Abstract
Synthetic solutions of 2-chlorophenol (2-CP, 1 mM) were treated in an undivided continuous flow electrochemical reactor equipped with boron-doped diamond (BDD) electrodes. The process was conducted at different current densities (j = 0.10, 0.125, and 0.14 A cm−2), initial pH (4.0, 7.3, and 9.0), and volumetric flow rate (Q = 0.5, 1.0, and 1.5 L min−1). The results of this study showed that the best operational conditions were: j = 0.14 A cm−2, pH = 7.3, and y Q = 1 L min−1. Under these operational conditions the degradation and mineralization of 2-CP were, 100% and 96%, respectively, after 6 h of electrolysis time. The by-products were identified by UHPLC. Also, it was found that the electrochemical degradation of 2-chlorophenol follows a pseudo-first order kinetics. Furthermore, these results demonstrate that the electrolysis process employed in this work allows high percentages (96%) of mineralization of 2-CP, a relative low treatment cost ($ 3 MXN/ 2.5 L of synthetic solution), and that the process is applicable to remediate wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2-chlorophenol (2-CP) is one of the 65 compounds listed by EPA as high-priority air and water pollutant [18] because of its toxicity [12]. In the last years, chlorophenols have been employed in many industries (dyes, cosmetic, and drugs). Therefore, 2-CP removal from industrial effluents has become of paramount importance. Because the degradation of this compound is rather difficult by natural means, it tends to persist in the environment, so it is currently a public health topic [4]. For this reason, it is necessary to be efficiently eliminated from wastewater resources. For this purpose, there are processes that can be either physical or chemical. The latter have been proven to offer high water remediation efficiency [16]. Advanced Oxidation Processes (AOPs) fall into this category and they emerge as an alternative to eliminate organic pollutants such as 2-CP. There is, in this context, the electrochemical process, that offers the advantage of producing the hydroxyl radical (●OH) in greener form, which is a non-selective oxidant [25] with high degradation capability of organic (toxic and persistent) and inorganic pollutants [21]. Moreover, the hydroxyl radical can be generated at the anode surface by the electrochemical reaction given by Eq. (1) [17, 23] or by direct oxidation of hydroxyl ion (OH−) according to Eq. (2). Equation (1) might take place at a pH around 9.0 [13] and reaction (2) is expected to proceed at an pH ≥10 [34],
Some studies of anodic degradation of 2-CP have been performed employing different electrodes like porous carbon felt [29], Ti coated with SnO2 (Ti/SnO2) [30], Ti coated with PbO2 (Ti/PbO2) [30], Pt/Ti [35], platinum (Pt) [14], carbon black diamond composite with 20% carbon black (20CBD) [1, 2], carbon fibers [36], activated carbon composite (ACC) [1], carbon nanotubes/Agarose/Indium oxide (CNTs/AG/ITO) [22], and boron-doped diamond (BDD) [35]. In this context, the BDD anode has emerged as a promising electrode for organic pollutants removal from wastewater [25]. This can be ascribed to the high amount of hydroxyl radicals produced and physisorbed onto its surface due to the O2 overvoltage inherent to BDD electrode [10]. These adsorbed radicals are responsible for the oxidation of organic compounds [7]. This leads to a rapid and efficient destruction of organic pollutants. The high oxidation efficiency of organic pollutants by BDD (●OH) anode has been demonstrated in many studies [2, 24, 28]. In electrochemical flow reactors, BDD anode has been reported to work in combination with different cathode materials (such as stainless steel (AISI 304) [31], platinum coated with titanium (Pt/Ti) [24], stainless steel [26], cupper (Cu) [35] and stainless steel or platinized plates [10]), such arrangements have led to achieve high organic compounds removal values, close to 100%. Electrochemical flow reactors can also be used in other water treatment processes such as electro-Fenton (EF), photoelectron-Fenton (FEP) and solar photoeleectro-Femton (SPEF). These technologias employing a BDD electrodes as anode and gas diffusion electrode as cathode (GDE) [10].
