Abstract
The anodic degradation of 1,4-benzoquinone (BQ), one of the most toxic xenobiotic, was investigated by electrochemical oxidation at boron-doped diamond anode. The electrolyses have been performed in a single-compartment flow cell in galvanostatic conditions. The influence of applied current (0.5–2 A), BQ concentration (1–2 g dm−3), temperature (20–45 °C) and flow rate (100–300 dm3 h−1) has been studied. BQ decay kinetic, the evolution of its oxidation intermediates and the mineralization of the aqueous solutions were monitored during the electrolysis by high-performance liquid chromatograph (HPLC) and chemical oxygen demand (COD) measurements. The results obtained show that the use of diamond anode leads to total mineralization of BQ in any experimental conditions due to the production of oxidant hydroxyl radicals electrogenerated from water discharge. The decay kinetics of BQ removal follows a pseudo-first-order reaction, and the rate constant increases with rising current density. The COD removal rate was favoured by increasing of applied current, recirculating flow rate and it is almost unaffected by solution temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
1,4-benzoquinone (BQ) is a multifunctional molecule with highly toxic characteristics causing erythema and localised tissue necrosis; it is irritating to the eyes and respiratory system, and it inhibits the function of biological wastewater plant. There are two main causes of the presence of BQ in the environment: (i) it is released in effluent during its use in the production of hydroquinone, dyes, insecticides, photographic processes, pharmaceuticals and leather industries; and (ii) it is an intermediate in the oxidation of benzene and other phenols.
Despite BQ is one of the most toxic xenobiotic and it is widely found in wastewaters, only few studies have been reported on its treatment method. These include electrochemical coagulation (Can and Bayramoglu 2010), photochemical oxidation (Shevchuk and Kirsho 1981), biodegradation (Kumar et al. 2011) and electrochemical oxidation (Pulgarin et al. 1994; Feng et al. 1995; Houk et al. 1998; Bock and MacDougall 1999; Yoon et al. 2007).
The anodic degradation of BQ was studied for the first time by Pulgarin et al. (1994) using Ti/IrO2 and Ti/SnO2 anodes showing that the nature of the anode is the most important parameter. With Ti/IrO2, the ring rupture occurred resulting in the accumulation of carboxylic acids as final products, while at Ti/SnO2 BQ and carboxylic acids are oxidised to CO2.
Feng et al. (1995) and Houk et al. (1998) studied the incineration of BQ using a quaternary metal oxide anode in the absence of a soluble electrolyte, using a solid-polymer electrolyte. The anode showed a good reactivity for the incineration of BQ, but the electrolysis time was excessively long (i.e. 64 h).
Acidic solution of BQ has been treated using amorphous Ni–Nb–Pt–Sn anode, obtaining the removal of 23 % of BQ after 3 h of electrochemical oxidation (Pierna et al. 2001).
Yoon et al. (2007) studied the degradation of BQ in a flow-through cell with carbon fibre and found that the removal efficiency increased with applied current and time. In particular after 12 h of electrolysis at 175 mA, 99.23 % of BQ was removed but only 41.65 % were mineralised to CO2.
In the last decades, many papers demonstrated that the complete mineralization of organic compounds can be obtained with high efficiency by direct electrolysis using synthetic boron-doped diamond (BDD) as anode material (Panizza and Cerisola 2009a, 2005; Martínez-Huitle and Ferro 2006). With this electrode, which present an inert surface with low adsorption properties and a wide potential window for water discharge, the organics and their intermediates are oxidised to CO2 by •OH radicals electrogenerated from water discharge:
It is reported that many organic compounds such as phenols (Elaoud et al. 2011; Panizza and Cerisola 2009b; Rodrigo et al. 2001; Iniesta et al. 2001; Canizares et al. 2005; Morao and Lopes 2004; Panizza et al. 2000), dyes (Panizza and Cerisola 2008; Canizares et al. 2006; Faouzi et al. 2006; Martinez-Huitle and Brillas 2009; Hammami et al. 2008; Panizza and Cerisola 2007), drugs (Guinea et al. 2009; Flox et al. 2006; Brillas et al. 2005; Ozcan et al. 2008; Ciriaco et al. 2009; Zhao et al. 2009) and real effluents (Martínez-Huitle et al. 2012; Panizza and Martinez-Huitle 2013; Panizza et al. 2006; Canizares et al. 2007a; Canizares et al. 2009, 2007b) have been successively removed using BDD, even with current efficiency approaching 100 %.
