Abstract
The present work deals with the production of liquid emulsion membrane (LEM) accomplished by applying hydrodynamic cavitation-based process for the extraction of Chromium(VI) from wastewater. Aliquat 336 was used as the carrier during membrane transport. Diesel and Span 80 were used as diluent and emulsifier, respectively. NaOH (as a stripping agent in the internal phase) was used in emulsion preparation with the use of orifice (1 mm diameter)-based hydrodynamic cavitation process. Chromium(VI) removal from heavy metal-containing water was examined with the influence of LEM production time, amount of carrier, surfactant concentration, treat ratio (which is the ratio of feed phase to emulsion membrane phase) and type of diluent. The LEM synthesized using hydrodynamic cavitation process showed the possibility of complete removal of Chromium(VI) from the feed phase containing heavy metals, i.e. Chromium(VI). The optimal conditions were observed to be 2 min emulsification time, 1.00% carrier concentration, 3% surfactant concentration and treat ratio of 1:05. Therefore, the application of hydrodynamic cavitation for the production of LEM with excellent stability can be considered as a novel process for the extraction of Chromium(VI) from wastewater.
Article Highlights
-
Efficient preparation of ELM with Aliquat 336 using hydrodynamic cavitation.
-
97.86% extraction of Cr(VI) in 10 min with prepared LEM by hydrodynamic cavitation.
-
Intensified interfacial mass transfer area of LEM prepared by hydrodynamic cavitation.
-
Intensified extraction of Chromium(VI) from the feed phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewater generated from numerous process industries like electroplating, leather tanning, textile, steel, and mining is found to contain heavy metals (Vetrimurugan et al. 2017). Release of these heavy metals in water bodies has adverse effects on the environment. Heavy metals are highly toxic to all living organisms and inhibit various biological processes occurring in the water (Sandrin and Maier 2003). Amongst the various heavy metals, chromium is predominantly found to be present in trivalent Cr(III) and hexavalent Cr(VI) oxidation states. Cr(VI) is proved to be more poisonous because of its strong oxidizing nature (Goyal et al. 2011). Chromium(VI) can enter the cell membrane exerting its noxious impact on the cell itself and results in cancer (GIL et al. 2006). Acute effects of Cr(VI) are stomach and skin irritation, whereas chronic effects of Cr(VI) are damage to kidney and liver, epigastric pain, dermatitis and nerve tissue damage (Kotaś and Stasicka 2000; Owlad et al. 2009). Consequently, it is essential to eliminate chromium wastewater before releasing it to water bodies.
Several successful attempts of Cr(VI) removal and its recycling have been stated in the literature by various techniques. These approaches are chemical precipitation (Song et al. 2004; Wang et al. 2007), ion exchange (Fan et al. 2013), photo-catalysis (Zhang et al. 2017), non-dispersive solvent extraction (Hafiane et al. 2000), electro-dialysis (Ali Kumbasar 2009), coagulation/flocculation (Song et al. 2004), etc. Major problems like a high initial capital requirement, operational and maintenance cost, metal sludge handling and disposal, a large inventory of solvents and struggle for automation have limited the use of these methodologies (Ali Kumbasar 2009; AL-Othman et al. 2012; Noah et al. 2018). Recently, separation processes based on liquid emulsion membranes have been popular for the separation of heavy metals (Lee 2013; Mokhtari and Pourabdollah 2015; Davoodi-Nasab et al. 2018; Ferreira et al. 2019). Many successful attempts of selective pollutant separation using liquid emulsion membrane (LEM) for pollutants such as dye, metals, and phenols have been reported (Mortaheb et al. 2008; Dâas and Hamdaoui 2010; Kumbasar 2010; Ahmad et al. 2012; Bahloul et al. 2013). The method consists of one-step extraction and stripping of pollutant from the waste water (Goyal et al. 2011). LEM possesses distinct advantages like higher solute diffusion rates through the membrane due to higher interfacial area for mass transfer and wide selectivity (Kumbasar 2010). The success of higher removal efficiency depends mainly on the emulsification method and membrane composition. Zhang et al. have reported the use of hydrodynamic cavitation as an effective way of producing oil in water emulsions below 100 nm size with higher stability. Parbat et al. (2020) have illustrated the use of an efficient method for the preparation of LEM which is a hydrodynamic cavitation based process, which showed almost 100% separation of cobalt(II) from wastewater in substantially less time. The increase in the interfacial area for mass transfer is attributed to the reduction in the droplet size of the LEM produced with the help of hydrodynamic cavitation, which is the main reason for the fast separation. In hydrodynamic cavitation, a moving liquid is made to pass through the orifice or venturi plates to produce the emulsion droplets (Bethi et al. 2017; Kumar et al. 2018). The method produces distinct benefits like lower energy requirement and ease of scale-up (Saharan et al. 2012; Bethi et al. 2016; Zhang et al. 2016). Typical physical factors like microstreaming, intense shearing and turbulence are accountable for the decrease in the droplet size (Bethi et al. 2016; Kumar et al. 2018). Further, this decrease in the droplet size results in substantial enhancement into the interfacial mass transport of the pollutants from the wastewater (i.e. feed phase) to the internal phase (stripping phase).
