Abstract
This work focuses on recycling of rice husk for production of activated carbon/Fe3O4 nanocomposite. Moreover, the synthesized composites were applied for elimination of methylene blue dye from wastewater. The proposed nanocomposite can replace pure activated carbon which is expensive and has some difficulties during its reusability. First, the activated carbon was extracted by thermal and chemical activations of rice husk ash. On the other hand, the activated carbon/magnetite nanocomposite was prepared by co-precipitation method using the suitable iron salts. The qualitative phase identification of prepared composite was performed by X-ray technique (XRD) and confirmed by Fourier Transform Infrared Spectroscopy (FT-IR). The morphology and particle size of prepared activated carbon and its composite were investigated by transmission electron microscope (TEM). The batch-adsorption method was utilized for studying the elimination of methylene blue dye from wastewater. Various factors like adsorbent dosage, pH of reaction, initial dye concentration, and duration of reaction were examined. The results showed that the prepared nanocomposite was almost amorphous as indicated from XRD patterns. Also, its particle size was very small (5–10 nm) and lower than the activated carbon alone (40–80 nm). Regarding the removal percentage of methylene blue dye from waste water, 98% was the maximum obtained percentage under the condition: pH 7, adsorbent dosage 0.1 g/100 mL, 50 ppm MB and 20 min. contact time at 25 °C. Furthermore, the maximum adsorption reaction followed the pseudo-second-order type and the Langmuir isotherm model.
Highlights
-
The recycling of rice husk for production of activated carbon/Fe3O4 was successfully conducted.
-
The synthesized composites were applied for elimination of methylene blue dye from wastewater.
-
The maximum removal percentages of methylene blue from wastewater was 98%.
-
The adsorption reaction followed the pseudo-second-order type and the Langmuir isotherm model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, a great interest was directed to environmental pollution to preserve the living organisms and ecological system from distortion. With the development of industry, huge amounts of liquids or solids wastes are withdrawn in the environment; especially in water systems. Water is the most important system in the environment and its corruption tends to several problems for all living organisms (Iqbal et al. 2019). There are many contaminants for water systems as nutrients (nitrogen and phosphorus), heavy metals, hydrocarbons, organic matters, microorganisms (bacteria, viruses, protozoa, and parasites), and dyes (Akpor et al. 2014; Azam et al. 2022; Khan et al. 2019; Lima et al. 2021; Yao et al. 2022).
The dye fabrication is considered as an important area in the chemical industries. The global production of dyes is about 850,000 tons every year. Generally, the synthesized dyes have many applications in many industries like paper-printing, textile, pharmaceutical, food, cosmetics, and leather. During different processes of textile industry, around 10–15% of dyes are withdrawn in water (Hassaan and Nemr 2017). Therefore, a huge amount of wastewater contaminated by dyes is produced and lost during the dyes industry. Dyes are usually categorized into three types, namely, anionic, non-ionic, and cationic dyes, while they are also classified according to the function groups into azo, anthraquinone, phthalocyanine, indigo, nitro, and sulfur. The cationic dyes as methylene blue, methyl violet, and crystal violet are the most toxic ones for the environment, since it includes cyclic organic rings (Hassaan and Nemr 2017). It is well recognized that the microorganisms that live in water systems need sunlight to generate energy, but the existence of coloring source like dye leads to preventing the light to penetrate; this consequently makes inconsistency in the ecosystem (Iqbal et al. 2015). Except the vat and disperse dyes, most of the dyes can dissolve in water and contain some toxic metals like chromium, cobalt, nickel, and copper (Dawood et al. 2014). Methylene blue is a solid dye and basic in nature; it can be applied for many applications, but it has toxic effect on the microorganisms, so it should be removed from wastewater. In water, it forms methylene blue and chloride ions which can be removed by many adsorbents (El Qada et al 2006). Many approaches have been approved for wastewater treatment; these included chemical and physical approaches like coagulation–flocculation, precipitation, advanced oxidation, ion exchange, membrane filtration, etc. The most interesting method used for eliminating dyes from wastewater is the adsorption technique because of its lower price, simplicity, and high efficacy; it proceeds without sludge and waste formation, extensive commercially available adsorbents, as well as the dye can be recovered (Andreou and Pashalidis 2018; Khan et al. 2020; Qureshi et al. 2022; Dehghani et al. 2017, 2018).
