Abstract
This study delves into the most recent advancements in computational fluid dynamics (CFD) and fluid–structure interaction (FSI) modeling applied to cardiovascular engineering. The application of FSI methods serves to overcome the constraints inherent in individual finite element analysis (FEA) and CFD techniques by simultaneously considering both fluidic and structural domains. The present review also explores One-Way and Two-Way FSI implementations across diverse cardiovascular systems, including scenarios involving aortic aneurysms and coronary arteries. A wide spectrum of cardiovascular applications is addressed, encompassing subjects such as hemodynamics in both healthy and diseased vessels, optimization of stent designs, evaluation of the effects of device interventions during stent deployment, modeling thrombosis and conducting risk assessments, analysis of heart valve dynamics, and assessment of cardiovascular devices such as ventricular assist devices. Furthermore, the study delves into assessing computational resource requirements, strategic implementation approaches, and potential directions for solving FSI-related issues within the context of cardiovascular systems. The integration of CFD modeling within cardiovascular medicine has profoundly transformed the landscape of biomedical device research and development. However, the challenges pertaining to regulatory aspects must be effectively addressed to ensure the widespread integration of CFD modeling within cardiovascular medicine. Successfully overcoming these challenges and adeptly addressing limitations will facilitate the seamless amalgamation of these pioneering techniques, ushering in a new era of precision medicine within cardiovascular healthcare.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Computational Fluid Dynamics (CFD) plays a crucial role in the field of Cardiovascular Engineering by providing valuable insights into the complex flow patterns and hemodynamics within the cardiovascular system and has been used in disease diagnosis, medical device design, treatment planning and drug delivery. Moreover, it aids in examining systems involving fluid dynamics, heat transfer, mass transfer, and related physical phenomena, including chemical reactions (Theodorakakos et al. 2008). CFD is widely utilized in aerodynamics, turbomachinery, hydrodynamics, and meteorology (Versteeg and Malalasekera 2007). In the context of bioengineering, this multidisciplinary approach combines principles from fluid mechanics, physiology, and engineering to better understand and optimize the performance of the heart and blood vessels. CFD can potentially revolutionize how we study, diagnose, and treat cardiovascular diseases, ultimately improving patient outcomes. It enables researchers and clinicians to create detailed blood flow models within the arteries, veins, and heart chambers (Morris et al. 2013). This involves the intricate physiological flows within the cardiovascular system and extends to developing patient-specific cardiovascular models and therapeutic interventions (Sermesant et al. 2012). By solving the Navier–Stokes equations, CFD can predict variables such as velocity, pressure, and wall shear stress, providing a comprehensive view of the fluid dynamics in the cardiovascular system in both healthy and diseased conditions (Morris et al. 2016). CFD-based techniques in the context of cardiovascular hemodynamics also help to analyze the blood flow within blood vessels and the heart using physical principles governing biological fluid flow. It can assist in diagnosing cardiovascular diseases by analyzing blood flow patterns. For instance, it can identify disturbed flow or recirculation regions that may indicate atherosclerosis, aneurysms, or stenosis. These simulations help clinicians make more informed decisions about treatment options.
Furthermore, engineers can use CFD to design and optimize medical devices like stents, heart valves, and artificial blood vessels. By simulating the interaction between these devices and blood flow, researchers can ensure they are safe and effective, reducing the need for extensive animal or clinical testing. CFD simulations are employed in planning interventional procedures, such as angioplasty or stent placement. Surgeons can use patient-specific CFD models to determine the optimal approach and device selection, improving the precision and success rate of the procedure. CFD aids in the development of drug delivery systems by predicting how pharmaceutical agents travel through the circulatory system. This is crucial for targeted drug delivery, where medications are delivered directly to the affected areas, minimizing side effects and increasing treatment efficacy. Personalized medicine is a growing trend in cardiovascular engineering. CFD allows for the creation of patient-specific models based on medical imaging data, enabling tailored treatment plans that account for individual variations in anatomy and physiology. By analyzing flow patterns and stresses within blood vessels, CFD can help predict the risk of cardiovascular events, such as thrombosis or rupture of aneurysms. This information can guide clinicians in managing and monitoring high-risk patients. CFD-based simulations can serve as educational tools for training surgeons and healthcare professionals. They provide a realistic environment for practising complex cardiovascular procedures, enhancing skills and improving patient safety. In summary, Computational Fluid Dynamics can be a powerful tool in Cardiovascular Engineering, offering a deeper understanding of the hemodynamics within the cardiovascular system, aiding in disease diagnosis, treatment planning, medical device design, and advancing our knowledge of cardiovascular physiology. As technology continues to evolve, CFD will likely play an even more significant role in improving the diagnosis and treatment of cardiovascular diseases, ultimately enhancing patient care and outcomes.
