Abstract
TiO2 modified with silver nanoparticle catalysts was prepared by radiolysis method using gamma ray from Co-60 source. The characteristic of prepared samples was determined by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and transmission electron microscopy (TEM). XRD patterns of Ag/TiO2 catalysts indicated the peak of silver metal, the XPS analysis showed that Ag existed as Ag metallic in structure of Ag/TiO2 catalyst, and the size of Ag nanodeposited on TiO2 surface was about 1 to 4 nm. The size of silver nanoparticle increased when the initial concentration of dopant increased. Photocatalytic activity of TiO2 and Ag/TiO2 for azo dye methyl red (MR) under visible light was conducted. The comparison results of MR degradation efficiency of TiO2 and Ag/TiO2 showed that Ag/TiO2 catalysts were higher than that of TiO2 under the same conditions. TiO2 doped with 2% Ag content has the highest photodegradation efficiency and showed a 49% increase in the MR photodegradation as compared to the pure TiO2. In addition, Ag/TiO2 prepared by radiolysis method was highly reusable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among toxic organic substances, textile dyes and industrial dyes are one of the groups of organic substances that are increasingly harmful to the environment [1]. Azo dyes are one of the most dangerous textile wastes. There are numerous methods for effectively degrading textile dyes from wastewater including advanced oxidation processes [1, 2], biodegradation [3], ozonation [4], adsorption, and the membrane process [5,6,7,8,9,10].
Currently, the method of degrading toxic organic substances using semiconductors is an effective method that has been widely studied. Semiconductors are materials that have a band gap between their valence band and conduction band. When they absorbed energies greater than or equal to their band gap, electrons from the valence band transfer to the conduction band, forming generated electrons in the conduction band and generated holes in the valence band. Charge carriers react with substances absorbed on surface of the semiconductor. Among semiconductors, TiO2 is the most commonly used because of its numerous advantages, including low cost, nontoxicity, and high photocatalytic efficiency [11,12,13]. The limitation of TiO2 is a large band gap energy and the recombination of the generated holes and electrons.
There are several studies that have been conducted to lower the band gap energy and avoid recombination of the generated holes and electrons [14], including the modification of TiO2 with noble metals, such as Au, Ag, and Pt. Suwarnkar et al. synthesized TiO2 doped with Ag using sol–gel method–assisted microware and found that increasing Ag contents from 0.0 to 0.25% mol resulted in smaller crystallite size. The highest methyl orange degadation efficiency was discovered in TiO2 doped with 0.25 mol% Ag [15]. The nanosized Ag-TiO2 were prepared by a single-step sol–gel method using titanium tetraisopropoxide (TTIP) and silver nitrate as precursors and aqueous ammonia as a reduction agent. The results of phathlic acid degradation showed that TiO2 doped with 0.75% Ag had the highest phthalic acid degradation efficiency under UV irradiation [16]. Nagaraj et al. synthesized Ag-doped TiO2 using photon-induced method and the photodegradation of methylene blue revealed that Ag/TiO2 completely degraded methylene blue [17]. Grabowska et al. used irradiation method to modify titanium dioxide with silver nanoparticles for phenol degradation. The results showed that titania modified with silver nanoparticles demonstrated higher photocatalytic activity than pure TiO2 under both UV and visible irradiation [18]. Radiolysis is a powerful method for synthesizing nanoparticles with control over their size and shape [18,19,20,21]. The size of the particles can be easily controlled by varying the concentration of silver ion [18]. The choice of the absorbed dose must be carefully chosen in order to control cluster size and crystal structure by adjusting the nucleation and growth steps for metallic cluster [22].
Among Ag/TiO2 synthesis methods, radiolysis method has many advantages including the ability to synthesize Ag-modified TiO2 at room temperature without the use of other reducing agents, and the aqueous solvent was dissociated by γ-ray into reducing radical (e−aq) which reduced silver ions to Ag0. In the irradiated solution, the reduction process is uniform, resulting in small, monodispersed Ag particles [23].
Methyl red is an azo dye that is commonly found in the textile industry. A significant amount of wastewater contains methyl red, which is potentially toxic to the environment and some of these toxic substances can be stored in food, causing human toxicity. Among methyl red degradation methods, the method using Ag/TiO2 to degrade methyl red was reported to be highly effective in completely degradating methyl red [24, 25].
For the aforementioned reasons, the study has synthesized TiO2 (P25) modified with silver nanoparticles prepared by radiolysis method (using γ-60Co as a resource), and the photocatalytic activity of TiO2 and Ag/TiO2 in methyl red degradation under visible light and the reused of Ag/TiO2 photocatalysts were also investigated and discussed.