In view of this, the present investigation deals with the application of hydrodynamic cavitation based process with orifice plate (as constriction geometry) for the creation of small and stable emulsion which is LEM. LEM obtained was further used for the removal of Chromium(VI) from the wastewater (feed phase). Factors like cavitation (emulsification) time, loading of surfactant and carrier, treat ratio and diluent used were studied for obtaining maximum chromium removal.
Experimental
Materials
Analytical grade chemicals were used for membrane preparation and extraction of Chromium(VI) without any further purification. 0.1M NaOH (Merck Ltd, Mumbai) solution was used as an internal phase and commercial grade diesel and kerosene were used as a diluent which was procured from the local market. Span 80 (sorbitan monooleate) having an HLB value of 4.3 was obtained from Merck Ltd, Mumbai, and was used as an emulsifier. The carrier used for Chromium(VI) transport was Aliquat 336 (Hi-Media Lab. Pvt. Ltd., Mumbai). Potassium dichromate was employed for the production of feed solution which was procured from S. D. Fine-Chem Limited, Mumbai, India. All the experiments were performed using distilled water.
LEM Preparation Experimental Setup
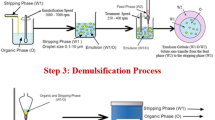
Hydrodynamic cavitation applied for the production of LEM is as represented in Fig. 1. The hydrodynamic cavitation setup comprising of 1 lit holding tank equipped with a centrifugal pump (1.28 kW, 2800 rpm) was used. Stainless steel pipe fittings were used in the fabrication of cavitation set-up. The orifice plate (1 mm hole diameter) was fixed on the mainline. To avoid leakages, the flanges and the gasket were provided on the mainline.
The bypass line was provided with a control valve to obtain the required flow and the pressure upstream of the orifice. Pressure gauges were provided to record the upstream and downstream pressure of the orifice. LEM preparation was accomplished in this hydrodynamic cavitation setup at various emulsification times, which was further applied for Chromium(VI) removal from wastewater. Hydrodynamic cavitation results in the sudden rise in membrane temperature which was subsequently decreased to room temperature by providing an ice bath.
Hydrodynamic Cavitation-Based Process for the Production of W/O Emulsion (LEM)
A similar procedure reported by Parbat et al. (Parbat et al. 2020) for the production of LEM assisted with hydrodynamic cavitation was applied. The selected constituents for the production of membrane phase were carrier Aliquat 336 (with 0.5–1.5% v/v concentrations) with surfactant Span 80 (at varied concentrations 2.0, 3.0 and 4.0% v/v) in diluent (i.e. Diesel and kerosene). A three-blade turbine was used to mix these constituents for 10 min to form a 600-mL membrane phase which is a homogeneous mixture or coarse emulsion and was shifted to holding tank of experimental setup. Then, the addition of 200-mL internal aqueous phase, which was 0.1 M NaOH aqueous solution, was steadily accomplished to the holding tank containing membrane phase having internal to membrane phase ratio of 1:3 (by volume). Further, the centrifugal pump was started with a fully opened valve located on the by-pass line, which results in the complete distribution of the internal phase (stripping phase) into the membrane phase. Initially, a light milky solution was obtained then the by-pass line valve was closed which resulted in the efficient cavitation process for the preparation of LEM. The cavitation time for the preparation of LEM was selected up to 10 min and LEM samples were withdrawn at 2-min intervals from the holding tank. These samples were cooled to 25 °C and then used for the Chromium(VI) removal from wastewater (feed phase).