Finite Difference Method (FDM), Finite Element Method (FEM), and Finite Volume Method (FVM) are some of the numerical methods commonly employed to solve the complex non-linear partial differential equations in CFD. Each proposed numerical method discretizes the fluid domain into discrete elements in space and time domain. FDM involves approximating the derivatives with finite differences, while FEM approximates the solution over each element using piecewise polynomial functions (Peiró and Sherwin 2005). FVM evaluates the numerical fluxes using a control-volume approach (Peiró and Sherwin 2005). However, CFD based on the Finite volume method is not capable of capturing the effect of pulsating fluid pressure gradient, and other forces (like intramural stresses in case of abdominal aortic aneurysms) will have on the deformation of the adjacent structure of blood vessel walls (Mendez et al. 2018). Similarly, finite element analysis (FEA) cannot develop a model to understand the structural deformation caused by fluid flow behavior if fluid dynamics are not considered, which is intrinsically dependent on the structural behavior of blood vessels and hearts. To overcome this limitation, the Fluid–structure interactions (FSI) concept is introduced to understand cardiovascular engineering problems, combined with CFD and FEA (Borowski 2018). Based on the proposed techniques, we study the fluid flow behavior and structural deformation (in terms of compliance and elasticity of the blood vessel walls) and their interactions. In the context of patient-specific modeling, the segmented 2D images obtained from CT scans or MRI analyses can be stacked and merged to form a 3D geometric model (Wang 2017a; Jahed 2018; Mendez et al. 2018). Such procedures are well-defined in cardiovascular CFD problem layout and can be used to form physiologically correct FSI models. These FSI models help to study clinical parameters that cannot be easily measured but form an important factor affecting the onset, development, and therapy of various diseases (like aortic aneurysm and aortic valve disease in which it is crucial to investigate vessel wall biomechanics like wall shear stress distribution and its resulting deformation) (Molony et al. 2010; Chen and Luo 2018). FSI studies can also help provide critical physical insights using patient-specific models in conditions like atherosclerosis, wherein elevated wall shear stress (WSS) promotes endothelial dysfunction, inflammation, and plaque formation. WSS cannot be measured directly but can now be studied using FSI (Drewe 2017; He 2017). In order to understand the numerical context of both the CFD and FSI-based approaches, the essential governing equations in terms of fluid dynamics interface and solid mechanics interface are explained in the following sections. Despite the abundance of literature on CFD/FSI in cardiovascular physiology, it lacks to explain the mathematical background behind these modeling strategies and comprehensive comparisons and benchmarks to ascertain the optimal balance between accuracy, computational efficiency, and adaptability across diverse cardiovascular scenarios. So, these studies aim to provide an understanding of computational fluid dynamics (CFD) and fluid–structure interaction (FSI) in cardiovascular physiology to comprehensively assess the advancements and applications of these techniques in studying the complex hemodynamics of the cardiovascular system. The major objective is to provide an in-depth analysis of how CFD and FSI methodologies contribute to understanding the interplay between fluid flow behavior and solid structures within the cardiovascular system.
The present review summarizes the numerical background behind CFD and FSI-based approaches, evaluates the strengths and limitations of different CFD and FSI-based approaches in terms of their accuracy, efficiency, and suitability to specific problem layouts, highlights technological advances in terms of numerical simulation algorithms like parallel processing and computational power like cloud-computing. It also outlines previous research investigating various cardiovascular phenomena using CFD/FSI techniques. Finally, the review highlights areas to study, like heart valve dynamics, stent optimization, thrombosis modeling, etc., for advancing the field of CFD and FSI application in cardiovascular physiology.