The aim of this work is to study the degradation of a solution containing BQ using a flow cell with a BDD anode. The effect of the main operating parameters, such as current density, flow rate, organic concentration and temperature on chemical oxygen demand (COD) decay and BQ removal has been investigated.
Experimental
The synthetic solution was prepared dissolving different amounts of BQ (C6H4O2) in distilled wastewater in 0.5 M HClO4. HClO4 was chosen as supporting electrolyte, because it does not generate some oxidising species liable to react with organics, as occurs using Cl− medium (i.e. generation of Cl2) or SO4 2− medium (i.e. production of S2O8 2−).
The boron-doped diamond (BDD) thin-film electrode was supplied by Adamant Technologies (Switzerland). It was synthesised by hot filament chemical vapour deposition technique (HF CVD) on single crystal p-type Si wafers. The filament temperature ranged from 2,440 to 2,560 °C, while the substrate temperature was 830 °C.
The obtained diamond film had 1 μm thickness. In order to stabilise the electrode surface and obtain reproducible results, the diamond electrode was pre-treated by anodic polarisation in 1 M HClO4 at 10 mA cm−2 for 30 min. Following this treatment the surface became hydrophilic.
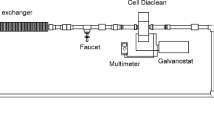
Bulk oxidations were performed in a one-compartment electrolytic flow cell under galvanostatic conditions using an AMEL 2055 potentiostat/galvanostat. BDD was used as the anode, and stainless steel as the cathode. Both electrodes were square, each with 50-cm2 geometrical area and 1-cm inter-electrode gap. The solution was stored in a 0.4-dm3 thermo-regulated glass tank and circulated through an electrochemical reactor by a centrifugal pump with different flow rates in the range of 100–300 dm3 h−1.
Current efficiency (CE) for anodic oxidation was calculated from COD values, using the following relationship (Panizza et al. 2008):
where COD t and COD t+Δt are chemical oxygen demands at times t and t + Δt (in gO2dm−3) respectively, I is the current (A), F is the Faraday constant (96,487 Cmol−1), V is the electrolyte volume (dm3), and 8 is the oxygen equivalent mass (geq.−1).
The specific energy consumption (E C, in kWh m−3) was obtained as follows:
where U cell is the average cell voltage (V), I is the current (A), t is the electrolysis time (s) and V is the volume of the treated solution (dm3).
Evolution of BQ concentrations during electrolyses was monitored by high-performance liquid chromatography (HPLC) using spectra monitor 3100 chromatograph equipped with UV detector at 254 nm and fitted with an Alltech C18 5u column (length 250 mm, i.d.4.6 mm) at 25 °C. The analyses were carried out isocratically with an acetonitrile/water 25:75 (v/v) mixture as mobile phase at a flow rate of 1 cm3 min−1.
Results and discussion
The effect of several operational parameters including applied current, initial organic concentration, flow rate and temperature on COD and BQ removal was studied during the electrochemical treatment using BDD electrodes.
