Abstract
Ultrasound-assisted emulsification–microextraction followed by inductively coupled plasma–optical emission spectrometry was utilized for simultaneous pre-concentration and trace detection of lead, bismuth, and iron in water samples. Disodium N,N\(^\prime\)-bis(salicylidene)ethylenediamine and trichloroethylene were used as chelating agent and extraction solvent, respectively. The parameters of interest were volume of extraction solvent, temperature, and concentrations of salt and chelating agent. A fractional factorial design was developed to identify these parameters and how they vary with one another. The results demonstrated that the concentrations of salt and chelating agent affected extraction efficiency. Subsequently, a central-composite design was used to acquire optimum levels of effective parameters. The optimal conditions were 160.7 mg L\(^{-1}\) and 1.77% (w/v) for concentration of chelating agent and salt, respectively. The linear dynamic ranges were determined to be 1–1000 \(\upmu\)g L\(^{-1}\) for Pb and Bi, and 10–1000 \(\upmu\)g L\(^{-1}\) for Fe. The correlation coefficient (\(R^2\)) was 0.990–0.995. The limits of detection were 0.54–0.78 \(\upmu\)g L\(^{-1}\). The relative standard deviation (concentration \(=\) 200 \(\upmu\)g L\(^{-1}\), \(n = 8\)) was in the range of 2.0–4.3%. This method was successfully applied for the trace detection of Pb, Bi, and Fe in freshwater samples of waterfall and spring and satisfactory relative recoveries (96.2–99.6%) were obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals have the potential to be caustic to human health, non-biodegradable, and often than not accumulate in vital human organs, where they can negatively affect the body over a long period of time. Urbanization, industrial development, and heavy traffic lead to contamination of waters with heavy metals [1]. Among the heavy metals, lead is one of the most perilous ones, and in the recent years, concerns have increased over the concentration of lead in drinking and natural waters. Lead has reportedly given rise to harmful effects in the body such as reduction of enzymatic activity, kidney dysfunction, and neuromuscular difficulties [2]. Bismuth has been used in pharmaceuticals for the treatment of Helicobacter pylori-induced gastritis. While bismuth was proven useful for remedying symptoms of gastritis, negative effects on human health have been reported. These side effects include nephropathy, osteoarthropathy, hepatitis, and disorders of the nervous system. The widespread use of bismuth in pharmaceuticals has led to an increased exposure by the environment [3]. Iron is vital to most living organisms. It is an essential part of hemoglobin: the red coloring agent of the blood that transports oxygen through our bodies. A high concentration of iron, however, can be harmful, because it is reversibly oxidized and reduced. This property, while essential for its metabolic functions, makes iron potentially hazardous because of its ability to participate in the generation of powerful oxidant species such as hydroxyl radical [4]. Numerous studies have assessed the association between the iron status and risk of coronary heart disease. A hereditary haemochromatosis or iron-overload condition has been identified as an independent risk factor for myocardial infarction and cardiovascular mortality [5]. Inductively coupled plasma–optical emission spectrometry (ICP–OES) is recognized as a sensitive technique for the trace detection of heavy metals. However, in complex environmental samples, the low concentration of heavy metals (\(\upmu\)g L\(^{-1}\) levels) together with the high concentration of interfering matrix components often requires an enrichment step combined with a matrix separation for an accurate, sensitive, and precise determination of heavy metals. The development of conventional liquid–liquid extraction (LLE) and solid-phase extraction (SPE) methods was limited [6] due to disadvantages such as large solvent consumption, large secondary wastes, time intensive, and complex equipment. Solid-phase microextraction (SPME) [7, 8] and liquid-phase microextraction (LPME) were developed to address these disadvantages [9,10,11]. Although SPME