In this work, the electrochemical oxidation of 2-CP was carried out in an electrochemical flow reactor equipped with BDD electrodes (both electrodes, cathode an anode). Also, the effect of current density, initial pH, and volumetric flow rate on electrochemical oxidation and mineralization of 2-CP were studied. Additionally, the byproducts were determined by UPHLC when the electrochemical flow reactor was operated at the best conditions. The kinetic order was also determined. Moreover, the cost of the electrochemical oxidation of 2-CP per unit of removed TOC was computed as a function of energy consumption and electrolysis time.
Materials and methods
Reagents
All the following reagents were supplied by Sigma Aldrich: 2-CP 99% purity, Na2SO4 99% purity, sulfuric acid (H2SO4) 95–98% purity, and sodium hydroxide (NaOH) 97% purity. The initial concentration of 2-CP in the synthetic solutions was 1 mM (72 mg/L of TOC) and the concentration of electrolyte support (Na2SO4) was 0.1 M. All solutions were prepared with distillated water. To adjust the pH of the synthetic solutions, 0.1 M NaOH and 0.1 M H2SO4 aqueous solutions were prepared.
Equipment
Energy was supplied by a Gwinstek GW GPR-351OHD single output DC power supply. The total organic carbon (TOC) in samples was measured by a Shimadzu TOC analyzer. The identification of by-products and remaining products from electrochemical oxidation of 2-CP was performed by a Thermo Scientific Vanquish liquids chromatograph. The solution pH was monitored with a Hanna HI2210 potentiometer.
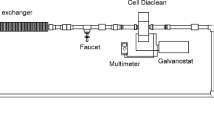
The electrochemical flow reactor was described in detail in [9]. In this work the distance between electrodes was 1.1 cm. The anode and cathode were of BDD material with a thickness of 5 μm supported on Niobium (Nb), which was supplied by Metakem™ company. Major details of the electrochemical flow reactor are given in Table 1.
The electrochemical flow reactor (FM01-LC) without division is shown in Fig. 1. The synthetic solution with electrolyte was placed in a polycarbonate reservoir with a total volume of 3 L. An MFG pump, model 2-MD-HC, of 300 rpm, was coupled to the reservoir lower part and used to supply the 2-CP synthetic solution. The volumetric flow rate was measured with a 4TSL01 rotameter from King Instruments Company. The flow lines were set-up with 1.27 cm inner diameter pipe; the pipeline and accessories were of polyvinyl acrylate.
Methodology for the electrolysis process and chemical analysis
The electrochemical oxidation of 2-CP was carried out in an electrochemical flow reactor (FM01-LC) coupled with BDD electrodes (both cathode and anode) at different operation conditions. Current density, volumetric flow rate and initial pH of synthetic solution were varied. The mineralization of 2-CP was followed by TOC analysis. For this purpose, many samples at different times were taken. It is worth noticing that all experiments were performed by triplicate; therefore, in the results only the average values are presented.
The percentage of mineralization efficiency (% MCE) was computed from the TOC data by using Eq. (3) [15, 33],
where F is the Faraday constant (96,487 C mol−1), Vs is the solution volume (L), Δ(TOC)Exp is the decline of experimental TOC value during electrolysis (mg L−1), 4.32 ×107 mg s mol−1 h−1 is a conversion factor, m is the number of carbon atoms, I is the applied current (A), n is the number of electrons, and t is the electrolysis time (h).
The number of electrons consumed per each organic molecule (2-CP) was taken as 26 considering its total mineralization towards CO2 and inorganic ions,
Specific energy consumption per mass unit of TOC (theoretical EC/TOC) was estimated by using Eq. (5) (according to [8]),
where Ecell is the mean cell voltage (V), Vs is the synthetic solution volume (L), Δ(TOC)Exp is the decline of experimental TOC value during the electrolysis (mg L−1), I is the applied current (A), and t is the electrolysis time (h).