There are many industrial and biomass wastes that can be utilized as sources for the production of adsorbents for removing a variety of contaminates or toxic materials from water and wastewater (Besharati et al. 2018). One of the most important biomasses is the rice husk which after burning it forms rice husk ash. It includes reactive carbon and silica, so it can be used as excellent source for production of activated carbon which contains many groups on its surface as –COOH, –OH, –NH2, etc., that make it a significant adsorbent for dyes and heavy metals (Besharati et al. 2018; Sugashini and Begum 2015). AC as adsorbent for dyes has high surface area, porosity, and adsorption capacity, but it is relatively expensive and it has difficulties during its usability. Thus, its production from waste materials in the presence of magnetic materials is recommended to overcome these drawbacks.
The application of nanoscale magnetic particles has attracted the attention of many researchers. Extremely, nanoparticles have favorable characteristics such as more atoms are located on their surfaces and they have a remarkable high surface area (Andreou and Pashalidis 2018; Suri et al. 2010). Magnetite and magnetic carbon nanoparticles have attracted a great interest in the field of environmental remediation due to their advantages as high thermal and mechanical stability as well as they can be rapidly separated from the solution by installing a magnetic separator (Kitkaew et al. 2018; Chen et al. 2018).
The main objective of the current work is to prepare activated carbon/magnetite nanocomposites to be utilized as adsorbent for removing of methylene blue from wastewater. The activated carbon was first extracted from rice husk waste, and then mixed with aqueous solution of iron slats to precipitate activated carbon/magnetite nanocomposites. The prepared nanocomposites were applied as adsorbents to remove methylene blue dye from wastewater by adsorption method. All parameters like reaction pH, duration of reaction, adsorbent dosage, and dye concentration were examined. Furthermore, type and order of reaction were estimated.
Materials and Experiments
Materials
First, the rice husk ash was prepared by burning the Egyptian rice husk, collected from different farms, in a muffle furnace for 2 h under argon atmosphere to be ready for preparation of activated carbon. Representative sample (50 kg) was collected from different farms in Egypt then quartered for many times until getting the final repressive sample (5 kg). While, the iron salts as FeSO4⋅7H2O and FeCl3⋅6H2O NaOH, cationic-methylene blue dye, HCl, and H3PO4 were supplied by Sigma-Aldrich, USA. The wastewater sample was supplied by Abu Rawash treatment plant, Cairo, Egypt. The properties of wastewater sample are illustrated in Table 1.
Chemical Preparation of Activated Carbon/Fe3O4 Nanocomposite
First, the activated carbon was prepared by thermal and chemical activation methods described elsewhere (Cheenmatchaya and Kungwankunakorn 2014; Zawrah et al. 2019, 2020). The prepared carbon was milled by high-energy ball mill model MTI SFM-1(QM-3SP2), to obtain nanopowder.
To prepare the proposed composite, 4 g FeSO4.7H2O and 8 g FeCl3 were mixed with 300 mL deionized water and warmed at 90 °C. 4 g of prepared nanoactivated carbon was mixed with iron dissolved salts. 200 mL of 2.5 M NaOH was dropped-wise slowly to precipitate magnetite during 2 h completely. The prepared activated carbon/Fe3O4 nanocomposite was washed by deionized water and separated by centrifuging. This method depends on decantation of liquid and then collecting the precipitate. Finally, the prepared nanocomposite was dried in heating-drier at 60 °C for 24 h. Figure 1 shows the schematic presentation for preparation method of activated carbon/Fe3O4 composite. This method gives high productive efficiency (Altıntıg et al. 2017).
Investigation of Activated Carbon and Its Composite with Fe3O4
To identify the qualitative phase composition and nature (crystalline or amorphous) of prepared nanoactivated carbon and its composite with magnetite, X-ray diffractometer model Philips PW1390, Holland, was used. To confirm the material composition and detect the functional groups, Fourier transform infrared spectroscopy was applied via the KBr pressed disc method. The spectra were recorded on Mattson 1000, Unicam infrared spectrophotometer, UK, in the range (400–4000 cm−1). The particle size and shape of fabricated nanoactivated carbon and its composite with magnetite were tested by transmission electron microscopy, model Philips, EM, 208, Holland. It provides excellent knowledge about the internal structure of materials like crystal structure, shape and size of grains, as well as the stresses in the structure.
