Abstract
Due to the rapid development of China’s regeneration industry, secondary aluminium ash (SAA) has been extensively produced. The reuse of SAA and Y2O3 doping was studied in this research. This proved that SAA can turn into a raw material for gehlenite/magnesia-alumina spinels. Furthermore, doping with Y2O3 can aggrandize the densification feature of gehlenite/magnesia-alumina spinels. The densification of the gehlenite/magnesia-alumina spinel without Y2O3 was lower than that of the doped spinel in the temperature range of 1573 to 1773 K. At 1673 K, 3 wt% Y2O3 was added to the gehlenite/magnesia-alumina spinel. It had a density of 2.05 g·cm−3 and a compressive strength of 91.2 MPa. Generally, 3 wt% Y2O3 was added, and the sintering temperature at 1673 K was appropriate. The elevation of the densification feature was also attributable to the solubility of Y2O3 and the formation of a low-viscosity liquid phase such as YCaAl3O7. The SAA can be reused for the recovery of gehlenite/magnesia-alumina spinels. Doping it with Y2O3 can broaden its reutilization in new water-resistant ceramic materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dregs of aluminium ash have already been popularly produced in China because the aluminium industry has grown. Nevertheless, in a landfill, over 90% of secondary aluminium ash (SAA) is handled without deeper management. In the natural environment, the negative influence of environmental quality has increased over the past few years [1, 2], and it is meaningful to recycle and reduce SAA. Al, MgAl2O4 (MA) spinels, and corundum are the major compounds of SAA. SAA uses minimal types of additional reagents with low costs that can be used as a raw material for spinels [3, 4]. There are many advantages of magnesium aluminium spinels, including a high melting point, mechanical properties, strong thermal and spalling properties, and excellent chemical performance. These characteristics make MA a main material for many adhibition applications in furnaces. It is necessary to use lining materials for the base and sidewall of the furnace [3]. However, the preparation of magnesium aluminate spinels is still an expensive process and should be supported by high-spirited and new technical techniques, which would lower the cost of raw materials and improve the quality of the outcome.
According to the characteristics of secondary aluminium ash raw materials, the ingredients can be slightly changed to prepare refractory materials [5, 6]. This can realize resource utilization. In recent years, to prepare a lightweight refractory material with both refractory properties (high strength) and reduced mass, the USA, Japan, and other countries successively developed phases that combine with melilite with a high melting point [7, 8]. MA is a kind of magnesium aluminium oxide material with a high melting point (2408 K), good thermal shock resistance, impact resistance, good electrical insulation performance, and strong alkali erosion resistance [9, 10]. Melilite is a class of silicate minerals that are composed of Al, Mg, and iron calcium silicate/sodium silicate, and its general chemical composition formula is (Ca, Na)2(Al, Mg, Fe)[(Al, Si)SiO7]. Gehlenite (Ca2Al2SiO7 (C2AS)) and akermanite (Ca2Mg(Si2O7)) are two important minerals in the melilite group. Melilite has a low density, a small thermal expansion coefficient, and a low thermal conductivity. Melilite is rarely the main crystal phase of refractory materials because its melting point is not very high, but in improving the compressive strength and hydration resistance of materials, it has a vital function [11]. In conclusion, we can prepare refractory materials in which the main crystal phase is a magnesia-alumina spinel and the combined phase is melilite from secondary aluminium. If the result holds up, the potential harm of secondary aluminium ash to the environment can be reduced, and the field of refractory preparation can also be broadened.
Rare earth oxides (REOs) are valid annexing agents that have been diffusely used in chinaware, biotechnology, powder metallurgy, new energy, and chemical technology. WC-MgO bulk composites are doped with La2O3. The flexural strength and Vickers hardness are enhanced [12]. CeO2 is one of the most reactive rare earth metal oxides and has some features, such as a large surface area, a large radius, and strong chemical resistance. It has been widely applied in conformational ceramics, such as oxide and nonoxide ceramics and functional ceramics [13, 14]. WC-Al2O3 compounds adulterated with 0.1% CeO2 showed a fining microstructure and enhanced machine capability compared with nondoped composites [15]. Different scholars have studied the improved sinterability of high-quality spinels doped with 5 wt% Y2O3 [16, 17]. Sintering was found to be improved by using rare earth oxides such as 4 wt% Yb2O3 and Dy2O3 [18]. Densification of the specimens with Y2O3 and Nd2O3 was attributed to the formation of complex oxides, which activated the crystal lattice [19]. La2O3 and CeO2 formed a liquid phase, which accelerated the densification of the crystal lattice [19]. The grain boundary energies decreased when the spinel was doped with 3 mol% Y2O3, Gd2O3, and La2O3 from grain boundary restraint detection [20].