Materials and methods
Sample preparation
TiO2 with diameter ranging from 10 to 40 nm (Degussa, Germany) were added into 5 mL ethanol (> 99.9%, China) and 95 mL distilled water. Then, AgNO3 (> 99.8%, China) was carefully added into the above mixture with different mass ratios of Ag/TiO2 of 0.5; 1.0; 1.5 and 2%. After 60 min of stirring, the mixture was placed into a gamma chamber with γ-60Co source on GC-5000 (BRIT, India) with dose rate of 3.0 kGy/h. The absorbed radiation dose is determined by the concentration of Ag, and the saturated absorbed radiation dose for 1 mmol/L Ag+ is 2 kGy [23]. The mixtures were centrifuged, then washed with distilled water until pH constant and dried at 60 °C for 12 h. The obtained TiO2-modified Ag were named as AT0.5, AT1.0, AT1.5, and AT2.0, respectively.
Characterization of TiO2 and TiO2 modified with Ag catalysts
The phase identification of a crystalline material was determined using X-ray diffraction (XRD) on D8 Advanced diffractometer (Brucker, Germany) with a Cu Kα radiation (λ = 0.15418 nm). The sizes of pure TiO2 and Ag-doped TiO2 catalysts were characterized by transmission electron microscopy (TEM) on JEM 1010 instrument (JEOL, Japan). The elemental composition and chemical state of catalysts were obtained by X-ray photoelectron spectroscopy analyses (XPS) using a ULVACPHI instrument outfitted with Al Kα X-ray source. The weight percentage of Ag in Ag/TiO2 was analyzed using an atomic absorption spectrometer (AAS) on AA-6300 instrument (Shimadzu, Japan).
Photocatalytic degradation activity
Photocatalytic degradation of methyl red by TiO2 and TiO2 modified with Ag catalysts were carried out under visible light using a 150-W halogen lamp as the light source. Firstly, 0.05 g of the catalyst was added to a 50-mL beaker containing methyl red (MR) at a concentration of 10−5 M. After stirring the mixture in the dark for an hour to achieve adsorption and desorption equilibrium, it was illuminated with halogen lamp. After period of irradiation time: 20, 40, 60, 80, 100, and 120 min, the mixture was centrifuged at 6000 rpm for 15 min to remove catalysts particles. The methyl red solution that remained after the reaction was measured using absorbance spectroscopy method (UV–Vis) to determine the concentration (at wavelength 410 nm). The experiment was carried out three times.
Results
Catalyst characteristic
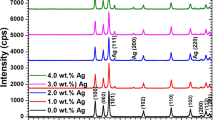
The crystalline phases of pure TiO2 and Ag/TiO2 were determined by X-ray diffraction. Typical XRD diagrams for TiO2 (P25), AT0.5, AT1.0, AT1.5, and AT2.0 are shown in Fig. 1. The XRD patterns of Ag/TiO2 samples exhibited all of typical TiO2 peaks. Diffraction peaks located at the 2θ values of 25.25, 37.85, 48.16, 53.86, 55.01, 62.68, 68.95, 70.37 and 75.24 corresponding to the crystal planes (101), (004), (200), (105), (211), (204), (116), (220) and (215) of anatase phase of TiO2 [26], whereas the peaks at 2θ values of 27.38, 36.02, 41.22, and 56.48 correspond to the crystal planes (110), (101), (111), and (220) representing the rutile phase of TiO2 (JCPDS card no. 21–1276) [26]. The XRD patterns of AT1.0 and AT0.5 have weak peak at 2θ value of 44.21. Furthermore, XRD patterns of AT1.5 and AT2.0 revealed two peaks at 2θ values of 38.05 and 44.21, which correspond to the crystal planes (111) and (200) shown for metallic silver (JCPDS card no.04–0783) [24]. The crystallite size of TiO2 and Ag-modified TiO2 was calculated from XRD data by using the Debye-Scherer formula [27]:
where L is the crystallite size (nm), β is FWHM, k = 0.89, and λ = 0.15418 nm. The results illustrated in Table 1 showed that the crystallite sizes of TiO2 in the pure TiO2 and Ag-modified TiO2 samples were about 20–21 nm.