Mathematical Foundations of Cardiovascular Flow Simulation
In this section, we start with the essential governing equations related to the incompressible flow problem in the cardiovascular system. They are non-linear partial differential equations representing the conservation of mass (continuity equation) and momentum (Navier–Stokes equations) for the fluid flow. They are given by
and
Here, \({\varvec{f}}\) is the body force vector acting on the flow direction within the cardiovascular system (i.e., heart), σ is the Cauchy-Green stress tensor that varies depending on the type of fluid studied, v is the fluid velocity vector, and p is acting pressure, respectively.
The non-Newtonian properties of the blood are modelled using different constitutive relationships, such as the Carreau model, Casson model, and Cross model, respectively (Fung 1993; Neofytou and Tsangaris 2006; Lee and Steinman 2007). The stated constitutive equations are given by:
where \(\mu\) is the dynamic viscosity (in Pa-s), \({\mu }_{0}\) is the zero shear rate viscosity (in Pa-s), \({\mu }_{\infty }\) is the high shear rate viscosity (in Pa-s), \(\gamma\) is the shear rate (in s−1), \({\tau }_{y}\) is the yield shear stress (in Pa), n is the flow behavior index, \(\lambda\) is the relaxation time constant (in s), m is an arbitrary constant which equals to 2, respectively.
To solve the above mentioned essential governing Eqs. (1–2), we consider the pulsating pressure boundary condition as an inlet boundary condition, no-slip boundary condition where we assume rigid wall of the blood vessel network for wall boundary condition as well as suppress backflow boundary condition as an outlet boundary condition (i.e., P = 0).
In the context of solid mechanics interface in cardiovascular engineering problems, we account the domains related to the biological tissues which are active in this analysis. The proposed model based on solid mechanics interface represents the load with the total shear stress distribution by the fluid motion that accounts during the fluid dynamic analysis. So, the governing equation for solid mechanics interface is written as:
where, \({{\varvec{u}}}_{s}\) is the solid displacement velocity (in m/sec),\(\rho_w\) is the solid wall density (in kg.m−3), σ is the Cauchy Green-stress tensor, \({\varvec{f}}\) is the body force vector acting on the flow direction within the cardiovascular system, respectively.
In the case of a flow-structured coupled equation (where, we account both fluid and solid structure interface), we can consider ALE (Arbitrary Lagrangian Eulerian formulations) to account the deformability of fluid–structure domain (Bodnár et al. 2014). It offers certain advantages in terms of relating the structure mechanics and fluid mechanical interface based on the Lagrange representation of the moving surface. So, the governing equations related to ALE formulations take the form:
where, vG is the reference velocity of the moving surface and G denotes the associated reference surface which is moving in the Lagrangian formulation. In Eq. 7, \(\rho\) is defined in terms of the density of the structure and of the flowing medium. The Cauchy-Green stress tensor, \(\sigma\) defines
where, \(\tau_{ij} = - p\delta_{ij} + \mu \cdot \left( {\frac{\partial v_i }{{\partial x_j }} + \frac{\partial v_j }{{\partial x_i }}} \right)\). This is valid for incompressible flow and Stokes friction law.
To solve Eq. (7), the boundary conditions at the interface G, are defined in terms of two relevant boundary conditions i.e., the kinematic boundary condition and dynamic coupling condition, respectively. The kinematic boundary conditions states that the rate of deformation, \(\left( {v_i } \right)\) must be equal to the flow velocity vector, v at the interface, which becomes:
In addition, the dynamic boundary condition defined in terms of the Cauchy-Green stress tensor, σ with the shear stress vector, τ, at the interface with the normal outward vector, n:
In the context of ALE formulation, coupling models are concerned with the exchange of stresses with the hydrostatic pressure and the shear stress components of the friction.
