Abstract
Surface modification with a nanomaterial has been confirmed to be an effective strategy to enhance the visible-light photodegradation efficiency of titanium dioxide nanoparticles (TiO2-NPs). In this regard, we used silver as an additive into TiO2-NPs to improve their photodegradation activity under visible light irradiation. Here, a novel and eco-friendly process was developed to prepare the Ag-doped TiO2 nanohybrid and named as photon-induced method (PIM). The XRD technique showed that the prepared Ag-doped TiO2 has mixed phases of anatase and rutile. However, the rutile-only phase was detected for the pure TiO2-NPs at 700 °C of calcination. Ultraviolet–visible (UV–Vis) absorption spectra revealed a reduction in the energy bandgap of TiO2 after Ag doping. Besides, the addition of Ag resulted in a significant improvement of TiO2 morphology. Methylene blue (MB) dye was chosen to be an organic target to investigate the photocatalyst activity of the TiO2-NPs. In this regard, the degradation rate of MB was found to be 100% for the Ag-doped TiO2, which is higher than that of pure rutile TiO2. The incorporation of Ag additive plays a significant role in the improvement of TiO2 stability and photodegradation performance due to the surface plasmon resonance phenomenon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, nanomaterials (NMs) are an important type of advancing structure and attract great attention from research society and industries. Semiconductor NMs such as TiO2, zinc oxide, tin oxide, and so on are the most used materials for various applications due to their fascinating features [1,2,3]. Also, nanoparticles have been prepared and employed in last decades due to their size-hooked on chemical and physical properties [4,5,6]. The use of NM for biomedical applications is crucial because NMs tend to be at the lowest level and immediately permit the food chain of the ecosystem [7]. Recently, researchers draw superior interest to TiO2 and silver (Ag) incorporated TiO2 crystalline structure because of their excellent electrical, optical, and chemical characteristics [8,9,10]. The semiconducting TiO2 has an incredible photodegradation performance, which is mainly used as a photocatalyst due to its high light sensitivity, low environmental impact, strong oxidizing power, chemical inertness, and relative cheapness [11]. In addition, TiO2 can be employed as an antimicrobial agent to deactivate microorganisms because of its chemical and physical stability, good photocatalytic efficiency, and ease of fabrication [12]. Nevertheless, two drawbacks limit the use of TiO2, which are low absorption ability in the visible range can be absorbed only under UV light and high recombination of carrier charges [13].
A broad variety of noble metals may well be combined with the TiO2-NPs to prompt photodegradation performance of dyes under visible light [14]. To date, Ag is an inexpensive metal with unique electronic merits compared with other ones, which makes it a good candidate for employing as an additive into the TiO2-NPs [15]. Thus, Ag can impede the recombination rate of the carrier charges by receiving the TiO2 photo-induced charge, which serves as an electron accumulator [16]. Moreover, the wide energy bandgap of TiO2 can be easily tuned by Ag modification leading to increasing the absorption of visible light [17].
To increase the photodegradation performance, several interdisciplinary studies have been conducted on TiO2 [18,19,20]. As reported in the literature, photocatalytic performance of NMs depends upon their crystallinity [21], additive engineering [22], surface area, and hydroxyl group [23]. Besides, Ag incorporating on the surface of semiconductors is used to improve the photocatalytic performance by slowing down recombination rates [24]. Lin and coworkers prepared mesoporous TiO2 doped with Ag+-coated graphene (MT-Ag/GR) by a sol–gel and solvothermal methods as catalyst. The as-prepared hybrid of MT-Ag/GR showed stronger photodegradation abilities of MB dye than those realized with pure MT, MT-Ag, and MT/GR [25]. Siti and coworkers reported the development of a two-dimensional and porous metals-doped TiO2 hybrids for studying their photocatalytic activity. They revealed that the Ag additive enhances the crystalline structure and gives an external oxidation level into the system for an improved charge transport pathway and surface reaction. As a result, this hybrid structure enhanced the photocatalytic activity toward rhodamine B [26]. Recently, our group investigated the visible-light photocatalytic activity of pure TiO2 prepared with facile and green process named as photo-induced method (PIM). Our previous findings showed that pure, highly crystalline, and good TiO2-NPs catalysts can be achieved by suggested approach [27].