is a solvent-free process developed by Arthur and Pawliszyn, it is expensive, its fibers are fragile and have limited lifetimes. A major concern with using this method is sample carry-over [12]. Cloud point extraction (CPE) uses surfactants for the extraction of materials. While there are many benefits to using CPE, surfactant selection poses a problem for complex mixtures analyzed by analytical techniques such as GC and HPLC [13, 14]. In addition, the use of anionic surfactants often require salts and adjustments of pH [15]. A novel and high-performance microextraction technique termed dispersive liquid–liquid microextraction (DLLME) has been introduced by Assadi et al. [11]. In this method, an appropriate mixture of the extraction solvent and the disperser solvent is injected into the aqueous sample and a cloudy solution is formed. The cloudy state results from the formation of fine droplets of extraction solvent which is dispersed in the sample solution. The cloudy solution is centrifuged and the fine droplets are settled at the bottom of the conical test tube. The analytes of interest are extracted from the initial solution. Determination of analytes in the settled phase can be performed by conventional analytical techniques. The combination of DLLME and ultrasound radiations provides an efficient pre-concentration technique known as ultrasound-assisted emulsification–microextraction (USAEME) for detecting trace levels of analyte, developed by Regueiro et al. [16]. Ultrasonic (US) radiation is an efficient tool to facilitate the emulsification phenomenon and accelerate the mass-transfer process between two immiscible phases. The main difference between USAEME and DLLME is that USAEME does not require a disperser solvent. The absence of a disperser solvent enhances the extraction efficiency, effectively reducing the time required for sample preparation. In this work, USAEME has been optimized for the simultaneous microextraction and trace detection of Pb, Bi, and Fe in freshwater samples. This technique relies on the ability of Bi(II), Pb(II), and Fe(II) to form complexes with disodium N,N\(^\prime\)-bis(salicylidene)-ethylenediamine. USAEME was used to extract the complexes of interest, followed by analysis via inductively coupled plasma–optical emission spectrometry. Potential parameters affecting the USAEME and analytical performance were studied and optimized in detail. To the best of our knowledge, this is the first report concerning Disodium N,N\(^\prime\)-bis (salicylidene)-ethylenediamine as a complexing agent for simultaneous speciation of heavy metals using the USAEME method.
2 Experimental
2.1 Apparatus
A radial view Varian Vista-MPX simultaneous inductively coupled plasma-optical emission spectrophotometer (Australia) coupled to a slurry nebulizer and charge coupled device was utilized for the trace detection of heavy metal ions. The instrument parameters and selected wavelength for Bi, Pb, and Fe are summarized in Table 1. The pH levels of the solutions were measured with a 691 Metrohm pH-meter (Metrohm AG company, Herisau, Switzerland). Centrifuges were performed by a Hermel-Z 200A (Wehingen-Germany) and a furnace (Heraeus GMBH, Germany) was used for drying the precipitate phase.
2.2 Reagents and Standard Solutions
Analytical grade reagents and doubly distilled deionized water were used throughout all experiments. The complexing agent was synthesized by Dr. Salavati Niasari et al., at the University of Kashan, Iran. Other chemicals, such as tetrachloroethylene, chloroform, chlorobenzene, carbon tetrachloride, trichloroethylene, 1,2-dichloroethane, methanol, sodium chloride, bismuth Subnitrate, lead(II) nitrate, and Fe wire with the purity higher than \(99\%\) were purchased from Merck (Darmstadt, Germany). Standard stock solution of Bi(III), Pb(II), and Fe(III) (1000 mg L\(^{-1}\)) was prepared by dissolving appropriate amount of pure grade of Bi\(_5\)O(OH)\(_9\)(NO\(_3\))\(_4\), Pb(NO\(_3\))\(_2\), and Fe wire, respectively, in 1 mol L\(^{-1}\) nitric acid. The standard working solution was diluted daily prior to use. The laboratory glassware was kept in \(8\%\) nitric acid for at least 24 h and subsequently washed with doubly deionized water.