Chemical analysis
The identification of by-products and remaining products from the electrochemical oxidation of 2-CP, was conducted by UHPLC, comparing their retention time with the ones from standard compounds according to the methodology proposed in [3]. An ultra-high-performance liquid chromatograph (UHPLC, Thermo Scientific Vanquish model) with a diode array detector was employed. Also, for the analysis of aromatic compounds, a mobile phase constituted by a mixture of water:methanol (V:V) 80:20 and 213 μl of solution 5 mM of H2SO4, was used. The data analysis was carried out in the Chromeleon 7.2 software package.
Results and discussion
Effect of current density on 2-CP mineralization
Figure 2(a) shows the effect of applied current density on normalized TOC. It can be seen that the initial TOC removal rate, given by the slope at time zero, does not depend on applied current density. This can be ascribed to a starting-up period of the oxidation process where this might be limited by ●OH radical production at the electrodes surface. After this initial period (30 min), a direct correlation between applied current density and TOC removal rate is actually observed. Thus, at j = 0.14 A cm−2, the TOC was practically removed (96%) in 6 h while after the same treatment time only 86 and 84% of mineralization was achieved by applying a j = 0.11 and 0.125 A cm−2, respectively. This behavior can be ascribed to hydroxyl radical (●OH) production being lower when applying lower current densities, i.e. j = 0.11 and 0.125 A cm−2. Hence, as expected, the hydroxyl radical (●OH) production increases when applied current density increases [6]. Therefore, as j = 0.14 A cm−2 leads to achieve the maximum mineralization percentage (96%), the effect of pH and volumetric flow rate on the standardized mineralization was studied at such operating condition. Despite the results shown in Fig. 2(a), it can be seen in Fig. 2(b), that the MCE% decreases when the current density applied increases. Actually, the best MCE% was achieved at a j = 0.11 A cm−2. The percentages values of MCE at 2 h and 6 h are 8.14% and 7.2%, respectively. This behavior is due to by-products formation (such as short chain carboxylic acids), which are rather difficult to be degraded even in the presence of hydroxyl radicals (●OH). Additionally, the low values of MCE% are associated to an increase in the nontoxic reactions, such as oxidation of BDD (●OH) to O2 [8, 20], the dimerization of ●OH given by reaction (6) [19] and their reaction with H2O2 to form other less oxidant species such as the hydroperoxyl group (\( {HO}_2^{\bullet } \) by reaction (7).
Figure 2(c) shows a profile of energy consumption (EC/TOC) as function of treatment time. In this Figure we can see that EC increases when current density increases (j). Also, the EC/TOC increases slowly when reaction time increases. The final values achieved were 2.9, 3.68, and 3.78 kWh (g TOC)−1 for j of 0.11, 0.125, and 0.14 A cm−2, respectively. From these results, it can be concluded that a current density of 0.11 A cm−2 favors the MCE and decreases the overall energy consumption of the electrochemical process, but mineralization is lees.
Effect of pH
In this section, the effect of pH on the mineralization of 2-CP was investigated when the volumetric flow rate was 1 L min−1 and j = 0.14 A·cm−2 at different pH0. Figure 3(a) shows that after 6 h of electrolysis time a 96%, 93.5%, and 93.8% of mineralization extent is achieved for pH0 of 7.3, 4, and 9, respectively. From these results, it can be concluded that the effect of pH0 on the 2-CP mineralization extent is negligible. Hence, it is not necessary to modify the pH of the synthetic solution of 2-CP and this is in agreement with previous reports [28].
In Fig. 3(b), it can be seen that the MCE% reaches the final values of 6, 6.45, and 6 for a pH0 of 4, 7.3, and 9, respectively. This is because at pH0 of 7.3 there is a greater production of ●OH than with pH0 of 4 and 9. Therefore, the MCE% and the process efficiency increase when there is a large ●OH production.