In this work, the series of objectives were as follows. A gehlenite/magnesia-alumina spinel was synthesized from SAA. The effects of Y2O3 doping on the gehlenite/magnesia-alumina spinel were characterized. The mechanisms of densification improvement by Y2O3 doping on the gehlenite/magnesia-alumina spinel obtained from SAA were evaluated.
Experimental procedure
Materials
In Jiangxi Province, China, we collected secondary aluminium ash (SAA). The ash was preprocessed by drying, selection, and ball milling to obtain a uniform particle size. Repeated impact during the ball-milling process resulted in a large number of microscopic defects, such as lattice distortions, dislocations, twinned crystal structures, and strain energy in the powders [21]. The mineral phases of the ash were Al, α-Al2O3, MA, and AlN (Fig. 1). Chemical phase analysis showed that Al and α-Al2O3 accounted for 23.67 wt% and 23.57 wt% of the ash, respectively, with 2.19 wt% AlN and 9.10 wt% MA (Table 1). The analytical grade agents used for analysis and detection included CaO, MgO, and Y2O3.
Experiment
After disposal, the D50 of SAA was 23.42 μm. In this study, SAA (70.8 wt%) was blended with CaO (18.58 wt%) and MgO (10.62 wt%) in a mixing machine for 1 day. The powders were put into a mould with a 3% bonder at 15 MPa, and then, the powders were pressed into bricks (diameter of 2.5 cm and thickness of 1 cm). The raw bricks were placed in an electric stove for sintering in the range of 1373 to 1773 K for 3 h in air (Fig. 2). The sintering temperature curve of the experiment is shown in Fig. 3. The right and left sides of the stove were sealed with a quartz plug to keep the stove warm. The stove was cooled to 773 K at a rate of 3–4 K/min. The bricks were tested at room temperature. The addition amount was 3 wt% Y2O3. The mass fractions of SAA, CaO, MgO, and Y2O3 were 68.8%, 18.0%, 10.3%, and 2.9%, respectively.
Sample analysis
The surface microcosmic morphology of the powder was recorded by scanning electron microscopy (SEM, Nova NanoSEN 230). X-ray diffraction (XRD, TRAX, Rigaku) was employed using a Cu Kα radiation source and a 10°/min scanning speed to analyse the crystal structures of the powders. The particle size distribution was examined by a laser particle analyser (OMEC LS-POP, China). The elements in SAA were examined by X-ray fluorescence (XRF-AXIOS, PANalytical, Netherlands). The powders were placed in a hydraulic press (YLJ-40 T, China) to obtain bricks. The compression strength was examined by an electrohydraulic compression machine (TYA-100C, China). The bulk density of the bricks was calculated using the Archimedes principle.
Results and discussion
Effect of sintering temperature on the characteristics of the gehlenite/magnesia-alumina spinel
The XRD patterns of the gehlenite/magnesia-alumina spinel from SAA at different sintering temperatures are shown in Fig. 4. The main phases in the spinel were C2AS and MA in the temperature range of 1373 to 1773 K and C2AS, MA, and Al2O3 at 1373 K. The crossover of SiO2 enhanced the incorporation of the framework with the MA stroma. The silica compositions began to melt at a high temperature, and the liquid phase formed. SiO2 softened and turned into a liquid amorphous glass phase. The liquid sintering process is due to the enhanced wetting between particles [22]. From XRD analysis, the main crystalline phases were C2AS and MA. However, Al2O3 disappeared at 1473–1773 K. During the sintering process, the mass transfer was accelerated because of the liquid phase [23]. The results showed that CaO and SiO2 could easily be turned into the liquid phase. CaO and SiO2 allowed densification by recrystallization in the presence of the liquid phase [23]. The intensities of C2AS and MA were slightly different at 1673 K and 1773 K.