2.3 USAEME Procedure
A 10 mL aqueous solution containing 200 \(\upmu\)g L\(^{-1}\) of Bi(III), Pb(II), and Fe(III) was first prepared and its pH adjusted to 7.0 in a screw cap glass test tube. Next, an 0.8 mL solution of \(2.0 \times 10^3\) mg L\(^{-1}\) complexing agent was added. The glass tube was shaken on a glass stirrer to homogenize the solution. The tube was then immersed in an ultrasonic bath, and \(100~ \upmu\)L of trichloroethylene (extraction solvent) was injected into it within 2 min using a \(100~ \upmu\)L Hamilton syringe. The test tube was kept in the ultrasonic bath for 8 min between sample runs. The resulting emulsion in the test tube was centrifuged at 4000 rpm for 6 min. The sedimentary organic phase at the bottom of the test tube was separated from the aqueous phase and evaporated at \(80\,^\circ\)C in an oven. Finally, the residue resulting from the evaporation of the organic phase was dissolved in 1 mL of 1 M nitric acid and then Bi, Pb, and Fe concentrations were determined by ICP–OES.
2.4 Sample Preparation
Freshwater samples were collected in polytetrafluoroethylene (PTFE) tubes and their pH were adjusted to 7.0. Spring water was collected from spring of Fin garden, Kashan, Iran. Waterfall water was collected from Niasar waterfall, Kashan, Iran.
3 Results and Discussion
3.1 Selection of Extraction Solvent
In the USAEME method of extraction, the solvent should have desirable characteristics such as: the ability to efficiently extract the targeted compounds, have a higher density than water, form a stable cloudy solution, and have low solubility in water. Therefore, in this step, C\(_2\)Cl\(_4\), C\(_2\)HCl\(_3\), CCl\(_4\), C\(_6\)H\(_5\)Cl, and C\(_2\)H\(_4\)Cl\(_2\) were investigated as the extraction solvents. Among these solvents, trichloroethylene showed the highest efficiency, and was, therefore, selected as the extraction solvent (Fig. 1).
3.2 The Effect of pH is an Important Factor for Metal Extraction
pH was investigated by one-factor-at-a-time method. The effect of pH versus recovery is shown in Fig. 2. The complexing agent is a phenolic salt which can be dissolved in water; however, in acidic pH, its ionic form converts to its phenolic organic form which is water immiscible, leading to a small rate of recovery. The optimum pH with the highest rate of recovery is demonstrated to be 7.0. It is also desirable not to have optimum pH at very basic pH ranges, since the recovery of extractions with the chelating agent at high levels of pH may be interfered by precipitation of metals with hydroxyl anion. In this work, we want the recoveries to be dependent upon complexation of the metals with the chelating agent not any other phenomenon. As a result, the chelating agent (disodium N,N\(^\prime\)-bis(salicylidene)ethylenediamine) is especially useful for the extraction of related metals, since the optimum pH (7.0) is not basic at all. Therefore, the optimum pH was selected to be 7.0 for all the next experiments.
3.3 Optimization of Ultrasound-Assisted Emulsification–Microextraction
The use of multivariate experimental design techniques is becoming increasingly widespread in analytical chemistry. Multivariate designs, which allow the simultaneous study of several control variables, are faster to implement and are more cost-effective than traditional univariate approaches [17].