For further improvement of TiO2 photodegradation efficiency, we used Ag additive TiO2 and doped into TiO2 lattice using PIM. The prepared hybrid was systematically compared with standard TiO2 using a series of techniques. Most importantly, the photocatalyst activity of the prepared hybrid was measured using the photodegradation of MB organic under visible light irradiation.
2 Experimental procedures
2.1 Synthesis of Ag-doped TiO2
Briefly, 0.5 gm of silver nitrate (AgNO3, Merck) and 3 gm of titanium tetra isopropoxide (TTIP, Sigma-Aldrich) were added to 1 L of distilled water. After that, the mixed solution was carefully stirred under a halogen light for 5 days. Then, the mixture was dried at 100 °C for 12 h and sintered at 700 °C for 60 min. The standard TiO2 (Merck) sample was purchased and sintered at 700 °C for 60 min before further characterization.
2.2 Photocatalyst activity
The photocatalytic performance of pure and Ag-doped TiO2 nanohybrid was characterized through MB degradation experiment. An aqueous dispersion of MB (1 × 10–5 M) was mixed well with 0.05 g catalyst powder into a glass beaker containing 100 ml of distilled water. The obtained suspension was maintained under sunlight for measuring the photodegradation activity. Finally, the UV absorption spectra were measured at intervals of 5 min.
2.3 Characterization
UV–Vis spectroscopy was used to record the optical characteristics of films using Ocean Optics (CHEM2000-UV–VIS). The structural properties of films were conducted by the XRD technique with CuKα (λ = 1.54050 Ao) irradiation operated at 40 kV and 30 mA and (Shimadzu XRD-6100/7000 X-ray diffractometers). The morphology of samples was investigated using field emission SEM (Mira3). The FTIR spectra of samples were obtained using a Burker (IFS-125HR) device with KBr disc.
3 Results and discussion
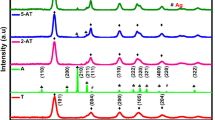
To characterize the crystal nature of the standard and Ag-doped TiO2 NPs, XRD measurement was performed in the range of 10°–75° (Fig. 1). The XRD shows the patterns of TiO2 and Ag-doped TiO2 NPs after sintering at 700 °C for 60 min. The X-ray pattern of the Ag-TiO2 hybrid matches with the standard TiO2 without any X-ray peaks from the Ag dopant (JCPDS card no. 21–1272) [8], therefore implying that the Ag additives are well loaded into the TiO2 lattice [11]. The X-ray pattern of Ag-doped TiO2 NPs shows a mixed phase of an anatase and rutile phase, while the standard TiO2 shows rutile-only phase. The average crystallite sizes of the standard TiO2 and Ag-doped TiO2 were found to be 60 nm and 25 nm, respectively, as determined by the Scherrer formula [28,29,30]. Also, XRD clearly exhibits TiO2 peaks without any related pattern corresponding to Ag.
The absorption spectra of pure and Ag/TiO2 hybrid were recorded using a UV–Vis spectrophotometer and are displayed in Fig. 2a. As shown, the absorption spectrum of the hybrid sample reveals a redshift at the absorption edge, which is ascribed from Ag addition. The energy bandgap of NPs was estimated by employing Tauc plot [31]. The plots of (αhν)1/2 vs energy (hν) are demonstrated in Fig. 2b. The bandgap can be directly obtained by extrapolating the line to the x-axis intercept. In this content, the energy bandgap of pure TiO2 was 2.87 eV, while Ag-doped TiO2 decreased to 2.7 eV. For hybrid NPs, this redshifting indicates the suppression of the recombination process of carrier charge and consequently enhanced visible light absorbance [4].
To further explore the impact of Ag doping on the structural properties of the prepared TiO2 NPs, FTIR analysis was studied. The FTIR spectra of standard and Ag-doped TiO2 samples are illustrated in Fig. 3. As shown, the peaks observed at ∼3426 cm−1 are assigned to the O–H vibration of the hydroxyl oxygen group [3]. By comparing with previous studies, the intensive peaks in the range of 744–726 cm−1 are corresponding to lattice vibrations of Ti–O–Ti bonds [10, 12], which further confirms that the Ag additive is dispersed well into the TiO2 lattice.