Chromium (VI) Removal from Wastewater (feed phase) Using LEM Prepared by Hydrodynamic Cavitation Process
The Chromium(VI) removal was accomplished from wastewater (feed phase). 100 ppm Chromium(VI) solution (feed phase) was prepared using potassium dichromate. 6 M HCl solution was gradually added to feed solution under magnetic stirring and pH of the feed solution (measured with pH meter) was maintained between 0.5 and 2.0 since chromium is active in the acidic medium. The addition of prepared LEM by hydrodynamic cavitation to the wastewater (feed phase) was accomplished in the varied ratio (e.g. 1:3, 1:5, 1:10 and 1:15) named treat ratio. A three-blade impeller was used for mechanically agitating the resultant mixture at a speed of 400 rpm. All the Chromium(VI) removal experiments were carried out by maintaining pH less than 2 resulting in creating pH differences in external and internal phases. This subsequently initiated a driving force for chromium diffusion (Goyal et al. 2011). Aliquots were withdrawn after a periodic interval of 2 min for 10 min, then the settling of the samples was accomplished with the help of separating funnel. This was done to provide two separate phases (i.e. treated water and separated emulsion phase comprising concentrated Chromium(VI) ions). Then the clear water phase was analyzed by atomic absorption spectrophotometer (Shimadzu AA-6701F model) and UV–Vis spectrophotometer (LABINDIA Analytical UV3200 model) to determine the extraction efficiency. The % removal efficiency of Chromium(VI) was estimated accordingly to
where C0 is the Chromium(VI) concentration (mg/L) at t = 0, CT is the concentration (mg/L) of Chromium(VI) in the samples at any time t
Results and Discussion
Facilitated Transport Mechanism Chromium(VI) Removal Using Aliquat 336
Aliquat 336 (R4N+Cl−) is oil soluble and is formed by methylation of mixed tri-octyl/decyl amine. The permanent positive charge present on quaternary ammonium structure (R4N+) has the ability to form salts with anions in acidic or slightly alkaline pH environment. Also at low pH, it has the ability to generate oil-soluble salts of anionic species. The presence of basic Nitrogen in Aliquat 336 can produce amine salts by reacting with organic and inorganic salts. These salts can further undergo ion exchange reactions with a variety of other anions (Kumbasar 2008). The reaction of Carrier Aliquat 336 (R4NCl) with NaOH (internal phase) is represented in following Eq. (2) which is well reported by Bhowal and Datta (2001) and Galan et al. (1994)
Further, as per the report of Bhowal and Datta (2001) and Kumbasar (2008), the chromate ions possibly are present in the aqueous medium in various ionic forms like \({\text{HCrO}}_{4}^{ - }\), \({\text{CrO}}_{4}^{2 - }\), \({\text{Cr}}_{2} {\text{O}}_{7}^{2 - }\), and \({\text{HCr}}_{2} {\text{O}}_{7}^{ - }\). Further, it has been reported that the total amount of chromium and the pH decide which actual chromium species will predominate in the aqueous phase. \({\text{CrO}}_{4}^{2 - }\) anion present in basic or marginally acidic solution while \({\text{Cr}}_{2} {\text{O}}_{7}^{2 - }\) anions dictate in acidic Cr(VI) aqueous solution. Moreover, \({\text{Cr}}_{2} {\text{O}}_{7}^{2 - }\) convert into \({\text{HCrO}}_{4}^{ - }\) anions in acidic aqueous solution at a lower concentration of Cr(VI). Therefore, in the present investigation, chromate ions will be present as \({\text{HCrO}}_{4}^{ - }\) in the external continuous phase at low initial concentration of Cr(VI). This reaction (2) indicates the presence of two carriers R4NCl and R4NOH–. Then both carriers R4NCl and R4NOH– combines with chromium ions at the outside edge of membrane–feed phase and form chromium–ammonium group complex as shown in
Further, to complete the stripping reaction in the stripping phase (internal membrane phase), the complex diffuses from the interface of the wastewater (feed phase) through the membrane phase. Sodium hydroxide present in the stripping (internal) phase reacts with the chromium complex. This discharges the chromium ions into the stripping (internal) phase. Further through the membrane phase, the H+ ion diffuses back to the wastewater (feed phase). The striping reaction similarly restores the carrier, because of less solubility of the carrier in the water phase which diffuses across the membrane phase.