Adsorption Study
The adsorption of methylene blue on activated carbon/magnetite nanocomposite was performed. First, a typical methylene blue solution with concentration 1000 mg/L was prepared without addition of composite as a control sample. Specific amounts of prepared composite were mixed with 100 mL of prepared methylene blue solution to prepare several samples with different composite dose. These samples were put on a shaker at 150 rpm at room temperature. Many factors, such as duration of reaction (0–60 min.), composite amount (50–350 mg/100 mL), pH of reaction (3–10), and concentration of methylene blue (5–150 mg/L), were investigated. At the end of each experiment, the samples were filtered on Whitman filter paper No. 40 to separate the composite. The remained amount of methylene blue was evaluated by spectrophotometer type Perkin-Elmer lambda 25, at 665 nm. The percent of removed methylene blue in solution was considered according to the following formula
Since, C0 and Cf (mg/L) are the original and ultimate dye concentrations, respectively.
The kinetic of adsorption reaction was examined by pseudo-first-order and pseudo-second-order models. The kinetic terms were estimated for each model by the suitable known formula. According to the drown results, the linear correlation coefficient (R2) determined using the linear regression was detected to identify the accurateness of proposed model. In general, when it is close to one, the model is fitting for the reaction. Furthermore, the obtained data were analyzed by Langmuir and Freundlich isotherms. All parameters related to these isotherms were evaluated.
Results and Discussion
Properties of Prepared Activated Carbon and Its Composite with Fe3O4
Phase Identification of Prepared Composite
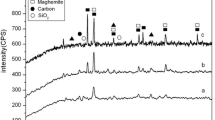
X-ray is a principal non-destructive tool for identifying the crystal structure and type of crystalline phases qualitatively for minerals, elements, and inorganic compounds after comparison of the acquired data with reference database. There is a specific diffraction pattern for each material which should match with the fingerprints of reference database. Figure 2 shows XRD patterns of prepared activated carbon and activated carbon/magnetite nanocomposite. It is well recognized that the ash of rice husk is composed mainly of amorphous carbon and silica; their percentages depend on the method of pyrolysis and the applied atmosphere. As seen in the patterns, low intensity peaks are detected, indicating that both materials are semi-crystalline or amorphous. Both materials exhibit small peaks of amorphous silica and carbonaceous materials. The patterns exhibit broad peak at 2-theta equal 22° which is corresponding to silica. The peaks of carbonaceous structures (hexagonal graphitic-carbon like reflections) are detected at 2-theta 25° and 43° (Omri et al. 2013). Some new and very small characteristic peaks at 2-theta equal of 35.3°, 42.9°, and 62.5° are appeared in the pattern of activated carbon/Fe3O4 nanocomposite. These peaks are characteristics for Fe3O4 (Ai et Al. 2011). Very small peaks at 2-theta equal of 24.2°, 35.7°, 40.9°, 49.5°, 54.1°, and 63.3° are also appeared in the pattern of composite; these peaks are typical peaks of rhombohedra-centered hematite (Reza and Ahmaruzzaman 2015).
Confirming the Composition by FT-IR
FT-IR is a diagnostic tool utilized to identify the functional groups in organic and inorganic materials. This tool determines the absorption of infrared radiation by the material against wavelength. For each functional group in the material, there are characteristic adsorption bands which are detected from IR spectrum. FT-IR spectra of prepared activated carbon and activated carbon/magnetite nanocomposite are presented in Fig. 3. It is clear that FT-IR spectrum of activated carbon exhibits four bands at 3456, 2907, 1620, and 1089 cm−1. The bands at about 3456 and 1620 cm−1 are ascribed to the stretching- and bending-vibration frequencies of OH group for adsorbed water molecule, respectively. Also, the band at 1620 cm−1 might assign to C=C or C=O bonds of aromatic ring or carboxylic acid typically for carbonaceous material. The detectable small band at about 2907 cm−1 is attributed to the existence of aliphatic C–H stretching of CH, CH2, and CH3 groups intermingled with the surface of carbon. The band at 1089 cm−1 is attributed to C–O-stretching vibration in RCOOH, ROH, PhOH, ROR, and RCOOR due to the oxidation of carbon. For activated carbon/Fe3O4 nanocomposite, the FT-IR spectrum exhibits all aforementioned bands in addition to two bands at 780 and 466 cm−1 but the band at 3456 cm−1 is intense due to the formed hydrated Fe3O4. The new formed bands are corresponding to iron oxides. These results are in agreement with the results obtained by Reza and Ahmaruzzaman (2015).