Model Constructions
In order to solve the above-mentioned governing equations, several numerical techniques based on finite volume or finite element methods have been employed (Cho and Kensey 1991). In the context of the numerical modelling approach, the fluid continuum is first discretized into smaller, discrete sub-domains termed elements, which is dubbed the meshing procedure. The governing equations are converted into a system of non-linear algebraic equations by integrating them over the complete set of elements. Figure 1 shows one of the numerical algorithms used in CFD in order to solve the momentum and mass conservation equations (where flow coupling is accounted for). These algebraic equations can be solved by iteration using computing clusters. The governing equations of fluid motion can now be solved under unsteady conditions (where the time scale is a dependent parameter) in complicated anatomies within an acceptable computing time due to developments in parallel computer processing. Accordingly, it is important to consider the time scales for blood flow (~milliseconds), solid deformation (~microseconds-hrs) and cardiac cycle (~seconds) while selecting the appropriate time step for the simulations. The time scale of blood flow refers to the characteristic time that it takes for fluid flow to change significantly within the cardiovascular system. It depends on factors such as the size of the cardiovascular system, velocity of blood flow, and viscosity of the blood. Similarly, in the context of the time scale of solid deformation, this refers to the time for solid structures within the cardiovascular system to deform or change shape significantly. This time scale strongly depends on the material properties of the solid and the magnitude of forces acting upon it. In other words, the time scale of cardiac cycles relates to the time that it takes for one complete heartbeat cycle, including the systole (contraction) and diastole (relaxation) phases. In humans, this time scale typically ranges from about 0.5 to 1.0 s, depending on factors such as heart rate and overall cardiovascular health. The time step should be small enough to accurately capture the flow dynamics of the cardiovascular system. Adaptive time-stepping techniques can also be employed here, where the time step dynamically adjusts based on the current state of the system, which is used to balance accuracy and computational efficiency. Resolving a time-accurate expression typically involves one or more days of calculations, taking into account a fluid domain discretized into one million elements (or more) and several cardiac cycles, each of which is divided into hundreds of time steps (Morris et al. 2013).
Flow chart of SIMPLE (semi-implicit method for pressure-linked equations) algorithm—one of the iterative solution schemes developed by Patankar and Spalding used in CFD (Patankar and Spalding 1983)
Numerical Workflow in Cardiovascular Engineering
The steps involved in the construction of the model and its obtained numerical solution using CFD are dubbed as workflow or toolchain. It broadly involves various steps explained below:
Clinical Imaging
The workflow of CFD in cardiovascular engineering requires the creation of a three-dimensional geometry of the desired vascular region from invasive or non-invasive images. Measurements of length and diameter of the blood vessels are obtained through different medical procedures (i.e., computed tomography, coronary angiography, intravascular images through optical coherence tomography or intravascular ultrasound) or MRI analyses that can help to construct digital models of cardiac chambers and blood vessels (Pennati 2013; Weese et al. 2013; Arbia et al. 2014).
Segmentation and Construction of Region of Interest
The process by which the clinical images are converted into digitally constructed geometries with defined physical bounds for a given region of interest is known as Segmentation. The region of interest is located using manual or automated segmentation algorithms that are a part of commercially available software i.e., ITK-SNAP, 3D slicer etc. (Pieper et al. 2004; Yushkevich et al. 2016). Both proposed software are used for medical image analysis, visualization and image-guided therapy research. It provides extensive capabilities for the segmentation, registration, and modelling of anatomical structures, making it suitable for creating patient-specific models for CFD simulations in cardiovascular engineering problems. The segmentation process develops with an initial triangular meshed model for the cardiac chamber or a blood vessel from raw medical images (from MRI and CT scans). This model can be refined by smoothing operations and triangle reductions (Cho and Kensey 1991; Barber and Hose 2005). Interactive segmentation methods combine automatic processes with user input to improve accuracy by integrating prior knowledge and allowing result validation and error correction. However, their effectiveness can be limited by the need for specific initial configurations and parameter adjustments. Edge-based and region-based level set methods, learning-based (using SVM and AI), graphical tools (Intelligent scissors), and energy minimisation are some of the algorithms being used for segmentation (Mishra et al. 2008; Zhao, F 2013).
The rest of the numerical steps are identical to the ones followed in conventional CFD problems (i.e., discretization, mesh generation, boundary conditions and initialization), so for further details, the readers are directed to standard textbooks (Versteeg and Malalasekera 2007). Among these numerical steps, mesh generation is a very important step in cardiovascular engineering problems. Several challenges are observed due to the complex geometry of the cardiovascular system and the dynamic nature of this system. The geometry of the physiological systems, such as the heart, arteries and veins, is intricate and irregular in shape. So, generating a high-quality mesh that accurately represents the complex geometry while maintaining computational efficiency is challenging. Similarly, physiological systems involve moving boundary conditions such as a beating heart or pulsating arteries. Mesh generation should account for these moving boundaries to accurately capture the dynamic of the flow phenomena, which adds to the complexity of the process. Mostly, ALE methods are employed to capture the moving mesh interface with the fluid flow while maintaining the fixed reference frame for solving the governing equations.