Overall, the formed complex, i.e. \({\text{NR}}_{4} {\text{HCrO}}_{4}\) diffuses across the membrane to the interface between the internal and membrane phase where it reacts with the internal reagent (sodium hydroxide) to release \({\text{HCrO}}_{4}^{ - }\) anion and the carrier (Kebiche-Senhadji et al. 2010). In the internal droplets, \({\text{HCrO}}_{4}^{ - }\), \({\text{CrO}}_{4}^{2 - }\) and \({\text{Cr}}_{2} {\text{O}}_{7}^{2 - }\) anions exist in equilibrium following the reactions given below:
Figure 2 indicates the transport mechanism of the carrier. The figure indicates the use of carrier for the Chromium(VI) ion transport to the internal phase. This process occurs from the feed phase (wastewater) through the membrane phase.
Influence of Cavitation Time on Removal Efficiency of Chromium(VI) in the Feed Phase
Hydrodynamic cavitation is able to provide more internal energy at less input and also produces uniform emulsion droplets. With an increase in cavitation time, the emulsion temperature increases (which was controlled with the coolant). This is due to the mechanical dissipations produced during the flow through an orifice plate. This further results in decreasing the interfacial tension and viscosity of the emulsion. As a result, this reduced viscosity produces a violent collapse of cavities to produce higher cavitation intensity and dispersion rates. During LEM preparation using hydrodynamic cavitation study, 600-mL organic phase contained 4% Span 80, 1.0% Aliquat 336 and rest diesel (all compositions were percent volume basis) was used. The internal phase (200 mL) comprised of 0.1 M NaOH was taken to maintain the internal (stripping) phase to an organic (membrane) phase ratio of 1:3. The LEM prepared with hydrodynamic cavitation process was applied further for the removal of Cr(VI) from 100 ppm Cr(VI) feed solution with a ratio of 1:5 (LEM/Feed phase) in the presence of mechanical stirring. Samples were drawn periodically (2 min) using a syringe for 10 min (overall time of removal process). The Cr(VI) removal efficiency for various cavitation times is as shown in Fig. 3. The figure indicates a marginal reduction in Cr(VI) extraction efficiency with LEM prepared with higher cavitation (processing) time.
Effect of LEM prepared at various cavitation (emulsification) time and with 1 mm orifice size on the removal of Chromium(VI). ([NaOH] = 0.1 M; Span 80 = 4% (v/v); [Aliquat 336] = 1.0% (v/v); Internal to membrane phase ratio (I/O) = 1:3; Diluent = Diesel; [Treat Ratio] = 1:5; agitation speed = 400 rpm)
For the membrane synthesis with 2-min synthesis time, the removal efficiency was observed to be 99.19%, which was found decreased to 93.17% for the LEM prepared by 10-min cavitation time. This may be due to an increase in internal globule size which is a result of more cavitation time that gives rise to re-coalescence. Further, as reported by Parbat et al. (2020), the use of hydrodynamic cavitation for the synthesis of LEM showed a reduction in the size of the emulsion which results in a considerably faster extraction process. Microstreaming, intense shearing and turbulence resulting because of hydrodynamic cavitation were found to be responsible for increased mass transfer area of LEM; this subsequently resulted in the higher Chromium(VI) removal.
Effect of Carrier Loading on Chromium(VI) Extraction Efficiency
The economy of LEM strongly depends on the carrier concentration. This is because of the carrier being the costliest among all the chemicals being used in the experimental investigation. An extractant or carrier is used to provide a huge quantity of carrier molecules at the existing interface of Cr(VI) ions. This also leads to a reduction in the reaction time and more number of extraction-stripping cycles are possible (Valenzuela et al. 2009). To investigate the influence of carrier loading on the Cr(VI) removal efficiency, Aliquat 336 loading was changed from 0.5 to 1.5%, keeping the remaining experimental conditions constant. Figure 4 depicts the influence of carrier loading on the removal efficiency of Chromium(VI) from wastewater (feed phase). The removal efficiency of Aliquat 336 with 0.5%, 1.0%, and 1.5% concentration was found to be 80.90%, 97.85%, and 95.65%, respectively, with 10-min extraction time and the LEM prepared with 6-min cavitation time. The increased removal efficiency for 1.0% was due to the higher loading of the carrier resulting to fomation of a larger amount of the extractant complexes at the donor-membrane interface. The higher concentration of carrier in the membrane phase results in the formation of more Cr–carrier complex at the exterior of the membrane phase and external feed (wastewater) phase interface that expedites the transportation of the Chromium(VI) ions to the innermost interface of stripping and membrane phase that enhances the extraction efficiency. Further increase in the concentration of Aliquat 336–1.5% (v/v) resulted in a decrease in removal efficiency of Chromium(VI) to 95.65%. This is attributed to an increase in the Aliquat 336, which increases the viscosity of the membrane phase and making Cr(VI) remain in complex form resulting in a decrease in the diffusion coefficient of extracted species (Ali Kumbasar 2009; Zaheri and Davarkhah 2017). The application of hydrodynamic cavitation in the preparation of LEM improves the interfacial area for mass transfer which further increases the Chromium(VI) extraction and was observed to be higher.