The microstructures of gehlenite/magnesia-alumina spinel powders sintered at 1473–1773 K were shown (Fig. 5). From Fig. 5 (a–d), the grains were breezed tiny at 1473 K for 3 h. The grain boundary size of C2AS and MA turned obviously with an increase in temperature to 1673 K. Likeness, from Fig. 5 (c–d), the crystalline grains were homogenous. And the MA linked another as the main phase at 1673 K. The main phase of the C2AS was a big square plate. The products were with little compact and homogeneous microstructures at 1773 K. So the 1673 K was appropriated. In all specimens, a glassy phase appeared, which was mainly due to the liquid glassy phase. The CaO and SiO2 components in the SAA decreased the viscosity of the glass phase, and that was good for the gehlenite/magnesia-alumina spinel synthesized during the course [24].
Effect of Y 2 O 3 on the gehlenite/magnesia-alumina spinel
The grain boundaries were observable from the microstructure of the powders with 3 wt% Y2O3 (Fig. 6). By forming a brittle phase because of Y2O3, new grain propagation occurred. The crystallite dimension was larger and clearer than that of the spinel without Y2O3. The grain size increases became obvious upon doping with Y2O3. Finally, the grain dimension distribution was more uniform and enlarged with 3 wt% Y2O3. Figure 6 shows the microstructure of the gehlenite/magnesia-alumina bricks with 3 wt% Y2O3 at 1473–1773 K. EDS (Table 2) and line scan analysis (Fig. 6) showed that Y was uniformly dispersed in the texting scope. The average length of elongated grains increased with 3 wt% Y2O3. Y2O3 is a crystal phase regulator and improves the microstructure. Y2O3 addition influenced the densification and microstructure. The presence of YCaAl3O7 as a second phase could be beneficial as it influences grain growth and reduces ion segregation at grain boundaries. This may be the reason for the different microstructure refinement trends of ceramic particles shown in Fig. 6 and Fig. 5 [25].
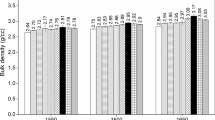
From Fig. 7, the results showed the bulk density and compressive strength of the gehlenite/magnesia-alumina spinel with and without Y2O3 doping and upon sintering at 1373–1773 K. The density of the gehlenite/magnesia-alumina spinel increased to 2.03 g·cm−3 at 1773 K. The density of the gehlenite/magnesia-alumina spinel increased dramatically from 1.78 to 2.03 g·cm−3 as the temperature increased from 1373 to 1773 K. The density of the gehlenite/magnesia-alumina spinel increased mildly when the temperature was 1773 K (from 2.02 to 2.03 g·cm−3). The compressive strength of the gehlenite/magnesia-alumina spinel also significantly increased from 69.1 to 75.2 MPa with an increase in temperature from 1373 to 1573 K, which was above the Chinese national standard value of 40 MPa for magnesia and magnesia-alumina refractory bricks (GB/T 2275–2007). When the temperature changed from 1673 to 1773 K, the compressive strength improved slightly from 89.8 to 90 MPa. This showed that 1673 K was suitable for synthesising gehlenite/magnesia-alumina.
In particular, the density feature of the Y2O3-doped gehlenite/magnesia-alumina spinel improved with sintering. The density of the gehlenite/magnesia-alumina spinel with 3 wt% Y2O3 was 2.05 g·cm−3 at 1673 K. The compressive strength of the gehlenite/magnesia-alumina spinel with 3 wt% Y2O3 showed a similar trend to the density trend. The compressive strengths of the samples doped with 3 wt% Y2O3 at 1373–1773 K reached 71.2, 75.7, 77.3, 91.2, and 92.3 MPa. The density and compressive strength of the Y2O3-doped gehlenite/magnesia-alumina spinel improved. Preparation of the Y2O3-doped gehlenite/magnesia-alumina spinel can expand the utilization of SAA in new ceramics.