3.3.1 Factorial Design
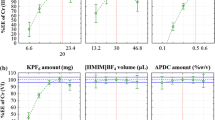
To detect influential factors, experimental designs for first-order models (factorial designs or Plackett–Burman designs) can be used. The most frequently used design with multiple factors is a factorial design. The possible combinations (\(=2^f\), f is the number of variables) of factor levels are used in a full factorial design. High-order interaction effects emerge when f is greater than 3 or 4, making them not to be important, since as the order of an interaction increases, it becomes less likely to be significant. Therefore, using a full factorial would not be necessary when the value of f is large. A fractional factorial applies a fraction of whole design points of the related full factorial [18]. Therefore, the number of experiments are reduced. A half-fractional factorial design (25-1) with 16 experiments was used for determination of the main factors affecting the extraction efficiency and their interactions. The factors and their related levels were chosen based on preliminary studies and experiments. The main factors, their symbols and levels are shown in Table 2. The experiments were run in a random manner to minimize the effect of uncontrolled variables [19]. To find the most important effects and interactions, normal plot was drawn using Design-Expert 8.0.3 (Fig. 3). According to normal plot and analysis of variance (ANOVA) table for selected factorial models, only C (concentration of chelating agent) and S (concentration of salt) are significant model terms. In the ANOVA table, p values less than 0.0500 indicate that model terms are significant. The p values for both C and S were less than 0.0001. The concentration of chelating agent and concentration of salt included in following optimization process, while the volume of extraction solvent, temperature, and time of ultrasonic was the factors that remained constant (\(100~ \upmu\)L, \(25\, ^\circ\)C and 10 min, respectively).
3.3.2 Optimization Design of the Effective Parameters
Factorial design does not provide information about interactions or squared terms due to exploring only two levels. After finding the main factors, it is often useful to obtain a more detailed model of a system using central-composite design (CCD). There are two primary reasons for this: the first is to find conditions that result in a maximum or minimum as appropriate. The second is to produce a detailed quantitative model to predict mathematically how a response relates to the values of various factors [20]. A CCD employs more than two levels to allow fitting of a full quadratic polynomial. In other words, CCD combines a two-level factorial design with additional points (star points) and at least one point at the center of the experimental region to obtain properties such as rotatability or orthogonality to fit quadratic polynomials. Center points are usually repeated to get a good estimate of experimental error (pure error). To determine the optimal C and S, a rotatable and orthogonal CCD was employed. A CCD is made orthogonal and rotatable by the choice of suitable axial point, “a”. The value of “a” needed to ensure orthogonality and rotatability can be calculated from Eq. (1):
where \(N_\mathrm{f} = 2f\) is the number of factorial points. Using Eq. (1) the axial spacing was \(\pm \,1.414\). Then, \(N_0\) was obtained using Eq. (2) equal to 8:
where \(N_\mathrm{a} (=2f)\) is the number of axial points. The total number of experimental runs (N) is obtained by Eq. (3), where f is the number of variables. Therefore, totally 16 experiments had to be run for the CCD:
The experiments were randomized to minimize the effect of uncontrolled variables. The factor levels \((-\,a, -\,1, 0, +\,1, +\,a)\) for concentration of chelating agent (C) are, 55, 80, 140, 200, 225 ppm and for salt effect (S) are, 0.63, 0.8, 1.2, 1.6, 1.77 w/v%. The design matrix with the responses is shown in Table 3.
The ANOVA data to evaluate the significance of the model equation and model terms are shown in Table 4. The model F value of 20.27 implies that the model is significant. There is only a \(0.01\%\) chance that a “Model F value” this large could occur due to noise. The second-order polynomial with the most reasonable statistics with higher F and R values and low standard error was considered as the satisfactory response surface model to fit the experimental data. This model that is shown in Eq. (4) consisted of two main effects: one-factor interaction effect and two curvature effects, where \(b_0\) is the intercept and the b terms represent those parameters of the model which are optimized iteratively to fit, or model the data:
The “lack of fit (LOF) F value” of 0.45 implies that the LOF is not significant relative to the pure error. The quality of fit of the polynomial model equation was expressed by correlation coefficient (\(R^2\), adjusted-\(R^2\), and “adequate precision”). \(R^2\) is a measure of the amount of variation around the mean explained by the model and equal to 0.910. The adjusted-\(R^2\) is adjusted for the number of terms in the model. It decreases as the number of terms in the model increases if those additional terms do not add value to the model [21]. It is equal to 0.865. Adequate precision is a signal-to-noise ratio. It compares the range of the predicted values at the design points to the average prediction error (Eq. 5). Ratios greater than 4 indicate adequate model discrimination. Here, the ratio of 16.270 indicates an adequate signal. This model can be used to navigate the design space:
where \(\hat{Y}\) is the predicted value, p is the number of model parameters (including intercept (\(b_0\)) and any block coefficients), \(\sigma ^2\) = residual MS from ANOVA table, and n is the number of experiments. For the graphical interpretation of the interactions, the use of three-dimensional plots of the model is highly recommended. Variables giving quadratic and interaction terms with the largest absolute coefficients in the fitted models were chosen for the axes of the response surface plots to account for curvature of the surfaces. This is useful to visualize the relationship between the responses and the experimental levels of each factor [22]. The response model is mapped against two experimental factors. Figure 4 shows that in the range of 55.15–160.67 mg L\(^{-1}\) chelating agent, the extraction efficiency increases. At higher concentrations of chelating agent, however, the extraction efficiency was determined to decrease. As the concentration of salt increases, the extraction efficiency also increases. This behavior may be attributed to the salting effect increase in the volume of the sedimentary phase. In these set of experiments, the optimization option of the design-expert 8.0.3 software package was used to obtain optimal condition of concentrations of chelating agent and salt. The optimum levels of factors are 160.67 ppm for concentration of chelating agent and 1.77 w/v% for concentration of salt.