Numerical Solution Methodology for CFD
CFD simulations necessitate a file with model inputs, including geometry, mesh, boundary conditions, and fluid properties, alongside details on numerical schemes such as discretization methods, pressure–velocity coupling, and turbulence modeling. Desired outputs like velocity and pressure profiles are specified before solving the governing fluid mechanics equations using a CFD solver (e.g., COMSOL Multiphysics, Ansys Fluent, openLB) until convergence. The flow chart of numerical solution metholdogy in the course of fluid flow behavior within the cardiovascular system is depicted in Fig. 2.
Schematic diagram of the flow chart of the numerical simulation involves solving the set of PDEs iteratively using discretization schemes like FVM/FEM. In time-dependent simulations, there exist certain criteria like Courant numbers to check the numerical stability of the obtained numerical solution (De Moura and Kubrusly 2013)
Numerical Solution Methodology for FSI
In the context of the FSI problem layout, the two approaches for FSI coupling between the fluid–solid interfaces are considered One-way and two-way coupling, respectively. The one-way coupling mechanism solves both the fluid and structural physics in a series manner, where the obtained numerical solution of one domain serves as an initial or boundary condition to the other domain (Benra 2011). On the other hand, in the two-way coupling method, both domains are solved parallelly until they converge at each iteration (Benra 2011; Pei 2012). The utility of both the proposed methods depends on the physical problem layout requirements and the computational resources available. The comparison between the two proposed approaches (i.e., one-way coupling and two-way coupling) is listed in Table 1.
Additionally, in one-way FSI, the obtained numerical results by solving the fluid domain using CFD first help calculate the acting forces on the vessel wall due to the fluid. By interpolating these fluid forces from nodes on the fluid domain to the nodes on the structural domain, we can predict the wall deformation rate using finite element analysis (FEA) in the second step (Benra 2011). However, in the real-case scenario, the vessel wall will distend (vasodilation), thus reducing the acting fluid pressure on the vessel wall instantaneously. As a result, the acting fluid pressure along the structural domain is artificially high, overestimating the distention (Benra 2011). Figure 3 provides a visual understanding of both the coupling processes.
Post-processing and Validation
Finally, the obtained numerical results need to be post-processed in an understandable form like velocity and pressure profiles to gain the physical insight into desired hemodynamic quantities (Chen et al. 2013). To test the reliability and accuracy of the CFD model in terms of model assumptions and essential governing equations, it is necessary to validate it by comparing the other obtained results with in-vivo phantom measurements or clinical measurements (Radaelli et al. 2008). The complete workflow can be visualized with the help of Fig. 4.
Visual abstract of the complete FSI workflow to determine the impact of transcatheter aortic valve replacement (TAVR). a CT images acquired during the standard TAVR clinical protocol are used to construct 3D patient-specific models. b The FSI-based evaluation compares TAVR performance to SAV and de-calcified normal AV. Sub-figures (c), (d), (e), (f), (g), and (h) show steps in the algorithm used to obtain numerical results followed by post-processing. (Reproduced from (Govindarajan et al. 2022))
Computational Resource Requirements
Important numerical factors such as the complexity of the model, the computational model used, and the availability of computational resources place constraints on various scenarios that can be studied through FSI problem layouts. Currently, this issue is tackled in two ways. Firstly, a large body of work is devoted in order to develop efficient numerical algorithms that require less computational power and give high accuracy. Secondly, a rise has been seen in the availability of computational resources, with researchers leveraging both small clusters in the lab and large computing clusters on their campus (Hirschhorn et al. 2020). Researchers are also implementing cloud computing since it allows access to high-end computational power without the need for any hardware systems. The following table gives an overview of computational requirements for various FSI studies (Table 2).
Applications of CFD in Cardiovascular Engineering
This section details the transformative role of Computational Fluid Dynamics (CFD) and Fluid–Structure Interaction (FSI) in unravelling the complexities of cardiovascular physiology. By merging cutting-edge simulation techniques with physiological insights, this section delves into the remarkable contributions of CFD/FSI applications in understanding blood flow dynamics, arterial mechanics, cardiovascular pathologies, etc.