Influence of Surfactant (Span 80) Concentration on Chromium(VI) removal
To form an emulsion with enhanced stability, the essential component surfactant is used and it acts as an emulsifying agent (Kumbasar 2010) during the production of LEM with the application of hydrodynamic cavitation. The influence of LEM prepared with various concentrations of surfactant Span 80 using hydrodynamic cavitation on Chromium(VI) extraction efficiency is represented in Fig. 5. The concentration of Span 80 was changed from 1 to 5% (v/v) during LEM production with the use of hydrodynamic cavitation. The reported results indicate enhancement in the removal efficiency from 81.90 to 97.86% with the use of produced LEM with increased loading of surfactant from 1 to 3%, respectively. This is attributed to the improved stability of LEM prepared with the hydrodynamic cavitation process due to increased surfactant concentration. Further, the figure also indicates the decreased extraction efficiency to 77.65% with the use of LEM prepared with higher loading (5%) Span 80. This may be because of increased concentration of surfactant which enhances mass transfer resistance for the transfer of Chromium(VI) from the wastewater (feed phase) to the internal (stripping) phase that further induces osmotic swelling resulting in reduced removal efficiency. The phenomenon of swelling is a consequence of the water molecules getting transported from wastewater (feed phase) to the internal (stripping) phase. This transport is through surfactant hydration, micelles, and reverse micelles (Shen et al. 1996; Goyal et al. 2011) at a higher concentration of surfactant. Ultimately, swelling yields the breakage of emulsion followed by transport of Cr(VI) ion in the feed phase. Therefore, LEM prepared with 3% Span 80 surfactant concentration showed 97.85% removal efficiency which is found to be optimum to produce a stable LEM.
Effect of surfactant (Span 80) concentration (%v/v) on the removal of Chromium(VI) ([NaOH] = 0.1 M; [Aliquat 336] = 1.0% (v/v); internal to membrane phase ratio (I/O) = 1:3; diluent = diesel; LEM prepared with 6 min emulsification time and with 1 mm orifice size; [treat ratio] = 1:5; agitation speed = 400 rpm)
Influence of LEM to Feed Phase (wastewater) Ratio on Chromium(VI) Extraction Efficiency
The effect of LEM on the feed phase (wastewater) is referred to as a treat ratio and is useful to determine the economics of the process. As per the process economics, usage of the least volume of emulsion membrane is always preferred for the treatment of the higher amount of feed water. To elucidate the effect of ratio, experiments were performed by varying the treat ratio from 1:3 to 1:15, maintaining LEM volume constant. In the investigation of the effect of treat ratio, an increase in feed phase decreases the probability of swelling and in turn membrane breakage. Also, this provides effective chromium removal because of higher Cr(VI) ion concentration for each globule at the interface. Figure 6 depicts the behavior of LEM to feed phase ratio on the percentage extraction efficiency of Chromium(VI). The resultant percentage chromium extraction efficiency enhanced from 86.87 to 97.86% when the treat ratio was found to be increased from 1:3 to 1:5, respectively. Further, increase in the treat ratio to 1:10 and 1:15, the percentage removal efficiency was found to be reduced to 57.57 and 51.85%, respectively. This decrease in removal efficiency was because of two probable reasons. One, the higher quantity of water in the feed phase resulted in higher swelling behavior resulting in membrane rupture. Another reason may be a large amount of water that consumed all the internal reagents required to react with the transported complex (Goyal et al. 2011).