3.4 Analytical Figures of Merit
A pre-concentration series of six experiments were executed under optimal conditions to achieve the calibration curves which are demonstrated in Fig. 5 for Bi, Pb, and Fe. The linear dynamic range (LDR) was determined to be 1–1000 \(\upmu\)g L\(^{-1}\) for both Bi and Pb, and 10–1000 \(\upmu\)g L\(^{-1}\) for Fe. The correlation coefficient (\(R^2\)) of the calibration curves was in the range of 0.990–0.995. The limits of detection (LOD), defined as LOD=\(\frac{3S_\mathrm{b}}{m}\) (where LOD, \(S_\mathrm{b}\) and m are the limit of detection, standard deviation of the blank and slope of the calibration curve, respectively) were 0.78, 0.54 and 0.60 \(\upmu\)g L\(^{-1}\) for Bi, Pb, and Fe, respectively. The percent relative standard deviations (RSDs, \(C=200~\upmu\)g L\(^{-1}\)) for eight replicated measurements in optimal conditions were 5.7, 5.4 and \(4.8\%\) for Bi, Pb, and Fe, respectively.
3.5 Interference Studies
Other ions might compete or interfere with the metals under study in this work by complexing with the chelating agent or disrupting the complexation phenomenon. This was investigated with the purpose of identifying the potential interferents. The corresponding results are listed in Table 5, confirming that the recoveries were acceptable in the presence of the excessive amounts of the possible interfering cations and anions.
3.6 Application to Real Samples
The extraction efficiency of the represented method was validated by monitoring Bi, Pb, and Fe concentrations in spring and waterfall samples. All samples were analyzed under optimal conditions. The results showed that the concentrations of Bi, Pb, and Fe in the selected samples were higher than the limit of detection of our proposed method. Likewise, for further investigation of the matrix effect on the extraction efficiency, the solutions were spiked with 50 \(\upmu\)g L\(^{-1}\) of Bi, Pb, and Fe. The relative recoveries from the samples were in the range of 96.2–99.6%. These results demonstrate that the spring and waterfall water sample matrices had little effect on the Bi, Pb, and Fe extractions (Table 6).
4 Accuracy of the Method
To ensure the accuracy and precision of this method, two certified reference materials were used for analysis of Bi, Fe, and Pb:NIST SRM 1643e (trace elements in water), and CRM-TMDW-500 (certified reference material of drinking water). The results show that the proposed method can be applied to the pre-concentration and determination of Bi, Fe, and Pb in freshwater samples (Table 7).