The CFD modeling can be used to assess the impact of coronary lesions in a non-invasive way, like virtual fraction flow reverse (vFFR) validated by experimental evidence. It can be further improved if realistic patient-specific boundary conditions (for myocardial resistance) with accurate geometry are used (Morris et al. 2013, 2015; Nørgaard et al. 2014; Tu et al. 2014). Aneurysm progression and rupture risk can be better analyzed by CFD complemented with FSI models and can be used as a pre-diagnosis non-invasive imaging tool to understand the experimental findings. The impact of low-image contrast structure of aortic aneurysm and wall motion is yet to be well understood in these types of problems (Molony et al. 2010; Georgakarakos et al. 2011; Gasser et al. 2014; Erhart et al. 2015). Valve prostheses (like mechanical valves) can be optimized and analyzed for thromboembolism using CFD modeling (Yoganathan et al. 2005). However, the dependence of predicted thrombotic potential on model validity as well as modeling tissue valve leaflets, are some of the challenges associated with such cardiovascular problems (Yoganathan et al. 2005; Sotiropoulos and Borazjani 2009; Simon et al. 2010). CFD can help predict intra-aneurysmal flow, jet impingement, stasis, and WSS using CT and MRI data in the case of cerebral aneurysm, but these obtained results need to be validated for rupture predictions (Radaelli et al. 2008; Peach et al. 2014; Schneiders et al. 2015). Stent design and stent-induced hemodynamic disturbance post percutaneous coronary intervention can be analyzed in terms of WSS using CFD models that may require time-resolved pulsatility and finer mesh thus, requiring longer run times (Wentzel et al. 2001; Suo Jin et al. 2004; LaDisa et al. 2005; Chien 2007; Jiménez and Davies 2009; Seshadhri et al. 2011; Lee and Smith 2012; Morlacchi and Migliavacca 2013). CFD models complemented with FSI can help monitor transvalvular hemodynamics by estimating transvalvular pressure drop and regurgitant fraction without the need for invasive procedures (De Hart et al. 2003; Astorino et al. 2012). Computed flow and pressure conditions from CFD models that use non-invasive boundary conditions obtained from CT and phase contrast MRI can help design therapeutic procedures for aortic dissection (Chen et al. 2013; Karmonik et al. 2013; Cheng et al. 2014). CFD models can also help in early diagnosis of heart failure by replicating pathophysiology like regional wall motion abnormality, dilated cardiomyopathy, myocardial infarction, etc., as well as characterizing complex hemodynamics like vortex flow, flow stagnation and thus, assessing thrombotic potential (Niederer et al. 2011; Smith et al. 2011; Aguado-Sierra et al. 2011; Sermesant et al. 2012; Brown et al. 2012a; Chan et al. 2013; Chiu 2014; Farag et al. 2014). Not only this, but it can also help predict responses (like aortic anomalies, univentricular circulation, pulmonary malfunctions, etc.) to putative surgical procedures in case of congenital heart disease using FSI and multiscale models (LaDisa et al. 2005; Pittaccio et al. 2005; Pennati 2013). CFD models can also serve as potential alternatives to invasive techniques like right heart catheterization and stratify complex pulmonary hypertension (PH) physiology into PH subcategories for better design of personalized treatment (Tang et al. 2012; Lungu et al. 2014; Qureshi et al. 2014; Kheyfets et al. 2015). Arterial wall shear stress abnormality can be linked to atherosclerosis, aneurysm, and post-stent neointimal hyperplasia by proper analysis of WSS distribution obtained from CFD simulation. It can also help predict aneurysm rupture location and in-stent restenosis severity (Suo Jin et al. 2004; LaDisa et al. 2005; Chien 2007; Seshadhri et al. 2011).
Though CFD simulations provide valuable insights into blood flow patterns, incorporating Fluid–Structure Interaction (FSI) enhances accuracy by considering the dynamic interactions between blood flow and vessel wall deformation, thereby bolstering cardiovascular medicine research and diagnosis. The following sub-sections make a detailed review of the application of FSI models for various complex cardiovascular physiologies.
Cardiac Valve Dynamics
Two-way FSI, particularly fully coupled, is used to simulate the interaction between valve movement and blood flow in a transient system, making it the standard approach for studying cardiac valve dynamics. The Immersed Boundary (IB) method is also preferred for this purpose due to the significant deformation of thin valve leaflets within the fluid domain. Implementing a mesh-conforming method would be computationally intensive and cumbersome due to the need for re-meshing (Wang 2017b; Sodhani 2018).