It can be seen in Fig. 3(c), that EC reaches the final values of 5.37, 3.78, and 5.35 kWh (g TOC)−1, for a pH0 of 4, 7.3, and 9, respectively. Where, the minimum energy consumption was achieved at pH0 of 7.3. Based on these results, the effect of volumetric flow rate on mineralization of 2-CP was studied when the electrochemical flow reactor was operated at pH0 of 7.3 and j of 0.14 A cm−2.
Effect of volumetric flow rate (Q)
Figure 4(a) shows the normalized TOC profiles at different volumetric flow rates at an electrolysis time of 6 h. The results show that the percentages of mineralization of 2-CP achieve the values of 90%, 96%, and 87% for a Q of 0.5, 1.0, and 1.5 L min−1, respectively. Hence, the maximum percentage of 2-CP mineralization was 96%, which was achieved at 1.0 L min−1. Meanwhile, the minimum percentage of 2-CP mineralization was 87%, which was achieved at Q = 1.5 L min−1. This can be explained in terms of contact time and turbulence, both affected by the volumetric flow rate. The ●OH radicals are produced onto the electrode surface where they oxidize the organic compounds. This oxidation is more likely to occur given the proper contact time and turbulence that promote the organic molecules reaching the electrode surface to be oxidized by those physisorbed radicals. When Q increases the turbulence increases and this favors the radicals desorption which is rather undesirable [24] since these radicals may extinct before encountering an organic molecule to oxidize. At this point is worth keeping on mind that the half-life time of the ●OH, 10−10 s [5], is rather short. In addition, when the electrochemical reactor operates at Q = 0.5 L min−1, the 2-CP in the synthetic solution is less likely to encounter the produced ●OH. Furthermore, it can be said that the best volumetric flow rate is Q = 1.0 L min−1 because the maximum percentage of mineralization of 2-CP was achieved at this volumetric flow rate. Hence, at this particular volumetric flow rate, the pollutant has enough time to react with the ●OH formed according to Eq. (1). This result was confirmed in a hydrodynamic study of the used electrochemical flow rate, which was reported in a previous work of our research group [32]. In such study, it was found that the best Q was 1.0 L min−1, which was achieved at an axial dispersion coefficient of 0.0005 m2 s−1 or with a Peclet number value of 40.
Figure 4(b) shows the MCE% for all tested volumetric flow rates. It is worth pointing out that the error on determining these values was lower than 1%. It is observed that the highest percentage of MCE (6.2%) was achieved at volumetric flow rates of 1 L min−1 and 1.5 L min−1. Meanwhile, a lower value of MCE (5.8%) was achieved at a volumetric flow rate of 0.5 L min−1. Given the error, however, this difference is not considered significant.
In order to establish the process controlling mechanism, i.e. mass transfer or current controlled, a comparison was conducted between the relative magnitude of limiting current density (jlim), as calculated in Eq. (8) and applied current density (japp). If jlim < japp, the electrochemical mineralization process is controlled by mass transfer (specifically by diffusion); the contrary, the electrochemical mineralization process is current controlled in agreement with [11].
where n is the number of electrons, F is the Faraday constant, km is the mean mass transfer coefficient, C0 is the 2-CP initial concentration. The mean transfer coefficient can be calculated from Eq. (9), where A is the geometric area of the electrode and Vs is the total volume of treated synthetic solution in batch mode.
As it can be seen in Table 2, for every Q, jlim<jApp, this indicates that the electrochemical mineralization was controlled by mass transfer.
Figure 4(c) depicts the energy consumption, and it can be observed that this achieved a value of 3.78 kWh (g TOC)−1 at a volumetric flow rate of 1 L min−1. Meanwhile, the energy consumption value was 4.10 kWh (g TOC)−1 at volumetric flow rates of 0.5 L min−1 and 1.5 L min−1. From these results, it can be concluded that the volumetric flow rate of 1 L min−1 is the best volumetric flow rate because the maximum mineralization of 2-CP and minimum energy consumption were achieved at this volumetric flow rate. Finally, based on all previous results, it can be said that the best operational conditions to perform the electrochemical oxidation of 2-CP were j of 0.14 A cm−2, pH of 7.3, and Q of 1 L min−1.