Figure 1 shows the trend of the COD and current efficiency (inset) with time during the anodic oxidation of 2 g L−1 of BQ at different current densities. For all the applied currents, the COD decreases up to the detection limit (about 15 mg L−1), indicating complete mineralization (>98 % COD removal) from overall reaction (Eq. 4) by means of its reaction with •OH electrogenerated on BDD surface from water discharge (Eq. 1):
Increasing the current from 0.5 to 1 A resulted in a faster COD removal rate (i.e. dCOD/dt), meaning that at low current, the process is under charge transfer control because a greater charge passing into the cell favours the oxidation of the pollutant and its intermediates.
The increase to 2 A does not produce significant improvement in the oxidation rate with respect to 1 A, and this behaviour indicates that at high current, the oxidation of BQ became under mass-transport control and an increase of the applied current mainly favours the secondary reaction of hydroxyl radicals decomposition to oxygen:
This was confirmed by the evolution of the current efficiency (Fig. 1, inset). In fact, at 0.5 A, at the beginning of the electrolysis, the CE remained about 100 %; this is a characteristic of electrolysis performed at a current below the limiting one, and under these conditions the oxidation of BQ is controlled by the rate at which electrons are delivered at the anode. On the contrary, at 2 A, CE is always below 100 % and it decreases with time, meaning that the oxidation was carried out at current density higher than the limiting one and the process was under mass-transport control.
The decay of BQ concentration during the electrolyses at different current densities has been followed by reversed-phase HPLC, and the results are reported in Fig. 2. It can be observed that BQ removal is slightly influenced by applied current, and in any condition, the BQ decays could be very satisfactory described by pseudo-first-order kinetics (Fig. 2, inset). The pseudo-first-order reaction can be attributed to the fact that in galvanostatic conditions, a stationary concentration of •OH radicals is reached at the electrode surface. From this analysis, it was found that the pseudo-first-order rate constant increases from 5.65 × 10−4 s−1 to 6.69 × 10−4 s−1 and 8.28 × 10−4 s−1 with increasing current from 0.5 to 1 and 2 A, respectively. From the data of Fig. 2, it is also possible to calculate the specific charge (Ah dm−3) necessary for the complete removal of BQ from the solution. The specific energy was 2.8, 4.5 and 9.4 Ah dm−3 for the electrolyses at 0.5, 1 and 2 A respectively. These values were lower than that obtained by Pulgarin et al. (1994) for the oxidation of BQ using IrO2 or SnO2 anodes (i.e. 100 and about 14 Ah dm−3, respectively). By comparing the results reported in Figs. 1 and 2, it is worth noting that BQ is removed more rapidly than the COD of the solution, meaning that some oxidation intermediates are formed. Hydroquinone and aliphatic carboxylic acids such as maleic, fumaric, acetic and oxalic acids have been identified as the main intermediates. These findings fit very well with the results reported by Pulgarin et al. (1994) using a SnO2 anode for the anodic oxidation of BQ. Figure 3 shows the influence of the current density on the evolution of oxidation intermediates, expressed as mg of carbon dm−3. At higher current density, lower amounts of intermediates are accumulated in the medium, since BQ oxidation is accelerated by reaction with more hydroxyl radicals. However, after 180 min, all the intermediates are mineralised to CO2 and water.
Effect of applied current on the evolution of BQ concentration during the electrolysis of 1 g dm−3 of BQ aqueous solution at applied current of (circle) 0.5 A, (triangle) 1 A and (box) 2 A. Conditions: T = 20 °C; flow rate = 300 dm3 h−1. The inset presents the corresponding kinetic analysis assuming a pseudo-first-order reaction
The influence of the recycle flow rate on COD removal during BQ oxidation at 1 A is shown in Fig. 4. The overall COD depletion is lower as the recycle flow rate is diminished; thus, meaning that at low recycle flow rate, the oxidation becomes a diffusion-controlled process. In fact, the increase in the recycle flow rate accelerates the transport of the organics towards the electrode surface, where they react with the electrogenerated hydroxyl radicals, and, consequently, they disappear from the solution more quickly and with higher current efficiency (Fig. 4, inset).