For further evidence of the composition and chemical state of the elements, TiO2 and AT1.5 samples also were measured by XPS analyses. The XPS results of TiO2 in Fig. 2 showed that pure TiO2 contained elements Ti and O. The XPS diagram of AT1.5 in Fig. 3 indicated that the sample contains elements Ti, O, and Ag. For more information on the state of Ag, the XPS measurement result of element Ag is shown in Fig. 4. The Ag3d XPS diagram showed the presence of two peaks with binding energy states (BE) of 368.25 and 374.25 eV, which are typical for the binding states of Ag 3d5/2 and 3d3/2 spin-orbital splitting photoelectrons of Ag. According to the splitting values, the chemical state of silver in AT1.5 sample is metallic silver (Ag0) [18]. There are no other chemical states of Ag. This is consistent with XRD results. Furthermore, two peaks with the binding energies of 459.55 eV and 465.40 eV indicated to Ti 2p3/2 and 2p1/2, respectively, that specify Ti4+ chemical states in these samples shown in Fig. 5 [28].
The morphology of TiO2, AT0.5, AT1.5, and AT2.0 samples was determined by transmission electron microscopy (TEM). The results in Fig. 6 showed that the shape and size of TiO2 particles do not change among these samples. The size of TiO2 from TEM was about 20 nm, which is similar to the result of crystallite size of TiO2 from XRD data. TEM image of AT0.5, AT1.5, and AT2.0 sample also showed that Ag particles with the size of 1–4 nm are attached on the surface of TiO2 particles. In general, increasing the percentage of silver precursor content from 0.5 to 2.0% increased Ag content in Ag/TiO2 samples resulting in larger the size of silver nanoparticle. Under the influence of reducing agent generated by gamma rays, Ag+ ions were reduced to Ag metallic with nanosize and these silver nanoparticles were modified on the TiO2 surface [29]. The Ag weight percentage in AT0.5, AT1.0, AT1.5, and AT2.0 was 0.39, 0.62, 1.2, and 1.41%, respectively, according to the AAS results. According to Grabowska et al., the size of Ag particles attached to the TiO2 surface increases from 1.3 to 1.5 nm when the initial Ag+ concentration increases from 0.5 to 1.0% [18].
Photocatalytic activity
The photodegradation activity of MR 10−5 M by TiO2 and Ag/TiO2 in visible light was investigated, with the results shown in Fig. 7. The MR degradation efficiency of all Ag/TiO2 samples were higher than that of TiO2. After 120 min of irradiation, the degradation efficiency of MR 10−5 M by TiO2 was 23.45% while AT0.5, AT1.0, AT1.5, and AT2.0 samples had MR degradation efficiency of 58.24%, 62.53%, 68.26%, and 71.96%, respectively. When the Ag content in the sample is higher, the photocatalytic activity under visible light improves. It was found that TiO2 doped with 2% Ag content has the highest photocatalytic efficiency. With 2 h of irradiation, the AT2.0/TiO2 catalyst showed a 49% increase in the MR photodegradation when compared to the pure TiO2. The ability of nanosize-doped metal to lower the band gap energy and thus shift the optical response to the visible light region was attributed as the reason. Furthermore, the nanosize TiO2-doped Ag particles inhibited the instinctive recombination of photogenerated electrons and holes within the catalysts, thereby increasing the photocatalytic efficiency [30].
In comparison to other studies, the photodegradation efficiency of Ag/TiO2 obtained in this study was found to be lower. After 90 minutes, the photodegradation efficiency of MR 7.4 x10−5 M by Ag/TiO2 synthesized by liquid impregnation method under solar radiation was greater than 85% [24]. The photodegradation efficiency of MR 7.5 × 10−5 M by Ag/TiO2 synthesized by sol-gel method was 83% after 60 minutes of solar irradiation [25]. However, the experiments for MR degradation by Ag/TiO2 in the preceding studies were conducted under solar and UV radiations, which typically offer higher removal efficiency than visible light. Furthermore, the radiolysis method for noble metal nanoparticle synthesis has the advantage of allowing nanomaterials to be synthesized at room temperature and this is a green method because toxic chemical-reducing agents are not used. Moreover, the radiolysis method has been shown to be a faster synthesis method, which aids in the reduction of power consumption and product prices [31].
Reuse of the photocatalyst
To determine the reusability of TiO2 modified by Ag nanosynthesized by gamma irradiation method, AT2.0 sample is then used to degrade MR and the photocatalytic degradation of MR was repeated up to four cycles. The results in Fig. 8 showed that after 5 times of using AT2.0 material, it still has a high catalytic activity, with the MR degradation efficiency of the fourth reused material sample yielding 66.2% compared to 71.96% of the initial material. After degradation of MR, AT2.0 material was reused four times and the catalytic activity decreased gradually but not significantly compared to the original sample.