By-products distribution
In order to establish the by-products and intermediate compounds of the electrochemical oxidation of 2-CP, the analysis of samples was performed by liquid chromatography. This was carried out at the best operating conditions found in this investigation. All by-products were identified unequivocally by comparing their retention time with the ones from standard compounds by liquid chromatography. Figure 5 shows the evolution of all compounds in the electrochemical oxidation of 2-CP with respect to time.
Figure 5 is the evidence that the first step on the oxidation 2-CP is the hydroxylation by ●OH that leads to the formation of aromatic by-products such as benzoquinone (BH), 4-chlorocatechol (4-CC), catechol (C) and hydroquinone (HQ). Concomitantly, although at a lower rate, Cl is removed from the 2-CP molecule and this produces phenol (Ph). All this can be observed in Fig. 5(a). Also, it can be seen that all compounds were destroyed by ●OH except the 4-CC, which remained, although in a very low amount (1.9 mg L−1), after the electrochemical treatment. Moreover, in Fig. 5(b), it can be seen that the formation of other by-products such as organic acids, are also formed during electrolysis of 2-CP. These organic acids are acetic, formic, fumaric, maleic, malonic, oxalic, and succinic. Furthermore, it can be concluded that the acids oxalic, maleic, formic, fumaric, and acetic, along with the 4-CC, are the compounds to be blamed for the remained 4% TOC. Among all, oxalic acid is the one with the highest concentration (2.5 mg L−1). It is well known that oxalic acid oxidation requires a high ●OH concentration
The aforementioned results suggest that 2-CP oxidation under the studied reaction conditions here, follows a reaction pathway rather similar to that previously proposed for 4-CP oxidation with BDD electrodes also [28]. In such a scheme, Cl atom removal might produce phenol as the first step. From Fig. 5a, it can be inferred though, that phenol is readily hydroxylated since it is barely detected during the whole treatment. Phenol hydroxylation leads to hydroquinone which is dehydrogenated to produce benzoquinone [3]. Subsequently the ●OH attacks the benzoquinone ring to produce carboxylic acids such as fumaric, maleic, and malonic, finally the oxidation of malonic acid yields to CO2 and H2O. Also, it is plausible that the hydroxyl radical (●OH) attacks the benzene ring and before Cl atom is removed, 4-chlorocatechol is formed. This is converted to catechol and its oxidation produces oxalic acid and subsequently produces formic acid so finally yields to CO2 and H2O. It is worth noticing that this reaction scheme is different to the pathway proposed by [1] who performed the degradation of 2-CP by using electrodes of black diamond carbon and activated carbon. The difference between the two pathways might be ascribed to the anodes having different degree of reactivity towards the electrochemical degradation [20].
For the sake of reference, Table 3 summarizes the main results regarding 2-CP electrochemical oxidation found in literature. It can be seen that in this study the percentage of mineralization was higher (96%) than in other works previously reported (e.g. 83.6% in [35]). Also, the degradation of 2-CP was 100%. Moreover, this study is the only one that reports the EC and %MCE with values of 3.78 kWh (g TOC)−1 and 6.2%, respectively. Furthermore, it can be seen that the degree of degradation depends on electrode type such as is reported in [35]. This author used two electrodes type, in the first one (Pt/Ti) and obtained TOC removal of 56.8% and in the second one (BDD) got a TOC removal of 83.6%. Based in the results obtained in [35] and in this study, it is worth mentioned that the use of BDD electrodes is a suitable alternative for the treatment of wastewater containing phenolic compounds (e.g. 2-CP).