Figure 5 shows the effect of the initial BQ concentration on COD and CE evolution during electrolysis at 25 °C by applying a current of 1 A and with a recycle flow rate of 300 dm3 h−1. Overall COD removal is achieved in all cases and the time for total mineralization increased with BQ concentration as expected from the presence of a greater amount of organic in the solution.
However, it is interesting to observe that at high initial concentration, at the beginning of the electrolysis, the COD decreased linearly with specific charge and CE remained about 100% (Fig. 5, inset), indicating that the electrolysis was under charge transfer control. On the contrary, at low concentration, COD decreases exponentially and CE is below 100 %, meaning that the oxidation was carried out at current density higher than the limiting one and the process was under mass-transport control.
The variation of the COD and BQ concentration during the electrolysis at 20, 35 and 45 °C when applying a current density of 1 A and with a recycle flow rate of 300 dm3 h−1 is presented in Fig. 6. In a medium that cannot generate oxidising species, such as HClO4, higher temperatures do not yield a significant increase of the oxidation rate of COD and BQ removal during the electrochemical process. This result is in agreement with other previous studies about the oxidation of naphthol (Panizza et al. 2001) and acid yellow (Rodriguez et al. 2009) on BDD. The small difference between the results obtained at different temperatures is only due to an increase of the diffusion rate with rising temperature due to the decrease of the medium viscosity.
From this kinetic analysis, it was found that the pseudo-first-order rate constant for BQ oxidation increases from 6.69 × 10−4 s−1 to 8.51 × 10−4 s−1 and 9.42 × 10−4 s−1 with increasing temperature from 20 to 35 and 45 °C, respectively.
The apparent activation energy for the electrochemical oxidation of BQ at the BDD electrode was found to be 10 kJ mol−1, which is close for a diffusion-controlled homogeneous reaction (typically less than 40 kJ mol−1 (Elaoud et al. 2011)).
For large application, it is also very important to estimate the treatment costs, and thus Fig. 7 reports the variation of specific energy consumption, calculated from Eq. (3), as function of COD removal, in the best operating conditions found previously (i.e. flow rate 300 dm3 h−1; T = 45 °C; I = 1 A). For low COD removal, the specific energy consumption increases almost linearly, while for COD removal higher than 80 % E C increased exponentially. This behaviour can be probably explained by the formation of more refractory products, such as carboxylic acids which are hardly oxidisable intermediates, and the decrease of organic content in the solution. Energy costs for total mineralization was about 49.5 kWh m−3.
Conclusions
Electrochemical oxidation using a BDD anode has been successfully applied to treat a solution containing BQ. The experimental results showed that:
-
Within the range studied, the almost total BQ and COD removal was obtained, regardless of the experimental conditions, due to the formation of hydroxyl radicals from the water discharge.
-
The oxidation of BQ is well described by a pseudo-first-order kinetic, because in galvanostatic electrolysis there is a stationary concentration of •OH radicals on the BDD surface.
-
At high BQ concentration, low current and high flow rate, the oxidation is controlled by charge transfer, while at low concentration, low flow rate and high current, it is under mass-transport control.
-
At low current, high current efficiency (i.e. 100 %) was obtained but high amount of intermediates were formed, while at high current, the efficiency was lower but less amount of intermediates were formed.
-
The COD removal rate increases with applied current and flow rate, while it is almost unaffected by solution temperature.