Mechanism of photocatalysis of Ag/TiO2
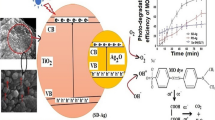
The proposed schematic illustration of photocatalytic mechanism of MR degradation by Ag/TiO2 under visible light in Fig. 9. Under visible light, electrons in the valence band were excited to the conduction band and formed photogenerated holes in the valence band of TiO2. These photogenerated electrons will be captured by Ag (due to the fact that the Fermi energy level of Ag is lower than the conduction energy of TiO2, forming a Schottky barrier in the contact zone of Ag and TiO2) and then reduced O2 on the surface to form •O2− radicals. Meanwhile, the photogenerated holes oxidize water on the surface to form •OH. The oxidized radicals •OH and •OOH are then formed as a result of the •O2− reaction. These oxidized radicals oxidize methyl red to degradation products [1].
Conclusions
Ag modified with TiO2 catalysts have been successfully synthesized by radiolysis method at room temperature. XRD and XPS results showed the presence of Ag metallic, while the TEM results revealed the small and homogeneous silver nanoparticles with sizes ranging from 1 to 4 nm attached to the surface of TiO2 nanoparticles. When increasing percentage of precursor content of silver from 0.5 to 2.0%, the size of silver nanoparticle increased. Under visible light, the photocatalytic activities of TiO2 and Ag/TiO2 in degradation of methyl red were studied. Under the same conditions, TiO2 modified with silver nanoparticles has higher photocatalytic activity than TiO2. TiO2 doped with 2% Ag has the highest photocatalytic efficiency, and it has an increase in MR degradation efficiency of 49% compared to pure TiO2. Furthermore, Ag/TiO2 catalysts are highly reusable. After four times of reuse, the MR degradation efficiency decreased by only 5.76%.
References
Konstantinou, I.K., Albanis, T.A.: TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: A review. Appl Catal B Environ 49, 1–14 (2004)
Kuo, W.G.: Decolorizing dye wastewater with Fenton’s reagent. Water Res 26, 881–886 (1992)
Sleiman, M., Vildozo, D., Ferronato, C., Chovelon, J.M.: Photocatalytic degradation of azo dye Metanil Yellow: optimization and kinetic modeling using a chemometric approach. Appl Catal B Environ 77, 1–11 (2007)
Slokar, Y.M., Le Marechal, A.M.: Methods of decoloration of textile wastewaters. Dyes Pigm 37, 335–356 (1998)
Derudi, M., Venturini, G., Lombardi, G., Nano, G., Rota, R.: Biodegradation combined with ozone for the remediation of contaminated soils. Eur J Soil Biol 43, 297–303 (2007)
Martin, M.J., Artola, A., Balaguer, M.D., Rigola, M.: Activated carbons developed from surplus sewage sludge for the removal of dyes from dilute aqueous solutions. Chem Eng J 94, 231–239 (2003)
Ahmad, A.L., Puasa, S.W.: Reactive dyes decolourization from an aqueous solution by combined coagulati-on/micellar-enhanced ultrafiltration process. Chem Eng J 132, 257–265 (2007)
Arslan, I., Balcioǧlu, I.A., Bahnemann, D.W.: Advanced chemical oxidation of reactive dyes in simulated dyehouse effluents by ferrioxalate-Fenton/UV-A and TiO2/UV-A processes. Dyes Pigm 47, 207–218 (2000)
Mo, J.H., Lee, Y.H., Kim, J., Jeong, J.Y., Jegal, J.: Treatment of dye aqueous solutions using nanofiltration poly-amide composite membranes for the dye wastewater reuse. Dyes Pigm 76, 429–434 (2008)
Rauf, M.A., Ashraf, S., Alhadrami, S.N.: Photolytic oxidation of coomassie brilliant blue with H2O2. Dyes Pigm 66, 197–200 (2005)
Cheng, B., Le, Y., Yu, J.: Preparation and enhanced photocatalytic activity of Ag/TiO2 core–shell nanocomposite nanowires. J Hazard Mater 177, 971–977 (2010)
Hashimoto, K., Irie, H., Fujishima, A.: TiO2 photocatalysis: a historical overview and future prospects. Jpn J Appl Phys 44, 8269 (2005)
Yang, H., Zhang, K., Shi, R., Li, X., Dong, X., Yu, Y.: Sol–gel synthesis of TiO2 nanoparticles and photocatalytic degradation of methyl orange in aqueous TiO2 suspensions. J Alloys Compd 413, 302–306 (2006)