Kinetic model
The concentration decay of 2-CP was followed by UHPLC analysis. Figure 6 shows that the concentration decay of 2-CP was given by ●OH production on the BDD electrodes. Also, this figure shows that BDD electrodes are able to remove 2-CP from the wastewater in a period of 4 h. An apparent first-order kinetic order and a rate constant k = 1.19178 h−1 were found by fitting the above concentration decay, with a high correlation coefficient (r2 = 0.981). Another way to compare the experimental values and those predicted by the model is through the reduced root-mean-square (RMS) given by Eq. (10). Small values of RMS lead to high agreement between the experimental values and those predicted by the model [19],
Where Cexp,i and Cpred,i are 2-CP experimental concentrations and predicted by model respectively, and N is the number of data.
In this case the RMS was 0.0257, which indicates that the experimental values are in high concordance with the predicted data. Meanwhile, there is plotted in Fig. 7, the comparison between the experimental data versus data obtained with the pseudo-first kinetic order model, with a high correlation between both of them. This behavior suggests that large amounts of ●OH are produced by BDD electrodes and react very fast with all by-products of the degradation of 2-CP to yield CO2 [27].
Associated cost
In this section the cost of electrolysis process was estimated at the best operating conditions of the electrochemical oxidation of 2-CP. The power is computed by the Eq. (11). Meanwhile, the energy is calculated by using Eq. (12). Also, the associated cost of the electrochemical oxidation of 2-CP is given by Eq. (13).
The electricity cost for industrial use in Mexico is $4.04 MXP/kWh when supplied by Comisión Federal de Electricidad (CFE, a Mexican company). Based on the referred cost, the associated cost of the electrochemical oxidation of 2-CP was of $0.06 USD/L ($1.2 MXP/L, based on $1USD/$20 MXP) of synthetic solution of 2-CP.
Conclusions
The complete electrochemical oxidation of 2-CP was successfully carried out in a continuous flow electrochemical reactor equipped with BDD electrodes. The degradation and mineralization percentages at the best operating conditions (j = 0.14 A cm−2, pH of 7.3, and Q = 1 L min−1) were 100% and 96%, respectively, at an electrolysis time of 6 h. The pH of the solution did not have an important effect on percentage of mineralization of 2-CP, so pH adjustment is unnecessary to eliminate the assessed phenolic compound in the synthetic solution through the methodology employed in the present investigation. By UHPLC analysis, under the best operating conditions of electrolysis reaction, 2-CP oxidation by-products were established. Degradation of 2-CP follows a pseudo-first-order kinetics, and the model has a high correlation with the experimental data (kinetic constant of 1.19178 h−1 with r2 of 0.981). The results of this study support the use of a continuous flow electrochemical reactor equipped with BDD electrodes to remove persistent organic compounds in wastewater treatment (e.g. the 2-CP) because the electrolysis process applied is cheaper ($ 0.06 USD/L) than conventional process. Furthermore, the use of BDD electrodes allows the use of relatively low current densities (0.14 A cm−2) and energy consumption (3.78 kWh (g TOC)−1. This offers the advantage of using sustainable power energies like solar cells with batteries as energy storage.