Y2O3 can be the key factor in the densification of bricks because of the large ionic radius of the Y3+. The glass network structure of the Y2O3 influenced the migration of other ions [19]. Y2O3 reacted with Al2O3, SiO2, or CaO in SAA, and the outcome reinforced intergranular fracture with an increase in the sintering temperature. In addition, ions moved to the intergrain void as the temperature increased. The holes were filled completely, which effectively reduced the residual number of closed pores in the sample [26]. The densification feature of the bricks with Y2O3 was superior to that of the gehlenite/magnesia-alumina spinel without Y2O3 (above 1473 K).
Mechanism of enhanced sintering with Y2 O 3 for obtaining gehlenite/magnesia-alumina spinels
Reaction set
XRD patterns of the 3 wt% Y2O3 samples sintered at 1473–1773 K are shown in Fig. 8. Thus, from the XRD analysis, the new compound that included Y ion was YCaAl3O7. The PDF card number of YCaAl3O7 is #49–0605. MA, C2AS, and YCaAl3O7 were the main phases in the sintered product. Al2O3 was the minor phase at 1473 K. YCaAl3O7 appeared at 1473 K and existed from 1473 to 1773 K. A liquid phase during sintering was beneficial for the densification feature. The large cation size of Y3+ could have caused distortion between layers and an expanded lattice. The presence of YCaAl3O7 as a second phase could be beneficial by influencing grain growth and reducing ion segregation at grain boundaries. YCaAl3O7 exhibited well-elongated grains in the matrix between MA and C2AS. YCaAl3O7 was helpful in the bonding of two or more MA and C2AS particles [25, 27].
Effect of Y 2 O 3 dissolution and exsolution
With MDI Jade 5.0 software, the lattice cell refinement function can be used to calculate the lattice constant changes. From Table 3, the lattice parameters at different temperatures were higher than those obtained with refinement, which may be attributed to the dissolution of Y2O3 and other compounds such as CaO and SiO2 [28, 29]. The lattice parameter of C2AS increased at 1473 to 1573 K and decreased at 1673 K. The lattice parameter of MA increased at 1473 to 1673 K and decreased at 1773 K. Dissolution and exsolution were related to diffusion. Diffusion enhances intermolecular motion, which is good for the densification of materials [30].
Figure 9 illustrates the mechanism underlying the enhancement in the characteristics of the gehlenite/magnesia-alumina spinel derived from SAA after Y2O3 doping. The densification feature of the gehlenite/magnesia-alumina spinel was attributed to the low-viscosity liquid phase. The dissolution and exsolution of Y2O3 occurred in the sintering process. Schmid [31] and Su [32] reported that Y3+ was concentrated in the intergranular regions. The ionic radius of Y3+ was large than the ionic radii of Al3+, Si4+, Ca2+, and Mg2+. The solute misfit strain energy was obtained because of the other ionic radii of Y3+ and other ions. The predominant driving force for the segregation of Y3+ moved to the crystalline grain boundaries. Y3+ diffused into the anomalous microscopic defects and filled the crystalline grain boundaries during the high-temperature sintering process. Dissolution of Y ion in the sintering process resulted in cation vacancies that reinforced movement and mass transfer [30, 33]. The Y ion in other ion sites caused lattice strain, which also reinforced the mass transport process for densification [18, 30, 34]. Y2O3 moved to the ion boundaries and then entered the gehlenite/magnesia-alumina spinel lattice. Interionic migration decreased the residual pores in the MA and C2AS boundaries and increased the density feature of the spinel. The Y2O3 can also reduce the coefficient of thermal expansion and the softening point of the glass phase. Several factors cooperatively enhance the characteristics of the gehlenite/magnesia-alumina spinel [35].
Conclusions
Secondary aluminium ash (SAA) can be used as the raw material of gehlenite/magnesia-alumina spinels. The density of the bricks changed from 1.78 to 2.03 g·cm−3, and the compressive strength changed from 69.1 to 90 MPa when they were sintered at a temperature ranging from 1373 to 1773 K for 3 h. The compressive strength of the gehlenite/magnesia-alumina spinel was above the Chinese national standard value of 40 MPa for magnesia and magnesia-alumina refractory bricks (GB/T 2275–2007). The density and compressive strength of the gehlenite/magnesia-alumina spinel doped with 3 wt% Y2O3 and sintered at 1673 K were 2.05 g·cm−3 and 91.2 MPa, respectively. The enhancing mechanism of Y2O3 doping contributed to the solubility of both Y2O3 and Y2O3-containing compounds, such as YCaAl3O7, which was turned into the low-viscosity liquid phase as the sintering temperature exceeded 1473 K.