TEM of Prepared Activated Carbon and Activated Carbon/Magnetite Composite
Figure 4 depicts TEM images of the extracted activated carbon and activated carbon/magnetite nanocomposite. It can be seen that the activated carbon (Fig. 4a) has homogeneous particle shape and size. The particles are mostly spherical with a few longer particles; their sizes are ranged between 40 and 80 nm. It is well known that the rice husk ash includes nanosilica particles, so the prepared activated carbon includes amorphous silica nanoparticles. On contrast, TEM image (Fig. 4b) of the activated carbon/Fe3O4 nanocomposite indicates the existence of inner components as black nanoparticles, namely, Fe3O4 (Sun et al. 2015). The overall morphology and size of this sample are homogenous. All the particle sizes are under 100 nm and reach less than 5 nm. The incorporation of Fe3O4 in the activated carbon leads to disperse of the particle and decrease the size. In addition, iron oxide can interact with the surface of activated carbon by chemical bonding which leads to change in the surface morphology of activated carbon/Fe3O4 nanocomposite (Darezereshki et al. 2013).
Adsorption Study of Methylene Blue by Activated Carbon/Fe3O4 Nanocomposite
To investigate the methylene blue removal efficiency by activated carbon/Fe3O4 nanocomposite, adsorption studies were conducted under different conditions as pH, contact time, adsorbent dosage, and initial dye concentration.
Effect of pH on Methylene Blue Dye Removal
It is well known that the pH of solution medium is significant parameter for evaluation of the adsorption efficiency during the adsorption process. It is almost effect on the dissociation of function groups existing on the surface of adsorbent, the amount of charges on surface, and adsorbent structure. It can also effect on the rate of ionization for adsorbate molecule and it is responsible for the high- or low-sorption efficiency of adsorbent (Crini et al. 2007).
Figure 5 shows the relationship between the pH and the removal percentage of methylene blue by activated carbon/Fe3O4 nanocomposite. It is appeared that the removal percentage rises gradually with raising the pH value and reaches its optimum value (95%) at pH 7 after which the removal percentages are constant. The control samples give negligible removal percentages. At lower pH value (i.e., pH 3), there are positive charges on the surface of activated carbon/Fe3O4 nanocomposite; these lead to rivalry between H+ ion and dye cation to touch the surface. Ultimately, the active sites on the activated carbon/Fe3O4 nanocomposite's surface are covered typically by the H+ ion and to a certain degree by the dye molecules. Therefore, the H+-ion limits the reaction between the cationic-methylene blue molecules (MB+) and the surface of activated carbon/Fe3O4 nanocomposite. This repulsive force delays the interaction of cationic MB+ molecules with the activated carbon/Fe3O4 nanocomposite's surface (Shah et al 2015). At pH 7, the positive charges on the surface of activated carbon/Fe3O4 nanocomposite decrease, so the cationic MB+ molecules have chance to adsorb on the active sites of adsorbent and reach their maximum. At pH more than 7, the active sites are full covered by methylene blue molecules, so there is no more adsorption can be occurred.
Effect of Composite Dosage on the Adsorption of Methylene Blue Dye
The influence of adsorbent dose on removal of MB by activated carbon/Fe3O4 nanocomposite is displayed in Fig. 6. As seen in the figure, the optimum dose for maximum adsorption of MB is 1 g after which very small decrease in removal percentage is occurred. This can be explained as: by increasing the amount of adsorbent up to 1 g, the active sites available for adsorption process increase which results in an increase in MB dye removal efficiency. After increasing the absorbent dose more than 1 g, the adsorption percentage of MB dye is relatively decreased till constancy. This is due to agglomeration of activated carbon/Fe3O4 nanocomposite nanoparticles and decreasing the surface area as well as the active sites (Gundogdu et al. 2012).
Effect of Adsorption Time on Removing of Methylene Blue
The effect of interaction time on removal of MB dye by activated carbon/Fe3O4 nanocomposite is shown in Fig. 7. It is clear from the figure that very fast adsorption rate is detected by removing of more than 80% of MB during the first 5 min; then, the adsorption speed is increased gradually until the equilibrium is attained. This means that the application of the proposed composite in the adsorption process is more effective. It can be also seen from the figure that there is no significant variation in removal percent of MB dye after 40 min contact time due to saturation of the active sites which does not allow further adsorption to take place (Lopez et al. 2010).