The FESEM micrographs of standard and Ag/TiO2 NPs are depicted in Fig. 4. In the pure TiO2 NPs, the NPs seem to be more aggregated, as revealed in Fig. 4a. Comparing with pure TiO2, this finding indicates that the shape and morphology of TiO2 NPs change with Ag addition. The effect of Ag incorporating on the morphology TiO2 is particles like structures, as shown in Fig. 4b. The average particle size of pure TiO2 and Ag-doped TiO2 was found to be 80–100 nm and 30–50 nm, respectively. Moreover, the FESEM observations demonstrate that the adding of Ag clearly altered in the morphology of the photocatalyst surface. The spongy and porous structure causes more surface area at high hardness that surely would be more effective for enhancing the light absorbance and photodegradation performance [32].
The photocatalyst performance was estimated using the UV–Vis measurements of MB degradation under visible light illumination. The NP precursor is mixed with organic MB to form a colloidal solution using stirring for 5 min in the dark conditions. Then, the mixed solution containing NPs and MB was irradiated using visible light illumination. Nevertheless, if the electrons and holes pass through the surface of the TiO2 without recombination, they can act a part in different oxidation and reduction reactions with adsorbed agents, such as organic species, oxygen, and water. As shown in Fig. 5, the electrons in the valence band can be energized to the conduction band, leaving a positive hole in the valence band of the photocatalyst. When a photocatalyst is illuminated by light with energy as the same to or greater than the bandgap energy, photocatalysis initiated by electron-doping with Ag does not disturb the crystal structure of anatase TiO2. The small size of Ag-TiO2 hybrid increased surface area as indicated by the FESEM measurements, with an increasing lifetime of photogenerated electrons and holes. This process is beneficial to enhance the photocatalytic efficiency. Through the evaluation, the rate constants among the Ag-doped TiO2 have shown to be markedly enhanced compared with standard TiO2 sample. This is due to the insignificant particle size of Ag-doped TiO2 and reduced recombination of electron–hole pairs, and bandgap narrowing [33, 34].
The photocatalytic activities of standard TiO2 and Ag-TiO2 hybrid calcined at 700 ºC are presented in Fig. 6. In comparison, the photodegradation curves of MB by Ag-TiO2 sample are 100% achieved within 30 min, whereas the standard TiO2 sample, only 10% degradation of MB is achieved within 30 min (standard TiO2 rutile phase only and large particle size so no photocatalytic activity). Thus, we find that Ag-TiO2 sample shows enhanced degradation in this case. This may be correlated with the size of the particles, and mixed-phase and the bandgap. Table 1 reveals the results of previous studies related with TiO2 photodegradation and compared with the results established in this work.
4 Conclusion
In summary, the novel and eco-friendly photo-induced method was used to prepare Ag-doped TiO2 nanoparticles with improved photodegradation activity. In this regard, high-quality TiO2 NPs were achieved with higher crystallinity and better morphology compared with pure TiO2. Ag-doping has a significant impact on the narrowing energy bandgap of TiO2 (decreasing from 2.87 eV to 2.7 eV), which improves the light absorption ability toward a visible light region as well as delaying the electrons-holes recombination rate. The obtained results indicate that the enhancement of photodegradation activity was acquired after Ag doping. The measurement of photodegradation of MB dye showed that the Ag-doped TiO2 NPs had 100% photocatalytic activity, while standard TiO2 achieved 10% after 30 min illumination. It was noted that the incorporation of Ag can effectively improve the photocatalytic activity of TiO2 by increasing its adsorption property, inhibiting charge recombination, and reducing the energy bandgap, which was due to the synergistic influence of TiO2 and Ag doping. This low cost and environment friendly approach can pave a new pathway for using semiconducting TiO2 in fabricating highly active nanostructured composites for water purification under solar irradiation.