References
Ajeel MA, Aroua MK, Daud WMAW (2015) Anodic degradation of 2-Chlorophenol by carbon black diamond and activated carbon composite electrodes. Electrochim Acta 180:22–28
Ajeel MA, Aroua MK, Daud WMAW, Mazari SA (2017) Effect of adsorption and passivation phenomena on the electrochemical oxidation of phenol and 2-Chlorophenol at carbon black diamond composite electrode. Ind Eng Chem Res 56(6):1652–1660
Amado-Piña D, Roa-Morales G, Barrera-Díaz C, Balderas-Hernandez P, Romero R, Martín del Campo E, Natividad R (2017) Synergic effect of ozonation and electrochemical methods on oxidation and toxicity reduction: phenol degradation. Fuel 198:82–90
Ba-Abbad MM, Takriff MS, Kadhum AAH, Mohamad AB, Benamor A, Mohammad AW (2017) Solar photocatalytic degradation of 2-chlorophenol with ZnO nanoparticles: optimisation with D-optimal design and study of intermediate mechanisms. Environ Sci Pollut Res 24(3):2804–2819
Baskin S, Salem H (1997) Oxidants, antioxidants and free radicals, Taylor & Francis
Borràs N, Oliver R, Arias C, Brillas E (2010) Degradation of atrazine by electrochemical advanced oxidation processes using a boron-doped diamond anode. J Phys Chem A 114(24):6613–6621
Brillas E, Sirés I, Arias C, Cabot PL, Centellas F, Rodríguez RM, Garrido JA (2005) Mineralization of paracetamol in aqueous medium by anodic oxidation with a boron-doped diamond electrode. Chemosphere 58(4):399–406
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev 109(12):6570–6631
Brown CJ, Pletcher D, Walsh FC, Hammond JK, Robinson D (1992) Local mass transport effects in the FM01 laboratory electrolyser. J Appl Electrochem 22(7):613–619
Cornejo OM, Murrieta MF, Castañeda LF, Nava JL (2019) Characterization of the reaction environment in flow reactors fitted with BDD electrodes for use in electrochemical advanced oxidation processes: A critical review. Electrochim Acta: 135373
Chang C-F, Chen T-Y, Chin C-JM, Kuo Y-T (2017) Enhanced electrochemical degradation of ibuprofen in aqueous solution by PtRu alloy catalyst. Chemosphere 175:76–84
Dercová K, Sejáková Z, Skokanová M, Barančíková G, Makovníková J (2007) Bioremediation of soil contaminated with pentachlorophenol (PCP) using humic acids bound on zeolite. Chemosphere 66(5):783–790
Enache TA, Chiorcea-Paquim A-M, Fatibello-Filho O, Oliveira-Brett AM (2009) Hydroxyl radicals electrochemically generated in situ on a boron-doped diamond electrode. Electrochem Commun 11(7):1342–1345
Ezerskis Z, Jusys Z (2001) Oxidation of chlorophenols on Pt electrode in alkaline solution studied by cyclic voltammetry, galvanostatic electrolysis, and gas chromatographymass spectrometry. Pure Appl Chem 73:1929
Guinea E, Arias C, Cabot PL, Garrido JA, Rodríguez RM, Centellas F, Brillas E (2008) Mineralization of salicylic acid in acidic aqueous medium by electrochemical advanced oxidation processes using platinum and boron-doped diamond as anode and cathodically generated hydrogen peroxide. Water Res 42(1–2):499–511
Huang CP, Chu C-s (2012) Indirect electrochemical oxidation of Chlorophenols in dilute aqueous solutions. J Environ Eng 138(3):375–385
Ikehata K, Jodeiri Naghashkar N, Gamal El-Din M (2006) Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: a review. Ozone Sci Eng 28(6):353–414
Keith L, Telliard W (1979) ES&T Special Report: priority pollutants: I-a perspective view. Environ Sci Technol 13(4):416–423
Kim S, Kim YK (2004) Apparent desorption kinetics of phenol in organic solvents from spent activated carbon saturated with phenol. Chem Eng J 98(3):237–243
Li X-y, Y-h C, Y-j F, Xie Z-m GJ-D (2005) Reaction pathways and mechanisms of the electrochemical degradation of phenol on different electrodes. Water Res 39(10):1972–1981