References
Hong, J.P., Wang, J., Chen, H.Y., Sun, B.D., Li, J.J., Chen, C.: Process of aluminum dross recycling and life cycle assessment for Al-Si alloys and brown fused alumina [J]. Trans. Nonferrous. Metals. Soc. China. 20, 2155–2161 (2010)
Lopez-Delgado, A., Tayibi, H., Perez, C., Alguacil, F.J., Lopez, F.A.: A hazardous waste from secondary aluminum metallurgy as a new raw material for calcium aluminate glasses [J]. J. Hazard. Mater. 165, 180–186 (2009)
Dmaschio, R., Fabbri, B., Fiori, C.: Industrial applications of refractories containing magnesium aluminate spinel [J]. Ind. Ceram. 8, 121–126 (1988)
P E Tsakiridis. Aluminum salt slag characterization and utilization--a review [J]. J. Hazard. Mater. 2012: 217–218.
B Dash, B R Das, B C Tripathy, BHATTACHARYA I N, DAS S C. Acid dissolution of alumina from waste aluminum dross [J]. Hydrometallurgy. 2008, 92(1–2): 48–53.
David, E., Kopac, J.: Hydrolysis of aluminum dross material to achieve zero hazardous waste [J]. J. Hazard. Mater. 209–210(4), 501–509 (2012)
Shinzato, M.C., Hypolito, R.: Solid waste from aluminum recycling process: characterization and reuse of its economically valuable constituents [J]. Waste Manage. 25(1), 37–46 (2005)
Tan, R., Khoo, H.H., Chen, A.: Study of a primary aluminum supply chain [J]. J. Clean. Prod. 13(6), 607–618 (2005)
Tavangarian, F., Emadi, R.: Synthesis and characterization of pure nanocrystalline magnesium aluminate spinel powder [J]. J. Alloy. Compd. 489(2), 600–604 (2010)
Bonnefont, G., Fantozzi, G., Trombert, S., Bonneau, L.: Fine-grained transparent MgAl2O4 spinel obtained by spark plasma sintering of commercially available nanopowders [J]. Ceram. Int. 38(1), 131–140 (2012)
Lv, C.S., Wang, J.W., Jia, Y.Z.: Effects of red mud content on the sintered red mud bricks [J]. J. Saf. Environ. 13(4), 98–100 (2013)
Ma, J., Zhu, S.G., Di, P., Zhang, Y.: Influence of La2O3 addition on hardness, flexural strength and microstructure of hot–pressing sintered WC-MgO bulk composites [J]. Mater. Des. 32(4), 2125–2129 (2011)
Qiu, G.M., Li, X.K., Qiu, T., Zhao, H.T., Yu, H.H., Ma, R.T.: Application of rare earths in advanced ceramic materials [J]. J. Rare Earths 25(2), 281–286 (2007)
Kong, L.B., Zhang, T.S., Ma, J., Boey, F., Zhang, R.F.: Mullite phase formation in oxide mixtures in the presence of Y2O3, La2O3 and CeO2 [J]. J. Alloy. Compd. 372(1–2), 290–299 (2004)
Qu, H.X., Wu, Q., Wen, H.Q.: Effect of CeO2 additives on the microstructure and mechanical properties of WC-Al2O3 composites [J]. Baosteel Tech. Res. 9, 17–22 (2015)
Baranova, T.F., Kurskaya, I.N., Dabizha, N.A., Petrov, Y.B., Lukin, E.S.: Sintering high-purity fusions of MgO and MgO·Al2O3 [J]. Refractories 22, 180–182 (1981)
Shi, Z.M., Pan, F., Liu, D.Y., Liang, K.M., Gu, S.R.: Effect of Ce4+-modified amorphous SiO2 on phase transformation towards α-cordierite [J]. Mater. Lett. 57, 409–413 (2011)
Sarkar, R., Tripathi, H.S., Ghosh, A.: Reaction sintering of different spinel composition in the presence of Y2O3 [J]. Mater. Lett. 58(16), 2186–2191 (2004)
Tian, Z.K., Wang, Z.F., Wang, X.T., Naihuo, C.: Effect of rare earth oxides on synthesis of magnesium aluminate spinel by reaction sintering [J]. Refractories 42, 326–329 (2008)
Hasan, M., Dholabhai, P., Dey, S., Uberuaga, B.P., Castro, R.H.: Reduced grain boundary energies in rare-earth doped MgAl2O4 spinel and consequent grain growth inhibition [J]. J. Eur. Ceram. Soc. 37, 4043–4050 (2017)
Suryanarayana, C.: Mechanical alloying and milling [J]. Prog. Mater. Sci. 46, 1–184 (2001)
E M Vlad, R V Buzduga, M D Buzduga, V Caloian, E F Plopeanu, C Pandelescu, C Dobresc, N Constantin. Experimental research on the effect of additives on the sintering process of alumina-based refractory materials [J]. J. Phys. Confer. Ser. 2021, 1781(012066).