References
S.A. Duha, R.M. Mohammad, K.A.M. Mustafa, Synthesis of multi-walled carbon nanotubes decorated with ZnO/Ag nanoparticles by co-precipitation method. Nanosci. Nanotechnol. 10, 127–133 (2020)
K.A.M. Mustafa, R.M. Mohammad, M.S. Jabir, S.A. Duha, Functionalization, characterization, and antibacterial activity of single wall and multi wall carbon nanotubes. IOP Conf. Ser. Mater. Sci. Eng. 757, 012028 (2020)
R.M. Mohammad, S.A. Duha, K.A.M. Mustafa, ZnO/Ag nanoparticles- decorated single-walled carbon nanotubes (SWCNTs) and their properties. Surf. Rev. Lett. 27, 1950123 (2020)
M.K.A. Mohammed, Carbon nanotubes loaded ZnO/Ag ternary nanohybrid with improved visible light photocatalytic activity and stability. Optik 217, 164867 (2020)
G. Nagaraj, K. Vairaprakasam, C. Chandraleka, T. Sivalingam, M.K.A. Mohammed, Green synthesis of Zn-doped Catharanthus Roseus nanoparticles for enhanced anti-diabetic activity. Mater. Adv., 1,3460–3465 (2020).
K.A.M. Mustafa, D.S. Ahmed, R.M. Mohammad, Studying antimicrobial activity of carbon nanotubes decorated with metal-doped ZnO hybrid materials. Mater. Res. Exp. 6, 055404 (2019)
D.S. Ahmed, M.K.A. Mohammed, Studying the bactericidal ability and biocompatibility of gold and gold oxide nanoparticles decorating on multi‑wall carbon nanotubes, Chem. Pap. 74, 4033–4046 (2020).
R.M. Mohammad, D.S. Ahmed, K.A.M. Mustafa, Synthesis of Ag-doped TiO2 nanoparticles coated with carbon nanotubes by the sol–gel method and their antibacterial activities. J. Solgel Sci. Technol. 90, 498–509 (2019)
G. Nagaraj, R.A. Senthil, K. Ravichandran, Firmness and band gap engineered anatase TiO2 nanoparticles for enhanced visible light photocatalytic activity. Mater. Res. Express 6, 095049 (2019)
G. Nagaraj, D. Brundha, C. Chandraleka, M. Arulpriya, V. Kowsalya, S. Sangavi, R. Jayalakshmi, S. Tamilarasu, R. Murugan, Facile synthesis of improved anatase TiO2 nanoparticles for enhanced solar-light driven photocatalyst. SN Applied Sciences 2, 734 (2020)
M.K.A. Mohammed, Sol-gel synthesis of Au-doped TiO2 supported SWCNT nanohybrid with visible-light-driven photocatalytic for high degradation performance toward methylene blue dye. Optik 223, 165607 (2020)
D.S. Ahmed, M.K.A. Mohammed, M.R. Mohammad, Sol-gel synthesis of Ag-doped titania-coated carbon nanotubes and study their biomedical applications. Chem. Pap. 74, 197–208 (2020)
M.K.A. Mohammed, A.K. Al-Mousoi, M.S. Mehde, A.M. Al-Gebori, Engineered electronic properties of the spin-coated MAPI for hole-transport free perovskite solar cell (HT-free PSC): spinning time and PSC performance relationship. Chem. Phys. Lett. 754, 137718 (2020)
G. Nagaraj, R.A. Senthil, R. Boddula, K. Ravichandaran, A Facile synthesis of anatase Ni2+ doped TiO2 nanorods with highly improved visible-light photocatalytic performance. Curr. Anal. Chem. 17, 279–284 (2021)
Taheri-Ledari, Reza, Seyedeh Shadi Mirmohammadi, Kobra Valadi, Ali Maleki, and Ahmed Esmail Shalan. RSC Advances 10, no. 71, 43670-43681 (2020).