Thus, FSI is a well-suited modeling technique for the dynamics of both prosthetic and native heart valves since their movements depend on the pressure and flow profiles within the heart chamber, influencing the hemodynamics significantly (as shown in Fig. 5).
FSI study used as a non-invasive monitoring and diagnostic tool for transcatheter aortic valve replacement. The visual abstract shows 3D geometries for the left atrium, left ventricle aortic valve, mitral valve, systemic circulation, and pulmonary circulation constructed using segmentation from CT images. Patient-specific image-based lumped parameter model was used to impose correct boundary conditions to simulate the effect of LV on the mitral valve model. The numerical results are validated using clinical cardiac catheterization and Doppler echocardiographic measurements (Reproduced from Khodaei et al. 2021)
Gao et al. used IB based two-way FSI coupled model of the mitral valve and left ventricle to study valve features, LV contraction, systolic ejection duration, peak aortic flow rate, and LV ejection fraction that showed good agreement with in vivo data (Gao 2017). Mao et al. used a fully-coupled smoothed particle hydrodynamics-finite element method (SPH—FE) based FSI model for aortic and mitral valve with left ventricle studying the opening and closing time as well as angle of the valves for their effect on intraventricular hemodynamics (Mao 2017). The obtained numerical results were validated with in vivo data. Arefin et al. used a two-way arbitrary Eulerian–Lagrangian FSI model to evaluate the effect of the angle between the aortic and mitral valves in terms of pulsating pressure distribution, wall shear stress, and hemodynamics in LV during the diastolic phase (Arefin 2017). Bahraseman et al. used a two-way ALE-based FSI model to evaluate the effect of exercise on aortic valve stroke work at different heart rates validated against literature (Bahraseman 2018). Deng et al. have made an ALE-based fully coupled FSI model to evaluate the effect of hypertrophic cardiomyopathy (HCM) that studies elements of interest in systolic anterior motion (SAM) in HCM in relation to septal myectomy surgery (Deng 2018). Govindarajan et al. used a fully coupled FSI model to investigate the biomechanics of the mitral valve and hemodynamics in LV, demonstrating the effect of leaflet dynamics on the functioning of the mitral valve, vortex ring, and fluid dynamics in LV during the diastolic phase (Govindarajan et al. 2018). Khalafvand et al. used ALE based fully coupled FSI model to evaluate the effect of mitral valve dynamics on interventricular flow (Khalafvand 2018).
FSI has also been used to model the aortic valves and the hemodynamics within the adjacent arterial systems. Some of the mentioned work in the following paragraph also reviews the turbulence models used by some authors to numerically solve the Navier–Stokes equation. In some of these cases, the k-Ω-based shear stress transport (SST) turbulence model is a better choice than the k-ε turbulence model since the former is less sensitive to grid resolution. This makes the k-Ω model more suitable for cases where computational resources are limited or where highly refined grids might not be practical. Thus, FSI analysis provides insights into valve performance (in terms of different flow parameters like aortic flowrate, ejection fraction, and ejection duration), geometric parameters like the mitral-aortic angle that can help in early diagnosis in cardiovascular pathophysiology, and an improved understanding of cardiac valve-related conditions.
Tango et al. used ALE based fully coupled FSI model of a healthy aortic valve that was modified to align with findings obtained from particle image velocimetry (PIV). The model can address the limitations inherent in the experimental configuration (Tango 2018). Gilmanov et al. used two-way FSI with a non-linear rotation-free shell finite element for the aortic valve to improve the model used for the material in the valve (Gilmanov 2016). Hasan et al. used IB based FSI model for the aortic root and descending aorta, providing numerical results for physiological pressures and flow rates that show good agreement with clinical data (Hasan 2017). Fedele et al. used a resistive immersed implicit surface, creating a 3D-0D FSI problem for developing a patient-specific aortic valve that provides data pertaining to peak diastolic pressure jumps across leaflets and hemodynamics (Fedele 2017). Cao et al. used the ALE-based FSI model to study the impact of coronary flow on wall shear stress in aortic valve leaflets, showing that coronary blood flow altered the flow patterns and WSS experienced by the valve surface (Cao 2016a). Mohammadi et al. published a series of works using a fully coupled FSI model of aortic valves incorporating smaller coronary structures and thoracic aorta, evaluating the impact of distal coronary arteries on velocity profiles and shear stress distribution (Mohammadi 2016, 2017a, b). Siguenza et al. used immersed thick boundary method (ITBM) based FSI and LES for turbulence modeling to study the impact of pulsatile flow on the aortic valve to assess the transition to turbulence downstream of the valve (Sigüenza et al. 2018). Stupak et al. used one-way FSI with turbulence models like k-ε and k-Ω to study patient-specific turbulent aortic valve flows, finding that the k-Ω to better than the k-ε model in comparison with reported simulation results (Stupak 2017).