Effect of Initial MB Concentration on Its Adsorption by Activated Carbon/Fe3O4 Nanocomposite
Figure 8 displays the dependence of MB dye removal percentage by activated carbon/Fe3O4 nanocomposite on its initial concentration (10–150 ppm). It is obvious that, as the initial dye concentration increases, there is a decrease in adsorption efficiency of dye from aqueous solution. The maximum dye removal percent (98%) is obtained up to 45 ppm dye concentration. The removal percentage becomes 96% with 50 ppm dye concentration. This is because there are limited available active sites, whereas the quantity of dye molecules rises with increasing original dye concentration. This leads to the consumption of active sites quickly, that affects on the removal percentage of the dye (Singh and Dawa 2014).
Application on Real Wastewater Examples
To inaugurate the validity of the suggested process, the process was applied for removal of MB in a real wastewater samples provided by Abu Rawash influent (Giza Governorate, Egypt). Two concentrations of MB dye (10 and 40 ppm) were spiked to the tested wastewater samples. The removal of MB dye from spiked solutions was successfully performed and up to 98% removal was achieved. Excellent removal percentage indicates that the complex matrix of wastewater samples does not interfere with the removal of MB.
Adsorption Kinetics Studies of MB by Activated Carbon/Fe3O4 Nanocomposite
The adsorption kinetic of methylene blue dye by activated carbon/Fe3O4 nanocomposite surfaces was recognized by examining the pseudo-first-order and pseudo-second-order kinetics models. The obtained results of kinetic parameters for MB adsorption on activated carbon/Fe3O4 nanocomposite are illustrated in Table 2 and presented in Figs. 9 and 10. It is indicated that the estimated qe value obtained from pseudo-first-order and pseudo-second-order kinetics models are 5.8 and 47.6 mg/g, respectively. Moreover, it can be observed from Figs. 9 and 10 that the correlation coefficient (R2) of pseudo-second-order model (R2 = 0.9996) is greater than that of pseudo-first-order model (R2 = 0.9886), indicating that the adsorption of MB onto activated carbon/Fe3O4 nanocomposite is fitting sound with the pseudo-second-order model. This means that the adsorption is chemisorption process, i.e., the electro-static interaction between the electrons in methylene blue dye molecules and activated carbon/Fe3O4 nanocomposite is the rate-limiting step (Phihusut and Chantharat 2017; Abuzerr et al. 2018).
Adsorption Isotherms of MB Dye onto Activated Carbon/Fe3O4 Nanocomposite
It is well recognized that the Langmuir and Freundlich adsorption isotherm models are the most common ones utilized to describe the equilibrium relationships between adsorbent and adsorbate interface in the adsorption process. Both of them are applied to examine the removal efficiency mechanism of methylene blue by activated carbon/Fe3O4 nanocomposite. The Langmuir model supposes that the adsorbate adsorbed homogenously in monolayer on equal sites of adsorbent, i.e., once an adsorbate species occupies a site, no more adsorption is occurred at that site. The Langmuir formula could be expressed as follows:
where qe is the amount of adsorbed methylene blue on activated carbon/Fe3O4 nanocomposite at equilibrium (mg/g), Qo is the quantity of adsorbed MB monolayer on composite, b is the adsorption equilibrium constant or Langmuir constant; it is associated with the adsorption energy (L/mg) and Ce is the equilibrium concentration (mg/L).
Figure 11 shows the linear relationship of specific adsorption (Ce/qe) and the equilibrium concentration (Ce) (mg/L) for the adsorption of methylene blue by activated carbon/Fe3O4 nanocomposite given the Langmuir model. The Langmuir coefficients Q0 and b can be estimated using the slope and intercept of the curve and are illustrated in Table 2. The Langmuir constant b (l/mg) can be correlated with the dimensionless constant (separation factor Rl) that is presented by the next formula (Hao et al. 2018)
where C0 is the primary concentration of dye (mg/L). The Rl values are used to define the kind of adsorption depending on its value range: Rl > 1 means unwanted adsorption, 0 < Rl < 1 gives favorable adsorption, Rl = 0 means irreversible adsorption, and Rl = 1 means linear adsorption. In the current study, the obtained Rl value is 0.0139 which means that the adsorption is favorable. Also, according to the higher R2 value (0.9923) for Langmuir isotherm model, this means that the chemical adsorption is the predominant mechanism, i.e., the methylene blue might chelate with numerous groups on the surface-activated carbon/Fe3O4 composite. Furthermore, the maximum Langmuir adsorption efficiency of methylene blue on activated carbon/Fe3O4 nanocomposite is calculated as 49.5 mg/g (Altıntıg et al 2017). This result indicates that the adsorption capacity of the prepared nanocomposite is increased due to increasing the surface area and micropores volume by incorporating of Fe3O4.