Taheri-Ledari, Reza, Jamal Rahimi, Ali Maleki, and Ahmed Esmail Shalan. New Journal of Chemistry 44, no. 45, 19827-19835 (2020)
B. Mohsen, C. Maryam, Ag-doped TiO2 nanocomposite prepared by sol gel method: photocatalytic bactericidal under visible light and characterization. JNS 2, 227–234 (2013)
K.H. Leong, B.L. Gan, S. Ibrahim, P. Saravanan, Synthesis of surface plasmon resonance) SPR) triggered Ag/TiO2 photocatalyst for degradation of endocrine disturbing compounds. Appl. Surf. Sci. 319, 128–135 (2014)
Nabih, Shimaa, Ahmed Esmail Shalan, Esraa Samy Abu Serea, Mona A. Goda, and Mohamed Fathi Sanad. Journal of Materials Science: Materials in Electronics 30, no. 10, 9623–9633 (2019)
O. Oluwafunmilola, M.M. Maroto-Valer, Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 24, 16–42 (2015)
M.M. Rashad, E.M. Elsayed, M.S. Al-Kotb, A.E. Shalan, J. Alloy. Compd. 581, 71–78 (2013)
R. Kumar, J. Rashid, M.A. Barakat, Colloids Interface Sci Commun 5, 1–4 (2015)
A. Hastir, N. Kohli, R.C. Singh, Mater Today Proce 4, 9476–9480 (2017)
T.N. Ravishankar, O.V. Mauricio de, T. Ramakrishnapp, R.T. Sergio, J. Dupontd, Ionic liquid assisted hydrothermal syntheses of Au doped TiO2 NPs for efficient visible-light photocatalytic hydrogen production from water, electrochemical detection and photochemical detoxification of hexavalent chromium (Cr6+), RSC Adv. 7, 43233 (2017)
L. Xiao, L. Youji, C. Feitai, X. Peng, L. Ming, Facile synthesis of mesoporous titanium dioxide doped by Ag-coated graphene with enhanced visible-light photocatalytic performance for methylene blue degradation. RSC Adv. 7, 25314–25324 (2017)
S.K. Md Saad, A.A. Umar, M.I.A. Umar, M. Tomitori, M.Y.A. Rahman, M.M. Salleh, M. Oyama, Two-Dimensional, Hierarchical Ag-Doped TiO2 Nanocatalysts: Effect of the Metal Oxidation State on the Photocatalytic Properties, ACS Omega, 3, 2579−2587 (2018).
G. Nagaraj, S. Tamilarasu, Visible light photocatalyst anatase phased TiO2 nanoparticles for enhanced antibacterial performance. J. Clust. Sci. (2020). https://doi.org/10.1007/s10876-020-01939-9
M.K.A. Mohammed, Studying the structural, morphological, optical, and electrical properties of CdS/PbS thin films for photovoltaic applications, Plasmonics, 15 1989–1996 (2020).
Mustafa K. A. Mohammed, Synthesis of transparent few layer graphene films using a dual flame approach for the ammonia gas sensor. Inorganic and Nano-Metal Chemistry, https://doi.org/10.1080/24701556.2020.1814336.
M.K.A. Mohammed, High-performance hole conductor-free perovskite solar cell using a carbon nanotube counter electrode. RSC Adv. 10, 35831–35839 (2020)
K.A.M. Mustafa, K.A. Ali, A.K. Haider, Deposition of multi-layer graphene (MLG) film on glass slide by flame synthesis technique. Optik 127, 9848–9852 (2016)
A.E. Shalan, M. Rasly, M.M. Rashad, J. Mater. Sci. Mater. Electron. 25(7), 3141–3146 (2014)
E. Vinodkumar, K.S. Michael, J.H. Steven, C.P. Suresh, Adv. Func. Mater. 21, 3744–3752 (2011)
L.-L. Tan, W.-J. Org, S.-P. Chai, RSC-Chem. Commun 50, 6923–6926 (2014)
M. Harikishore, M. Sandhyarani, K. Venkateswarlu, T.A. Nellaippan, N. Rameshbabu, Effect of Ag doping on antibacterial and photocatalytic activity of nanocrystalline TiO2. Procedia Mater Sci 6, 557–566 (2014)
S. Adilah, A. Thanita, C. Siriluk, Enhanced visible light photocatalytic activity of N and Ag doped and co-doped TiO2 synthesized by using an In-situ solvothermal method for gas phase ammonia removal. Catalysts 10, 251 (2020)
Acknowledgement
The authors thank Director Dr. P. Mohana Sundram, PG Extension Centre, Periyar University, Dharmapuri-636107, Tamil Nadu, and India.
Author information
Authors and Affiliations
Contributions
GN and MKAM wrote introduction and revised version HGA wrote SEM and XRD results, PS and SK completed experimental parts, ST supervised and revised the manuscript.
Corresponding author
Ethics declarations
Conflict and interest
The authors declare there is no conflict of interest in this research paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagaraj, G., Mohammed, M.K.A., Abdulzahraa, H.G. et al. Effects of the surface of solar-light photocatalytic activity of Ag-doped TiO2 nanohybrid material prepared with a novel approach. Appl. Phys. A 127, 269 (2021). https://doi.org/10.1007/s00339-021-04427-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-021-04427-7