Chatzitakis, A., Berberidou, C., Paspaltsis, I., Kyriakou, G., Sklaviadis, T., Poulios, I.: Photocatalytic degradation and drug activity reduction of chloramphenicol. Water Res. 42, 386–394 (2008)
Suwarnkar, M.B., Dhabbe, R.S., Kadam, A.N., Garadkar, K.M.: Enhanced photocatalytic activity of Ag doped TiO2 nanoparticles synthesized by a microwave assisted method. Ceram Int 40, 5489–5496 (2014)
Mogal, S.I., Gandhi, V.G., Mishra, M., Tripathi, S., Joshi, P.A., Shah, D.O.: Single-step synthesis of silver-doped titanium dioxide: influence of silver on structural, textural, and photocatalytic properties. Ind Eng Chem Res 53, 5749–5758 (2014)
Nagaraj, G., Mohammed, M.K., Abdulzahraa, H.G., Sasikumar, P., Karthikeyan, S., Tamilarasu, S.: Effects of the surface of solar-light photocatalytic activity of Ag-doped TiO2 nanohybrid material prepared with a novel approach. Appl Phys A 127, 1–7 (2021)
Grabowska, E., Zaleska, A., Sorgues, S., Kunst, M., Etcheberry, A., Colbeau-Justin, A., Remita, H.: Modification of titanium (IV) dioxide with small silver nanoparticles: application in photocatalysis. J Phys Chem C 117, 1955–1962 (2013)
Wishart, J.F., Rao, B.M.: Recent trends in radiation chemistry. World Scientific, Singapore (2010)
Belloni, J., Mostafavi, M., Remita, H., Marignier, J.L., Delcourt, M.O.: Radiation-induced synthesis of mono-and multi-metallic clusters and nanocolloids. New J Chem 22, 1239–1255 (1998)
Remita, H., Lampre, I., Mostafavi, M., Balanzat, E., Bouffard, S.: Comparative study of metal clusters induced in aqueous solutions by γ-rays, electron or C6+ ion beam irradiation. Radiat Phys Chem 72, 575–586 (2005)
Belloni, J.: Nucleation, growth and properties of nanoclusters studied by radiation chemistry: application to catalysis. Catal Today 113, 141–156 (2006)
Bui, D.D., Dang, V.P., Nguyen, N.D., Nguyen, T.K.L., Vo, T.K.L., Ngo, V.K.T., Nguyen, T.P.P., Nguyen, Q.H.: Preparation of colloidal silver nanoparticles in poly (N-vinylpyrrolidone) by γ-irradiation. J Exp Nanosci 3, 207–213 (2008)
Sahoo, C., Gupta, A.K., Pal, A.: Photocatalytic degradation of Methyl Red dye in aqueous solutions under UV irradiation using Ag+ doped TiO2. Desalination 181, 91–100 (2005)
Gupta, A.K., Pal, A., Sahoo, C.: Photocatalytic degradation of a mixture of Crystal Violet (Basic Violet 3) and Methyl Red dye in aqueous suspensions using Ag+ doped TiO2. Dyes Pigm 69, 224–232 (2006)
Mohammad, M.R., Ahmed, D.S., Mohammed, M.K.: Synthesis of Ag doped TiO2 nanoparticles coated with carbon nanotubes by the sol-gel method and their antibacterial activities. J Sol-Gel Sci Technol 90, 498–509 (2019)
Mohammad, M.R., Ahmed, D.S., Mohammed, M.K.: Studying antimicrobial activity of carbon nanotubes decorated with metal-doped ZnO hybrid materials. Mater Res Express 6, 1–15 (2019)
Rajender, G., Kumar, J., Giri, P.K.: Interfacial charge transfer in oxygen deficient TiO2-graphene quantum dot hybrid and its influence on the enhanced visible light photocatalysis. Appl Catal B Environ 224, 960–972 (2018)
Abedini, A., Daud, A.R., Hamid, M.A.A., Othman, N.K., Saion, E.: A review on radiation-induced nucleation and growth of colloidal metallic nanoparticles. Nanoscale Res Lett 8, 1–10 (2013)
Seery, M.K., George, R., Floris, P., Pillai, S.C.: Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J Photochem Photobiol 189, 258–263 (2007)
Viet, P.V., Sang, T.T., Hien, N.Q., Thi, C.M., Hieu, L.V.: Synthesis of a silver/TiO2 nanotube nanocomposite by gamma irradiation for enhanced photocatalytic activity under sunlight. Nucl Instrum Methods Phys Res B 429, 14–18 (2018)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thu, N.V.T., Dinh, K.D. Modification of TiO2 with Ag nanoparticles using gamma irradiation method for photocatalytic degradation of azo dye. J Aust Ceram Soc 57, 1563–1570 (2021). https://doi.org/10.1007/s41779-021-00648-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-021-00648-4