Regarding the Freundlich model, it assumes that the adsorption is happened on heterogeneous surfaces and mono- or multi-layers are formed according to the quantity of methylene blue adsorbed at equilibrium. The Freundlich linear formula can be expressed by the next one
where Kf is practically correlated to the adsorption capacity and 1/n is the adsorption strength. When n > 1, this means favorable adsorption (Omri et al. 2013; Aranfil and Kilduff 1999). By plotting lnqe against lnCe (Fig. 12), the intercept lnKf and slope 1/n can be estimated. The values of Kf, n and the linear regression correlation (R2) for Freundlich model are presented in Table 3; their values are 0.064, 1.73, and 0.941, respectively. According to the values of linear regression correlation (R2) in Langmuir and Freundlich isotherms, it is indicated that they are high and reach closely to one, but Langmuir is relatively higher, indicating that it is slightly fits well than the Freundlich model (Porkodi and Kumar 2007; Dhmees et al. 2019; Alhogbi et al. 2021). Generally, it can be concluded that the activated carbon/Fe3O4 nanocomposite is a good adsorbent for methylene blue dye owing to the existence of high active sites and electro-static interaction between its surface negative charge and cationic-methylene blue dye. Figure 13 shows the color of methylene blue after treatment with activated carbon/Fe3O4 nanocomposite at optimum conditions. The left flask contains methylene blue before treatment, while the right flask contains the remaining solution after removing of methylene blue by the proposed adsorbent; it is mostly clear like pure water. It is worth to mention that existence of common ions (K+, Na+, and Ca2+) in water surface can affect slightly on the adsorption process, since they can compete with the contaminants and decrease their adsorption (Hassaan and Nemr 2017; Iqbal et al 2015; Dawood et al. 2014; El Qada et al. 2006). Compared to many effective adsorbents published in the literatures (El Qada et al. 2006), the proposed activated carbon/magnetite nanocomposites have also good efficiency for removing of methylene blue from water.
Conclusion
In this article, activated carbon/Fe3O4 nanocomposite was successfully prepared utilizing rice husk as a source of activated carbon and iron salts as source for Fe3O4. This composite was also utilized successfully as adsorbent for removing of methylene blue from wastewater by fast and simple adsorption technique. The prepared magnetic composite could be well dispersed in water and can be easily separated by a magnet from the aqueous solution after adsorption of the dye. The optimized parameters for the adsorption process were: 0.1 g/100 mL of adsorbent dosage, pH 7, dye concentration 50 ppm, and contact time of 20 min. The maximum adsorption capacity for activated carbon/Fe3O4 was 49.5 mg/g and the maximum removal percentage of methylene blue dye from real wastewater samples reached 98%. The kinetic study indicated that the reaction proceeded by pseudo-second-order model reaction mechanism, while the Langmuir isotherm model was the predominant mechanism.
Data Availability
Not applicable.
