%. Allen et al. applied combination of MRI-PDFF, dampening ratio, and complex shear modulus among 83 patients, 37 of whom had NASH with only 3 having F3–4. In this study, it was shown that the damping ratio and shear stiffness correlated with lobular inflammation and hepatocellular ballooning. When combined with PDFF, these three parameters were able to assess for histologic NASH with and AUROC of 0.89, a sensitivity of 68%, specificity of 85%, PPV 0.73, and NPV 0.82 [57]. Data from a multicentre study of 99 patients who underwent MRI-PDFF, MRE, and liver biopsy and had an AUROC of 0.80 [58].
Combination approaches like these may hold the highest ability to detect earlier stages and grades of NASH but will need to be evaluated in larger populations in order to validate their use. A recent study demonstrated cost-effectiveness of multiparametric MRI compared to VCTE, wet biomarkers, and liver biopsy, and demonstrated a cost saving of 150,218 pounds per 1000 patients compared to biopsy to identify patients with NASH [50].
14.5 Conclusion
Current radiological techniques including VCTE, MRI-PDFF, MRE, and multiparametric MRI have good performance characteristics to grade hepatic steatosis and stage hepatic fibrosis. When individually applied to assess for underlying NASH, the performance characteristics become less robust with AUROCs mostly around 0.70–0.80 depending on the prevalence of NASH and advanced fibrosis in each cohort. Combination approaches that incorporate multiparametric MRI imaging protocols with MR-based elastography and serum biomarkers that can capture high-risk features of metabolic syndrome will likely be the highest yield. The primary drawback of this approach remains cost of MRI imaging and lack of accessibility of these highly specialized MRI-based protocols.
References
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57.
Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB. Fatty liver: imaging patterns and pitfalls. Radiographics. 2006;26(6):1637–53.
Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol. 2007;189(6):W320–3.
Khov N, Sharma A, Riley TR. Bedside ultrasound in the diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(22):6821–5.
Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–50.
Mottin CC, Moretto M, Padoin AV, Swarowsky AM, Toneto MG, Glock L, et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg. 2004;14(5):635–7.
de Moura AA, Cotrim HP, Barbosa DB, de Athayde LG, Santos AS, Bitencourt AG, et al. Fatty liver disease in severe obese patients: diagnostic value of abdominal ultrasound. World J Gastroenterol. 2008;14(9):1415–8.
Lin SC, Heba E, Wolfson T, Ang B, Gamst A, Han A, et al. Noninvasive diagnosis of nonalcoholic fatty liver disease and quantification of liver fat using a new quantitative ultrasound technique. Clin Gastroenterol. 2015;13(7):1337–45.e6.
Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Ledinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–30.
Petta S, Wong VW, Camma C, Hiriart JB, Wong GL, Marra F, et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology. 2017;65(4):1145–55.
Chan WK, Nik Mustapha NR, Wong GL, Wong VW, Mahadeva S. Controlled attenuation parameter using the FibroScan(R) XL probe for quantification of hepatic steatosis for non-alcoholic fatty liver disease in an Asian population. United European Gastroenterol J. 2017;5(1):76–85.
Wong VW, Petta S, Hiriart JB, Camma C, Wong GL, Marra F, et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol. 2017;67(3):577–84.
Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717–30.
Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188(5):1307–12.
Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239(1):105–12.
Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34(4):729–49.
Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol. 2016;65(5):1006–16.
Paige JS, Bernstein GS, Heba E, Costa EAC, Fereirra M, Wolfson T, et al. A pilot comparative study of quantitative ultrasound, conventional ultrasound, and MRI for predicting histology-determined steatosis grade in adult nonalcoholic fatty liver disease. AJR Am J Roentgenol. 2017;208(5):W168–w77.
Yokoo T, Serai SD, Pirasteh A, Bashir MR, Hamilton G, Hernando D, et al. Linearity, bias, and precision of hepatic proton density fat fraction measurements by using MR imaging: a meta-analysis. Radiology. 2018;286(2):486–98.
Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150(3):626–637.e7.
Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152(3):598–607.e2.
Middleton MS, Heba ER, Hooker CA, Bashir MR, Fowler KJ, Sandrasegaran K, et al. Agreement between magnetic resonance imaging proton density fat fraction measurements and pathologist-assigned steatosis grades of liver biopsies from adults with nonalcoholic steatohepatitis. Gastroenterology. 2017;153(3):753–61.
Petitclerc L, Sebastiani G, Gilbert G, Cloutier G, Tang A. Liver fibrosis: review of current imaging and MRI quantification techniques. J Magn Reson Imaging. 2017;45(5):1276–95.
Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47(2):380–4.
Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, et al. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52(2):206–10.
Bardou-Jacquet E, Legros L, Soro D, Latournerie M, Guillygomarc'h A, Le Lan C, et al. Effect of alcohol consumption on liver stiffness measured by transient elastography. World J Gastroenterol. 2013;19(4):516–22.
Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, et al. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55(3):666–72.
Liu H, Fu J, Hong R, Liu L, Li F. Acoustic radiation force impulse elastography for the non-invasive evaluation of hepatic fibrosis in non-alcoholic fatty liver disease patients: a systematic review & meta-analysis. PLoS One. 2015;10(7):e0127782.
Cassinotto C, Boursier J, de Ledinghen V, Lebigot J, Lapuyade B, Cales P, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63(6):1817–27.
de Ledinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60(5):1026–31.
Foucher J, Castera L, Bernard PH, Adhoute X, Laharie D, Bertet J, et al. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18(4):411–2.