Lim CH, Ang JJ, Lau S, Tay MG (2017) Optimization of hydroxyl radical production using electro-Fenton method for chemical oxygen demand reduction in diluted palm oil mill effluent. Water Environ J 31(4):578–583
Liu H, Zhang Z, Ren M, Guan J, Lu N, Qu J, Yuan X, Zhang YN (2018) Preparation of the CNTs/AG/ITO electrode with high electro-catalytic activity for 2-chlorophenol degradation and the potential risks from intermediates. J Hazard Mater 359:148–156
Michaud PA, Panizza M, Ouattara L, Diaco T, Foti G, Comninellis C (2003) Electrochemical oxidation of water on synthetic boron-doped diamond thin film anodes. J Appl Electrochem 33(2):151–154
Nava JL, Sirés I, Brillas E (2014) Electrochemical incineration of indigo. A comparative study between 2D (plate) and 3D (mesh) BDD anodes fitted into a filter-press reactor. Environ Sci Pollut Res 21(14):8485–8492
Oturan MA, Aaron J-J (2014) Advanced oxidation processes in water/wastewater treatment: principles and applications. A Review. Crit Rev Environ Sci Tech 44(23):2577–2641
Panizza M (2014) Anodic oxidation of benzoquinone using diamond anode. Environ Sci Pollut Res 21(14):8451–8456
Panizza M, Cerisola G (2009) Direct and mediated anodic oxidation of organic pollutants. Chem Rev 109(12):6541–6569
Peralta E, Ruiz M, Martinez G, Mentado-Morales J, Zarate LG, Cordero ME, Garcia-Morales MA, Natividad R, Regalado-Mendez A (2018) Degradation of 4-Chlorophenol in a batch electrochemical reactor using BDD electrodes. Int J Electrochem Sci 13(5):4625–4639
Polcaro AM, Palmas S (1997) Electrochemical oxidation of Chlorophenols. Ind Eng Chem Res 36(5):1791–1798
Polcaro AM, Palmas S, Renoldi F, Mascia M (1999) On the performance of Ti/SnO2 and Ti/PbO2 anodesin electrochemical degradation of 2-chlorophenolfor wastewater treatment. J Appl Electrochem 29(2):147–151
Ramírez C, Saldaña A, Hernández B, Acero R, Guerra R, Garcia-Segura S, Brillas E, Peralta-Hernández JM (2013) Electrochemical oxidation of methyl orange azo dye at pilot flow plant using BDD technology. J Ind Eng Chem 19(2):571–579
Regalado-Méndez A, Mentado-Morales J, Vázquez Carlos E, Martínez-Villa G, Cordero Mario E, Zárate LG, Skogestad S, Peralta-Reyes E (2018) Modeling and hydraulic characterization of a filter-press-type electrochemical reactor by using residence time distribution analysis and hydraulic indices. Int J Chem React Eng 16
Skoumal M, Arias C, Cabot PL, Centellas F, Garrido JA, Rodríguez RM, Brillas E (2008) Mineralization of the biocide chloroxylenol by electrochemical advanced oxidation processes. Chemosphere 71(9):1718–1729
Tarr MA (2003) Chemical degradation methods for wastes and pollutants: environmental and industrial applications. CRC press
Yoon J, Lee B, Choi S, Won M, Shim Y (2012) Electrochemical degradation of phenol and 2-chloro phenol using Pt/Ti and boron-doped diamond electrodes. B Korean Chem Soc 33(7):2274–2278
Yoon JH, Jeong ED, Shim YB, Won MS (2004) Anodic degradation of toxic aromatic compound in the flow through cell with carbon Fiber electrode. Key Eng Mat 277-279:445–449
Acknowledgements
Authors thank PRODEP and CONACYT for the financial support (Project DSA/103/14/11350, 2014; CUP: 2 IE1503 and CONACYT No. 269093). Also, Mayra Castellanos thanks PRODEP for the scholarship to complete her bachelor thesis (Through the items ID 32643, 32646, and 32651 of the project DSA/103/14/11350, 2014; CUP: 2 IE1503).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peralta-Reyes, E., Natividad, R., Castellanos, M. et al. Electro-oxidation of 2-chlorophenol with BDD electrodes in a continuous flow electrochemical reactor. J Flow Chem 10, 437–447 (2020). https://doi.org/10.1007/s41981-020-00079-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-020-00079-5