References
Abuzerr S, Darwish M, Mahvi AH (2018) Simultaneous removal of cationic methylene blue and anionic reactive red 198 dyes using magnetic activated carbon nanoparticles: equilibrium, and kinetics analysis. Water Sci Technol 2017(2):534–545
Ai L, Zhang C, Liao F, Wang Y, Li M, Meng L, Jiang J (2011) Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: kinetic, isotherm and mechanism analysis. J Hazard Mater 198:282–290
Akpor OB, Otohinoyi DA, Olaolu DT, Aderiye BI (2014) Pollutants in wastewater effluents: impacts and remediation processes. Int J Environ Res Earth Sci 3(3):050–059
Alhogbi BG, Altayeb S, Zawrah MF, Bahaidarah EA (2021) Preparation of activated carbon from palm-tree fiber wastes: studying the performance in re-mediating dyes from wastewater. Processes 9:416
Altıntıg E, Altundag H, Tuzen M, Sarı A (2017) Effective removal of methylene blue from aqueous solutions using magnetic loaded activated carbon as novel adsorbent. Chem Eng Res Des 122:151–163
Andreou MA, Pashalidis I (2018) Removal of crystal violet from aqueous solution by biofibers. Desalin Water Treat 112:90–93
Aranfil T, Kilduff JE (1999) Role of granular activated carbon surface chemistry on the adsorption of organic compounds. 1. Priority pollutants. Environ Sci Technol 33(18):3217–3224
Azam M, Wabaidur SM, Khan MR, Al-Resayes SI, Islam MS (2022) Heavy metal ions removal from aqueous solutions by treated Ajwa Date Pits: kinetic, isotherm, and thermodynamic approach. Polymers 14:914. https://doi.org/10.3390/polym14050914
Besharati N, Alizadeh N, Shariati S (2018) Removal of cationic dye methylene blue (MB) from aqueous solution by Coffee and Peanut husk modified with magnetite iron oxide nanoparticles. J Mex Chem Soc 62(3)
Cheenmatchaya A, Kungwankunakorn S (2014) Preparation of activated carbon derived from rice husk by simple carbonization and chemical activation for using as gasoline adsorbent. Int J Environ Sci Dev 5(2):171
Chen R, Wang P, Li M, Tian F, Xiao J, Fu X, Shi Y (2018) Removal of Cr (VI) by magnetic Fe/C crosslinked nanoparticle for water purification: rapid contaminant removal property and mechanism of action. Water Sci Technol 78(10):2171–2182
Crini G, Peindy HN, Gimbert F, Robert C (2007) Removal of CI basic green 4 (Malachite green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: kinetic and equilibrium studies. Sep Purif Technol 53(1):97–110
Darezereshki E, Bakhtiari F, Vakylabad AB, Hassani Z (2013) Single-step synthesis of activated carbon/γ-Fe2O3 nano-composite at room temperature. Mater Sci Semicond Process 16(1):221–225
Dawood S, Sen TK, Phan C (2014) Synthesis and characterization of novel-activated carbon from waste biomass pine cone and its application in the removal of Congo red dye from aqueous solution by adsorption. Water Air Soil Pollut 225(1):1818
Dehghani MH, Dehghan A, Alidadi H, Dolatabadi M, Mehrabpour M, Converti A (2017) Removal of methylene blue dye from aqueous solutions by a new chitosan/zeolite composite from shrimp waste: kinetic and equilibrium study. Korean J Chem Eng 34:1699–1707
Dehghani MH, Pourshabanian M, Heidarinejad Z (2018) Experimental data on the adsorption of Reactive Red 198 from aqueous solution using Fe3O4 nanoparticles: Optimization by response surface methodology with central composite design. Data Brief 19:2126–2132
Dhmees AS, Rashad AM, Eliwa AA, Zawrah MF (2019) Preparation and characterization of nano SiO2@CeO2 extracted from blast furnace slag and uranium extraction waste for wastewater treatment. Ceram Int 45:7309–7317
El Qada EN, Allen SJ, Walker GM (2006) Adsorption of methylene blue onto activated carbon produced from steam activated bituminous coal: a study of equilibrium adsorption isotherm. Chem Eng J 124(1–3):103–110
Gundogdu A, Duran C, Senturk HB, Soylak M, Ozdes D, Serencam H, Imamoglu M (2012) Adsorption of phenol from aqueous solution on a low-cost activated carbon produced from tea industry waste: Equilibrium, kinetic, and thermodynamic study. J Chem Eng Data 57:2733–2743
Hao Z, Wang C, Yan Z, Jiang H, Xu H (2018) Magnetic particles modification of coconut shell-derived activated carbon and biochar for effective removal of phenol from water. Chemosphere 211:962–969
Hassaan MA, El Nemr A (2017) Health and environmental impacts of dyes: mini review. Am J Environ Sci Eng 1(3):64–67
Iqbal M, Ahmad MZ, Bhatti IA, Qureshi K, Khan AJCI (2015) Cytotoxicity reduction of wastewater treated by advanced oxidation process. Chem Int 1(1):53–59