5 Conclusion
The results from this study indicate that the optimized USAEME procedure combined with ICP–OES could be used for the simultaneous separation, pre-concentration, and trace detection of Bi, Pb, and Fe in freshwater samples. One of the main advantages of using USAEME over DLLME is the reduction of toxic organic solvent consumption. Furthermore, this method is fast, simple, efficient, low cost, and reproducible. The rapidity of this technique is due to short extraction time and the infinitely large surface area between extraction solvent and aqueous phase after formation of cloudy solution by ultrasonic radiations. Subsequently, the equilibrium state is achieved quickly. Disodium N,N\(^\prime\)-bis(salicylidene)ethylenediamine was used as a chelating agent for the first time in this work. Experimental designs (FFD and CCD) were utilized for determining the optimal operating conditions of the pre-concentration stage (USAEME) to achieve the maximum extraction efficiency. Moreover, experimental design provided all the main factors, their effects, quadratic and interaction terms simultaneously for optimization. This was not possible by classical methods to gather information about the detailed effect of factors on each other and on the extraction efficiency to interpret the behavior of the system. Ultimately, the proposed method was successfully utilized to detect trace amounts of heavy metals in freshwater samples.
References
Melek E, Tuzen M, Soylak M. Analytica Chimica Acta. 2006;578:213.
Comitre ALD, Reis BF. Talanta. 2005;65:846.
Itoh S-I, Kaneco S, Ohta K, Mizuno T. Analytica chimica acta. 1999;379:169.
Swaminathan S, Fonseca VA, Alam MG, Shah SV. Diabetes care. 2007;30:1926.
Tuomainen T-P, Punnonen K, Nyyssönen K, Salonen JT. Circulation. 1998;97:1461.
Barrionuevo WR, Lanças FM. Bulletin of Environmental Contamination and Toxicology. 2002;69:123.
Arthur CL, Pawliszyn J. Analytical Chemistry. 1990;62:2145. https://doi.org/10.1021/ac00218a019.
Belardi RP, Pawliszyn JB. Water Quality Research Journal of Canada. 1989;24:179.
Flanagan R, Morgan P, Spencer E, Whelpton R. Biomedical Chromatography. 2006;20:530.
Berijani S, Assadi Y, Anbia M, Hosseini M-RM, Aghaee E. Journal of Chromatography A. 2006;1123:1.
Rezaee M, Assadi Y, Hosseini M-RM, Aghaee E, Ahmadi F, Berijani S. Journal of Chromatography A. 2006;1116:1.
Prosen H, Zupan-i-Kralj L. TrAC Trends in Analytical Chemistry. 1999;18:272.
R. Carabias-Martınez, E. Rodrıguez-Gonzalo, B. Moreno-Cordero, J. Pérez-Pavón, C. Garcıa- Pinto, and E. F. Laespada, Journal of Chromatography A 902, 251 (2000).
Ferrer R, Beltran J, Guiteras J. Analytica chimica acta. 1996;330:199.
Sicilia D, Rubio S, Pérez-Bendito D, Maniasso N, Zagatto E. Analytica chimica acta. 1999;392:29.
Regueiro J, Llompart M, Garcia-Jares C, Garcia-Monteagudo JC, Cela R. Journal of Chromatography A. 2008;1190:27.
Ferreira SL, Dos Santos WN, Quintella CM, Neto BB, Bosque-Sendra JM. Talanta. 2004;63:1061.
Sailer O. Statistical Papers. 2008;49:597.
Sereshti H, Karimi M, Samadi S. Journal of Chromatography A. 2009;1216:198.
R. G. Brereton, Applied chemometrics for scientists (John Wiley & Sons, 2007).
Sereshti H, Khojeh V, Samadi S. Talanta. 2011;83:885.
D. C. Montgomery, G. C. Runger, and N. F. Hubele, Engineering statistics (John Wiley & Sons, 2009).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sahraeian, T., Sereshti, H. & Rohanifar, A. Simultaneous Determination of Bismuth, Lead, and Iron in Water Samples by Optimization of USAEME and ICP–OES via Experimental Design. J. Anal. Test. 2, 98–105 (2018). https://doi.org/10.1007/s41664-017-0046-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-017-0046-0