References
Bock C, MacDougall B (1999) Anodic oxidation of p-benzoquinone and maleic acid. J Electrochem Soc 146:2925–2932
Brillas E, Sires I, Arias C, Cabot PL, Centellas F, Rodriguez RM, Garrido JA (2005) Mineralization of paracetamol in aqueous medium by anodic oxidation with a boron-doped diamond electrode. Chemosphere 58:399–406
Can OT, Bayramoglu M (2010) The effect of process conditions on the treatment of benzoquinone solution by electrocoagulation. J Hazard Mater 173:731–736
Canizares P, Lobato J, Paz R, Rodrigo MA, Saez C (2005) Electrochemical oxidation of phenolic wastes with boron-doped diamond anodes. Water Res 39:2687–2703
Canizares P, Gadri A, Lobato J, Nasr B, Paz R, Rodrigo MA, Saez C (2006) Electrochemical oxidation of azoic dyes with conductive-diamond anodes. Ind Eng Chem Res 45:3468–3473
Canizares P, Lobato J, Paz R, Rodrigo MA, Saez C (2007a) Advanced oxidation processes for the treatment of olive-oil mills wastewater. Chemosphere 67:832–838
Canizares P, Louhichi B, Gadri A, Nasr B, Paz R, Rodrigo MA, Saez C (2007b) Electrochemical treatment of the pollutants generated in an ink-manufacturing process. J Hazard Mater 146:552–557
Canizares P, Hernandez M, Rodrigo MA, Saez C, Barrera CE, Roa G (2009) Electrooxidation of brown-colored molasses wastewater. Effect of the electrolyte salt on the process efficiency. Ind Eng Chem Res 48:1298–1301
Ciriaco L, Anjo C, Correia J, Pacheco MJ, Lopes A (2009) Electrochemical degradation of Ibuprofen on Ti/Pt/PbO2 and Si/BDD electrodes. Elecrtrochim Acta 54:1464–1472
Elaoud SC, Panizza M, Cerisola G, Mhiri T (2011) Electrochemical degradation of sinapinic acid on a BDD anode. Desalination 272:148–153
Faouzi M, Canizares P, Gadri A, Lobato J, Nasr B, Paz R, Rodrigo MA, Saez C (2006) Advanced oxidation processes for the treatment of wastes polluted with azoic dyes. Electrochim Acta 52:325–331
Feng J, Houk LL, Johnson DC, Lowery SN, Carey JJ (1995) Electrocatalysis of anodic oxygen-transfer reactions: the electrochemical incineration of benzoquinone. J Electrochem Soc 142:3626–3632
Flox C, Cabot PL, Centellas F, Garrido JA, Rodriguez RM, Arias C, Brillas E (2006) Electrochemical combustion of herbicide mecoprop in aqueous medium using a flow reactor with a boron-doped diamond anode. Chemosphere 64:892–902
Guinea E, Brillas E, Centellas F, Canizares P, Rodrigo MA, Saez C (2009) Oxidation of enrofloxacin with conductive-diamond electrochemical oxidation, ozonation and Fenton oxidation. A comparison. Water Res 43:2131–2138
Hammami S, Bellakhal N, Oturan N, Oturan MA, Dachraoui M (2008) Degradation of Acid Orange 7 by electrochemically generated OH radicals in acidic aqueous medium using a boron-doped diamond or platinum anode: a mechanistic study. Chemosphere 73:678–684
Houk LL, Johnson SK, Feng J, Houk RS, Johnson DC (1998) Electrochemical incineration of benzoquinone in aqueous media using a quaternary metal oxide electrode in the absence of a soluble supporting electrolyte. J Appl Electrochem 28:1167–1177
Iniesta J, Michaud PA, Panizza M, Cerisola G, Aldaz A, Comninellis C (2001) Electrochemical oxidation of phenol at boron-doped diamond electrode. Electrochim Acta 46:3573–3578
Kumar P, Nemati M, Hill GA (2011) Biodegradation kinetics of 1,4-benzoquinone in batch and continuous systems. Biodegradation 22:1087–1093
Martinez-Huitle CA, Brillas E (2009) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B-Environ 87:105–145
Martínez-Huitle CA, Ferro S (2006) Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem Soc Rev 12:1324–1340