Effect of LEM to feed phase ratio (treat ratio) on the removal of Chromium(VI). ([NaOH] = 0.1 M; Span 80 = 4% (v/v); [Aliquat 336] = 1.0% (v/v); internal to membrane phase ratio (i/o) = 1:3; diluent = diesel; LEM prepared with 6 min emulsification time and with 1 mm orifice size; agitation speed = 400 rpm)
This is also due to a decrease in the amount of LEM at enhanced treat ratio which further reduces the number of carrier ions. Consequently, the decline in the removal/extraction of Chromium(VI) from the wastewater (feed phase) takes place. Also, the higher treat ratio is responsible for the increase in the number of Chromium(VI) ions in the limited number of LEM globules causing considerable swelling of the LEM globule. This process is more responsible for the leakage of Chromium(VI) ions back in the feed phase and thereby reduction in the removal/extraction efficiency was observed. Further, substantial decrement in the interfacial mass transfer area was observed at a higher treat ratio, which also is another reason for the reduction in the extraction efficiency.
Influence of LEM Produced with Diverse Diluents on the Chromium(VI) % Extraction Efficiency
Permeability and thickness of LEM are governed by two factors, namely density and viscosity of diluents. The type of diluent used in the preparation of LEM decides the stability of LEM and then Chromium(VI) extraction efficiency. The diluents used in the present study are diesel and kerosene for the preparation of LEM using the hydrodynamic cavitation process. The density of diesel (832 kg/m3) and kerosene (780–810 kg/m3) at 20 °C is nearly the same and also the viscosity 1.3–2.4 and 1.3 centipoise, respectively. Therefore, the properties of these diluents are nearly the same and so showed similar trends in the Chromium(VI) extraction efficiency. In the present study, the percentage extraction was found to be 97.86% and 97.43% with the use of diesel and kerosene diluents in the membrane synthesis using the hydrodynamic cavitation process (Fig. 7). This is attributed to the nearly similar properties i.e. viscosity and density of diesel and kerosene diluent.
Effect of type of diluent in the preparation LEM by hydrodynamic cavitation on the removal of Chromium(VI). ([NaOH] = 0.1 M; Span 80 = 4% (v/v); [Aliquat 336] = 1.0% (v/v); internal to membrane phase ratio (I/O) = 1:3; diluent = diesel; LEM prepared with 6 min emulsification time and with 1 mm orifice size; [treat ratio] = 1:5; agitation speed = 400 rpm)
Conclusions
In the present study, the successful synthesis of LEM was achieved by employing the use of orifice (1 mm)-based hydrodynamic cavitation process in very less time (optimal cavitation time = 6 min). For optimal condition, 97.86% Chromium(VI) removal from the wastewater (aqueous feed phase) was observed. The optimal conditions were 1.0% (v/v) Aliquat 336, 3% (v/v) Span 80, 0.1 M NaOH, 1:3 internal (stripping) to membrane phase ratio, 1:5 treat ratio and 6 min emulsification (processing) time with the application of hydrodynamic cavitation. The application of the hydrodynamic cavitation process for the production of LEM showed a higher extraction of Chromium(VI) in very less time. This intensifies the process of extraction of Chromium(VI) from the feed phase. Further, the concentrated Cr(VI) strip solution can be treated with alternative separation techniques like membrane separation/filtration for recovery of costly and toxic Cr(VI) materials.