X P Tan, S Q Liang.Thermal phase transformation of SiO2-Al2O3-ZrO2 glass [J]. J. Central South Univ. (Science and Technology). 2011, 42(1).
P Y Yan, W Y Yang. Study on the cementitious behaviour of CaO Al2O3 SiO2 glass [J]. J. Chin. Ceram. Soc. 1997, 25(4).
Ge, X.Z., Ge, Q., Ge, X.S., Ji, D.H., Huang, Y., Zhang, Z.L., Zhang, H.B.: Influence of La2O3 addition on microstructure and mechanical properties of Al2O3 ceramics [J]. Mater. Sci. Forum 956, 69–77 (2019)
Fu, P., Xu, Z.J., Chu, R.Q., Li, W., Xie, Q.: Application study actuality and development foreground of rare earth oxides in ceramics materials [J]. Ceramics. 12, 7–10 (2008)
Akin, I., Yilmaz, E., Sahin, F., Yucel, O., Goller, G.: Effect of CeO2 addition on densification andmicrostructure of Al2O3–YSZ composites [J]. Ceram. Int. 37, 3273–3280 (2011)
Naik, P.P., Tangsali, R.B.: Enduring effect of rare earth (Nd3+) doping and γ-radiation on electrical properties of nanoparticle manganese zinc ferrite [J]. J. Alloy. Compd. 723, 266–275 (2017)
Kim, T., Kim, D., Kang, S.: Effect of additives on the sintering of MgAl2O4 [J]. J. Alloy. Compd. 587, 594–599 (2014)
Sarkar, R., Das, S.K., Banerjee, G.: Effect of additives on the densification of reaction sintered and presynthesised spinels [J]. Ceram. Int. 29, 55–59 (2003)
Schmid, H.K., Pennefather, R., Meriani, S., Schmid, C.: Redistribution of Ce and La during processing of Ce(La)-TZP/Al2O3 composites [J]. J. Eur. Ceram. Soc. 10, 381–392 (1992)
Su, C.H., Duanmu, Q.D., Wang, Y.: Non-equilibrium thermodynamic analysis of the concentration distribution of rare earth oxide at alumina transparent ceramic crystal boundary [J]. J. Chin. Ceram. Soc. 26, 802–807 (1998)
Hirai, S., Murakami, H., Katayama, H.G.: Effect of additives on the formation of MgAl2O4 from MgO and Al2O3 [J]. J. Japan Inst. Metals. Mater. 55, 166–171 (1991)
Skomorovskaya, L.A.: Magnesia spinel ceramics alloyed with rare earth oxides [J]. Glass Ceram. 50, 165–168 (1993)
Wu, T.T.: Effect of rare earth oxides on the wear reaiatance and acid resistance of high-alumina ceramic [D]. Wuhan University of Technology, Wu Han (2017)
Funding
This work was supported by National Natural Science Foundation of P. R. China (Grants 52004113).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Duan, T., He, X. et al. Effect of Y2O3 doping on a gehlenite/magnesia-alumina spinel obtained by sintering secondary aluminium ash. J Aust Ceram Soc 58, 891–899 (2022). https://doi.org/10.1007/s41779-022-00740-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-022-00740-3