Martínez-Huitle CA, Dos Santos EV, De Araújo DM, Panizza M (2012) Applicability of diamond electrode/anode to the electrochemical treatment of a real textile effluent. J Electroanal Chem 674:103–107
Morao A, Lopes A, Pessoa de Amorim MT, Goncalves IC (2004) Degradation of mixtures of phenols using boron doped diamond electrodes for wastewater treatment. Electrochim Acta 49:1587–1595
Ozcan A, Sahin Y, Koparal AS, Oturan MA (2008) Propham mineralization in aqueous medium by anodic oxidation using boron-doped diamond anode: influence of experimental parameters on degradation kinetics and mineralization efficiency. Water Res 42:2889–2898
Panizza M, Cerisola G (2005) Application of diamond electrodes to electrochemical processes. Electrochim Acta 51:191–199
Panizza M, Cerisola G (2007) Electrocatalytic materials for the electrochemical oxidation of synthetic dyes. Appl Catal B-Environ 75:95–101
Panizza M, Cerisola G (2008) Electrochemical degradation of methyl red using BDD and PbO2 anodes. Ind Eng Chem Res 47:6816–6820
Panizza M, Cerisola G (2009a) Direct and mediated anodic oxidation of organic pollutants. Chem Rev 109:6541–6569
Panizza M, Cerisola G (2009b) Electrochemical degradation of gallic acid on a BDD anode. Chemosphere 77:1060–1064
Panizza M, Martinez-Huitle CA (2013) Role of electrode materials for the anodic oxidation of a real landfill leachate—comparison between Ti-Ru-Sn ternary oxide, PbO2 and boron-doped diamond anode. Chemosphere 90:1455–1460
Panizza M, Duo I, Michaud PA, Cerisola G, Comninellis C (2000) Electrochemical detection of b-naphthol on boron-doped diamond electrodes. Influence of anodic treatment. Electrochem Solid St 3:429–430
Panizza M, Michaud PA, Cerisola G, Comninellis C (2001) Anodic oxidation of 2-naphthol at boron-doped diamond electrodes. J Electroanal Chem 507:206
Panizza M, Zolezzi M, Nicolella C (2006) Biological and electrochemical oxidation of naphthalenesulfonates. J Chem Technol Biotechnol 81:225–232
Panizza M, Sires I, Cerisola G (2008) Anodic oxidation of mecoprop herbicide at lead dioxide. J Appl Electrochem 38:923–929
Pierna AR, Sistiaga M, Navascues C, Lorenzo A (2001) Electrochemical treatment of toxic compounds on the surface of amorphous Ni-Nb-Pt-Sn alloys. J Non-Crystalline Solids 287:432–436
Pulgarin C, Adler N, Peringer P, Comninellis C (1994) Electrochemical detoxification of a 1,4-benzoquinone solution in wastewater treatment. Water Res 28:887–893
Rodrigo MA, Michaud PA, Duo I, Panizza M, Cerisola G, Comninellis C (2001) Oxidation of 4-chlorophenol at boron-doped diamond electrodes for wastewater treatment. J Electrochem Soc 148:D60–D64
Rodriguez J, Rodrigo MA, Panizza M, Cerisola G (2009) Electrochemical oxidation of Acid Yellow 1 using diamond anode. J Appl Electrochem 39:2285–2289
Shevchuk I, Kirsho U (1981) Mutual effects in the cooxidation of phenols, quinone and benzopyrene. Izvesti NSV Tead Akad Toim Keem 30:29–33
Yoon J-H, Yang J, Shim Y-B, Won M-S (2007) Electrochemical degradation of benzoquinone in a flow through cell with carbon fibers. Bull Korean Chem Soc 28:403–407
Zhao X, Hou Y, Liu H, Qiang Z, Qu J (2009) Electro-oxidation of diclofenac at boron doped diamond: kinetics and mechanism. Electrochim Acta 54:4172–4179
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Panizza, M. Anodic oxidation of benzoquinone using diamond anode. Environ Sci Pollut Res 21, 8451–8456 (2014). https://doi.org/10.1007/s11356-014-2782-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2782-2