References
Ahmad AL, Kusumastuti A, Derek CJC, Ooi BS (2012) Emulsion liquid membrane for cadmium removal: studies on emulsion diameter and stability. Desalination 287:30–34. https://doi.org/10.1016/j.desal.2011.11.002
Ali Kumbasar R (2009) Extraction of Chromium(VI) from multicomponent acidic solutions by emulsion liquid membranes using TOPO as extractant. J Hazard Mater 167:1141–1147. https://doi.org/10.1016/j.jhazmat.2009.01.113
AL-Othman ZA, Ali R, Naushad R (2012) Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: adsorption kinetics, equilibrium and thermodynamic studies. Chem Eng J 184:238–247. https://doi.org/10.1016/j.cej.2012.01.048
Bahloul L, Ismail F, Samar ME (2013) Extraction and desextraction of a cationic dye using an emulsified liquid membrane in an aqueous solution. Energy Procedia 36:1232–1240. https://doi.org/10.1016/j.egypro.2013.07.139
Bethi B, Sonawane SH, Rohit GS et al (2016) Investigation of TiO 2 photocatalyst performance for decolorization in the presence of hydrodynamic cavitation as hybrid AOP. Ultrason Sonochem 28:150–160. https://doi.org/10.1016/j.ultsonch.2015.07.008
Bethi B, Sonawane SH, Potoroko I et al (2017) Novel hybrid system based on hydrodynamic cavitation for treatment of dye waste water: A first report on bench scale study. J Environl Chem Eng 5:1874–1884. https://doi.org/10.1016/j.jece.2017.03.026
Bhowal A, Datta S (2001) Studies on transport mechanism of Cr(VI) extraction from an acidic solution using liquid surfactant membranes. J Membr Sci 188:1–8
Dâas A, Hamdaoui O (2010) Extraction of anionic dye from aqueous solutions by emulsion liquid membrane. J Hazard Mater 178:973–981. https://doi.org/10.1016/j.jhazmat.2010.02.033
Davoodi-Nasab P, Rahbar-Kelishami A, Safdari J, Abolghasemi H (2018) Evaluation of the emulsion liquid membrane performance on the removal of gadolinium from acidic solutions. J Mol Liq 262:97–103. https://doi.org/10.1016/j.molliq.2018.04.062
Fan Y, Wang X, Wang M (2013) Separation and recovery of chromium and vanadium from vanadium-containing chromate solution by ion exchange. Hydrometallurgy 136:31–35. https://doi.org/10.1016/j.hydromet.2013.03.008
Ferreira LC, Ferreira LC, Cardoso VL, Filho UC (2019) Mn(II) removal from water using emulsion liquid membrane composed of chelating agents and biosurfactant produced in loco. J Water Process Engg 29:100792. https://doi.org/10.1016/j.jwpe.2019.100792
Galan B, Urtiaga AM, Alonso AI et al (1994) Extraction of anoins with Aliquat 336: chemical equilibrium modelling. Ind Eng Chem Res 33:1765
Gil R, Cerutti S, Gasquez J et al (2006) Preconcentration and speciation of chromium in drinking water samples by coupling of on-line sorption on activated carbon to ETAAS determination. Talanta 68:1065–1070. https://doi.org/10.1016/j.talanta.2005.06.069
Goyal RK, Jayakumar NS, Hashim MA (2011) Chromium removal by emulsion liquid membrane using [BMIM]+[NTf2]− as stabilizer and TOMAC as extractant. Desalination 278:50–56. https://doi.org/10.1016/j.desal.2011.05.001
Hafiane A, Lemordant D, Dhahbi M (2000) Removal of hexavalent chromium by nanofiltration. Desalination 130:305–312. https://doi.org/10.1016/S0011-9164(00)00094-1
Kebiche-Senhadji O, Tingry S, Seta P et al (2010) Selective extraction of Cr(VI) over metallic species by polymer inclusion membrane (PIM) using anion (Aliquat 336) as carrier. Desalination 258:59–65
Kotaś J, Stasicka Z (2000) Chromium occurrence in the environment and methods of its speciation. Environ Pollut 107:263–283. https://doi.org/10.1016/S0269-7491(99)00168-2
Kumar MS, Sonawane SH, Bhanvase BA, Bethi B (2018) Treatment of ternary dye wastewater by hydrodynamic cavitation combined with other advanced oxidation processes (AOP’s). J Water Process Eng 23:250–256. https://doi.org/10.1016/j.jwpe.2018.04.004
Kumbasar RA (2008) Studies on extraction of Chromium(VI) from acidic solutions containing various metal ions by emulsion liquid membrane using Alamine 336 as extractant. J Membr Sci 325:460–466. https://doi.org/10.1016/j.memsci.2008.08.009