Iqbal M, Abbas M, Nisar J, Nazir A, Qamar A (2019) Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: a review. Chem Int 5(1):1–80
Khan MA, Oterob M, Kazic M, Alqadamia AA, Wabaidura SM, Siddiquia MR, Alothmana ZA, Sumbuld S (2019) Unary and binary adsorption studies of lead and malachite green onto a nanomagnetic copper ferrite/drumstick pod biomass composite. J Hazard Mater 365:759–770
Khan MA, Wabaidur SM, Siddiqui MR, Alqadami AA, Khan AH (2020) Silico-manganese fumes waste encapsulated cryogenic alginate beads for aqueous environment de-colorization. J Clean Prod 244:118867
Kitkaew D, Phetrak A, Ampawong S, Mingkhwan R, Phihusut D, Okanurak K, Polprasert C (2018) Fast and efficient removal of hexavalent chromium from water by iron oxide particles. Environ Nat Resour J 16(1):91–100
Lima ÉC, Dehghani MH, Guleria A, Sher F, Karri RR, Dotto GL, Tran HN (2021) Adsorption: fundamental aspects and applications of adsorption for effluent treatment. In: Green technologies for the deflouridation of water, pp 41–88
Lopez JA, González F, Bonilla FA, Zambrano G, Gómez ME (2010) Synthesis and characterization of Fe3O4 magnetic nanofluid. Revista Latinoamericana de Metalurgia y Materiales 60–66
Omri A, Benzina M, Ammar N (2013) Preparation, modification and industrial application of activated carbon from almond shell. J Ind Eng Chem 19:2092–2099
Phihusut D, Chantharat M (2017) Removal of methylene blue using agricultural waste: A case study of rice husk and rice husk ash from Chaipattana rice mill demonstration center. Environ Nat Resour J 15(2):30–38
Porkodi K, Kumar KV (2007) Equilibrium, kinetics and mechanism modeling and simulation of basic and acid dyes sorption onto jute fiber carbon: eosin yellow, malachite green and crystal violet single component systems. J Hazard Mater 143(1–2):311–327
Qureshi SS, Shah V, Sabzoi Nizamuddin NM, Mubarak RR, Karri MH, Dehghani SR, Khalid M, Rahman ME (2022) Microwave-assisted synthesis of carbon nanotubes for the removal of toxic cationic dyes from textile wastewater. J Mol Liq 356:119045
Reza RA, Ahmaruzzaman M (2015) A novel synthesis of Fe2O3@activated carbon composite and its exploitation for the elimination of carcinogenic textile dye from an aqueous phase. RSC Adv 5(14):10575–10586
Shah I, Adnan R, Ngah WSW, Mohamed N (2015) Iron impregnated activated carbon as an efficient adsorbent for the removal of methylene blue: regeneration and kinetics studies. PLoS ONE 10(4):122603
Singh H, Dawa TB (2014) Removal of methylene blue using lemon grass ash as an adsorbent. Carbon Lett 15(2):105–112
Sugashini S, Begum KMMS (2015) Preparation of activated carbon from carbonized rice husk by ozone activation for Cr(VI) removal. New Carbon Mater 30(3):252–261
Sun P, Hui C, Khan RA, Du J, Zhang Q, Zhao YH (2015) Efficient removal of crystal violet using Fe3O4-coated biochar: the role of the Fe3O4 nanoparticles and modeling study their adsorption behavior. Sci Rep 5:12638
Suri J, Shaw LL, Zawrah MF (2011) Synthesis of carbon-free Si3N4/SiC nanopowders using silica fume. Ceram Int 37:3477–3487
Yao LW, Khan FSA, Mubarak NM, Karri RR, Khalid M, Walvekar R, Abdullah EC, Mazari SA, Ahmad A, Dehghani MH (2022) Insight into immobilization efficiency of lipase enzyme as a biocatalyst on the graphene oxide for adsorption of azo dyes from industrial wastewater effluent. J Mol Liq 354:118849
Zawrah MF, Shereefy El, El Sayed E, Khudir AY (2019) Reverse precipitation synthesis of ≤10 nm magnetite nanoparticles and their application for removal of heavy metals from water. SILICON 11(1):85–104
Zawrah MF, Halkaha MA, Eldessouky MMI, Mohamed GG (2020) Utilization of bagasse waste for production of AC and AC/Fe3O4 composite for removal of dye from wastewater. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-01169-z
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MFZ designed the plan of research work, wrote the article, and discussed the results, MIE-G (supervisor), MS (supervisor), MAE-S (supervisor), and Maged Ahmed (conducted the experiments).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zawrah, M.F., El-Gammal, M.I., Salem, M. et al. Recycling of Rice Husk for Preparation of Activated Carbon/Magnetite Nanocomposites for Removal of Methylene Blue from Wastewater. Int J Environ Res 17, 51 (2023). https://doi.org/10.1007/s41742-023-00542-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-023-00542-w