Vuppalanchi R, Siddiqui MS, Van Natta ML, Hallinan E, Brandman D, Kowdley K, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67(1):134–44.
de Ledinghen V, Wong VW, Vergniol J, Wong GL, Foucher J, Chu SH, et al. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan(R). J Hepatol. 2012;56(4):833–9.
Wong VW, Vergniol J, Wong GL, Foucher J, Chan AW, Chermak F, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107(12):1862–71.
Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51(2):454–62.
Gaia S, Carenzi S, Barilli AL, Bugianesi E, Smedile A, Brunello F, et al. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol. 2011;54(1):64–71.
Venkatesh SK, Ehman RL. Magnetic resonance elastography of liver. Magn Reson Imaging Clin N Am. 2014;22(3):433–46.
Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60(1):69–77.
Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13(3):440–451.e6.
Cui J, Heba E, Hernandez C, Haufe W, Hooker J, Andre MP, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology. 2016;63(2):453–61.
Chen J, Yin M, Talwalkar JA, Oudry J, Glaser KJ, Smyrk TC, et al. Diagnostic performance of MR elastography and vibration-controlled transient elastography in the detection of hepatic fibrosis in patients with severe to morbid obesity. Radiology. 2017;283(2):418–28.
Loomba R, Cui J, Wolfson T, Haufe W, Hooker J, Szeverenyi N, et al. Novel 3D magnetic resonance elastography for the noninvasive diagnosis of advanced fibrosis in NAFLD: a prospective study. Am J Gastroenterol. 2016;111(7):986–94.
Pavlides M, Banerjee R, Sellwood J, Kelly CJ, Robson MD, Booth JC, et al. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J Hepatol. 2016;64(2):308–15.
Wang QB, Zhu H, Liu HL, Zhang B. Performance of magnetic resonance elastography and diffusion-weighted imaging for the staging of hepatic fibrosis: a meta-analysis. Hepatology. 2012;56(1):239–47.
Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259(3):749–56.
Lee HW, Park SY, Kim SU, Jang JY, Park H, Kim JK, et al. Discrimination of nonalcoholic steatohepatitis using transient elastography in patients with nonalcoholic fatty liver disease. PLoS One. 2016;11(6):e0157358.
Sasso M, Chan WK, Harrison SA, Czernichow S, Allison MED, Tsochatzis EA, et al. Fibroscan-based score (FS3) to identify nash patients with NAS≥4 and F≥2: development in a NAFLD UK cohort-external validation in a Malaysian NAFLD cohort, a us screening cohort and a French bariatric surgery cohort. Hepatology. 2018;68:87A–8A.
Braticevici CF, Alexandru M, Tribus L, Razvan P, Ana P, Necula A, et al. Comparing of noninvasive tests in predicting diagnosis of nonalcoholic steatohepatitis. J Hepatol. 2018;68:S575.
Loomba R, Wolfson T, Ang B, Hooker J, Behling C, Peterson M, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60(6):1920–8.
Eddowes PJ, McDonald N, Davies N, Semple SIK, Kendall TJ, Hodson J, et al. Utility and cost evaluation of multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2018;47(5):631–44.
Allen AM, Yin M, Venkatesh SK, Mounajjed T, Kellogg TA, Kendrick ML, et al. Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts NAS score for NASH diagnosis. J Hepatol. 2017;66(1):S659–S60.
Pavlides M, Banerjee R, Tunnicliffe EM, Kelly C, Collier J, Wang LM, et al. Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver Int. 2017;37(7):1065–73.
Yin M, Glaser KJ, Manduca A, Mounajjed T, Malhi H, Simonetto DA, et al. Distinguishing between hepatic inflammation and fibrosis with MR elastography. Radiology. 2017;284(3):694–705.
Harrison S, Wilman H, Kelly M, Bachtiar V, Dennis A, Kelly C, et al. Prevalence and stratification of NAFLD/NASH in a UK and US cohort using non-invasive multiparametric MRI. J Hepatol. 2018;68:S550.
McKay A, Dennis A, Kelly M, Fallowfield JA, Hirschfield G, Neubauer S, et al. Multi-parametric MRI as a composite biomarker for NASH and NASH with fibrosis. Hepatology. 2018;68:954A–5A.
Gallego-Duran R, Cerro-Salido P, Gomez-Gonzalez E, Pareja MJ, Ampuero J, Rico MC, et al. Imaging biomarkers for steatohepatitis and fibrosis detection in non-alcoholic fatty liver disease. Sci Rep. 2016;6:31421.
Allen AM, Venkatesh SK, Mounajjed T, Kellogg TA, Kendrick M, McKenzie TJ, et al. Novel multiparametric magnetic resonance elastography (MRE) protocol accurately predicts early NASH and disease activity. Hepatology. 2017;66:104A.
Noureddin M, Sundaram V, Ayoub WS, Han MAT, Grotts JF, Saouaf R, et al. The performance of MRI-proton density fat fraction (PDFF) and MR elastography (MRE) in diagnosing nonalcoholic steatohepatitis (NASH) noninvasively. Hepatology. 2018;68:1327A–8A.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tincopa, M.A., Harrison, S.A. (2020). Noninvasive Diagnostic Approach to NASH: Radiological Diagnostics. In: Bugianesi, E. (eds) Non-Alcoholic Fatty Liver Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-95828-6_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-95828-6_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95827-9
Online ISBN: 978-3-319-95828-6
eBook Packages: MedicineMedicine (R0)