Kumbasar RA (2010) Selective extraction and concentration of chromium(VI) from acidic solutions containing various metal ions through emulsion liquid membranes using Amberlite LA-2. J Ind Eng Chem 16:829–836. https://doi.org/10.1016/j.jiec.2010.05.004
Lee SC (2013) Development of an emulsion liquid membrane system for removal of acetic acid from xylose and sulfuric acid in a simulated hemicellulosic hydrolysate. Sep Purif Technol 118:540–546. https://doi.org/10.1016/j.seppur.2013.07.032
Mokhtari B, Pourabdollah K (2015) Emulsion liquid membrane for selective extraction of Bi(III). Chin J Chem Eng 23:641–645. https://doi.org/10.1016/j.cjche.2014.06.035
Mortaheb HR, Amini MH, Sadeghian F et al (2008) Study on a new surfactant for removal of phenol from wastewater by emulsion liquid membrane. J Hazard Mater 160:582–588. https://doi.org/10.1016/j.jhazmat.2008.03.095
Noah NFM, Jusoh N, Othman N et al (2018) Development of stable green emulsion liquid membrane process via liquid–liquid extraction to treat real chromium from rinse electroplating wastewater. J Ind Eng Chem 66:231–241. https://doi.org/10.1016/j.jiec.2018.05.034
Owlad M, Aroua MK, Daud WAW, Baroutian S (2009) Removal of hexavalent chromium-contaminated water and wastewater: a review. Water Air Soil Pollut 200:59–77. https://doi.org/10.1007/s11270-008-9893-7
Parbat SA, Bhanvase BA, Sonawane SH (2020) Investigation on liquid emulsion membrane (LEM) prepared with hydrodynamic cavitation process for cobalt (II) extraction from wastewater. Sep Purif Technol 237:116385. https://doi.org/10.1016/j.seppur.2019.116385
Saharan VK, Pandit AB, Satish Kumar PS, Anandan S (2012) Hydrodynamic cavitation as an advanced oxidation technique for the degradation of acid red 88 dye. Ind Eng Chem Res 51:1981–1989. https://doi.org/10.1021/ie200249k
Sandrin TR, Maier RM (2003) Impact of metals on the biodegradation of organic pollutants. Environ Health Perspect 111:1093–1101
Shen J-Q, Yin W-P, Zhao Y-X, Yu L-J (1996) Extraction of alanine using emulsion liquid membranes featuring a cationic carrier. J Membr Sci 120:45–53. https://doi.org/10.1016/0376-7388(96)00158-5
Song Z, Williams CJ, Edyvean RGJ (2004) Treatment of tannery wastewater by chemical coagulation. Desalination 164:249–259. https://doi.org/10.1016/S0011-9164(04)00193-6
Valenzuela F, Araneda C, Vargas F et al (2009) Liquid membrane emulsion process for recovering the copper content of a mine drainage. Chem Eng Res Des 87:102–108. https://doi.org/10.1016/j.cherd.2008.05.010
Vetrimurugan E, Brindha K, Elango L, Ndwandwe OM (2017) Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Appl Water Sci. https://doi.org/10.1007/s13201-016-0472-6
Wang LK, Dahm DB, Baier RE, Ziegler RC (2007) Treatment of tannery effluents by surface adsorption. J Appl Chem Biotech 25:475–490. https://doi.org/10.1002/jctb.5020250610
Zaheri P, Davarkhah R (2017) Rapid removal of uranium from aqueous solution by emulsion liquid membrane containing thenoyltrifluoroacetone. J Environ Chem Eng 5:4064–4068. https://doi.org/10.1016/j.jece.2017.07.076
Zhang Z, Wang G, Nie Y, Ji J (2016) Hydrodynamic cavitation as an efficient method for the formation of sub-100 nm O/W emulsions with high stability. Chin J Chem Eng 24:1477–1480. https://doi.org/10.1016/j.cjche.2016.04.011
Zhang F, Zhang Y, Zhou C et al (2017) A new high efficiency visible-light photocatalyst made of SnS 2 and conjugated derivative of polyvinyl alcohol and its application to Cr(VI) reduction. Chem Eng J 324:140–153. https://doi.org/10.1016/j.cej.2017.05.009
Acknowledgements
This work was funded under Research Promotion Scheme [Sanction order no. 8-102/FDC/RPS(policy-1)/2019-20 dated 14th August 2020] by All India Council For Technical Education (AICTE), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Bolne, P.C., Ghodke, S.A. & Bhanvase, B.A. Intensified Hydrodynamic Cavitation-Based Process for the Production of Liquid Emulsion Membrane (LEM) for the Extraction of Chromium(VI) Ions. Int J Environ Res 15, 313–320 (2021). https://doi.org/10.1007/s41742-021-00322-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-021-00322-4