From the above works, it is possible to understand the utility of FSI to discern the functioning of the coronary system and its impact on cardiac valves in terms of hemodynamics, pulsatile flow, and stress distribution. This can help in patient-specific analysis (by performing accurate hemodynamic assessment) as well as risk assessment (or risk stratification) for treatment outcomes or device deployment.
Toma et al. used a fully-coupled smoothed particle hydrodynamics-finite element method (SPH—FE) based FSI model analyzing the chordae structure of the mitral valve, highlighting how the diameter and strain of the chordae influenced the area of the regurgitant orifice by exploring 51 potential points of chordae rupture (Toma 2016a, b). Khodaei et al. used a two-way ALE FSI model to study mitral valve prolapse in prolapse patients, showing an elevation in stress levels on the interior and posterior leaflets of the mitral valve compared to healthy individuals (Khodaei et al. 2017). Rupture of chordae occurs in other areas; it leads to increased tension, specifically on the chordae in these regions. Caballero et al. demonstrated that fully coupled FSI models proved better than structural valve models for transient valve dynamics for mitral valve prolapse post chordae rupture, revealing that basal/strut chordae bear the highest loads and chordae rupture leads to stress reduction in the basal/strut region (Caballero 2018). Feng et al. used immersed boundary-based FSI model using three different chordal structures (simple, pseudo-fibre, and complex) for chordae and mitral valve (Feng 2018). The FSI modeling of mitral valve-chordal rupture compared three different methods of incorporating the “pseudo-fibers” method into the structural model, revealing that this method provides the highest accuracy at the least computational cost. Hassani et al. used a two-way FSI model to simulate mitral valve mal coaptation demonstrating mitral valve dynamics with a focus on regurgitation and leaflet coaptation (Hassani 2018).
The FSI can also be used in studying mitral valve prolapse and regurgitation. The valve and its associated structures form a complex system, but the availability of a validated valve pathology model can greatly assist clinicians in making informed decisions regarding future treatment options (for instance, if the FSI simulation predicts a high risk of post-TAVR paravalvular leak due to inadequate sealing, the clinician might opt for surgical valve replacement instead). BAV occurs when two out of the three leaflets of the aortic valve are fused, resulting in a bicuspid valve configuration. BAVs are classified based on the pattern of leaflet fusion, with type-0 indicating equal-sized leaflets and type-1 indicating unequal-sized leaflets (Hirschhorn et al. 2020).
Cao et al. used ALE based two-way FSI model to study stress distribution and structural deformation of tricuspid and bicuspid aortic valve leaflets providing numerical results for WSS profile and leaflet deformation in normal tricuspid aortic valves (TAV), type-0 bicuspid aortic valves (BAVs), and type-1 BAVs, revealing abnormal aortic hemodynamics in areas at high risk of dilation (Cao 2016b). Pasta et al. used a fully coupled FSI model to study intramural stress studies in the aortic valve, giving a comparative analysis of 21 bicuspid aortic valve (BAV) and 13 tricuspid aortic valves (TAV) geometries, aortic dilation in BAV patients revealing notable differences in structural and hemodynamic characteristics between the two categories (Pasta 2017). Lavon et al. used ALE based two-way FSI model on studying the effect of non-fused cusp angle on the bicuspid aortic valve, revealing that the nondistensible fibrosis curve (NFC) angle influenced mechanical behavior, eccentric jet direction, and fluid/structural stresses, emphasizing the higher risk of early failure in BAVs with small NFC angles and the preference for a larger NFC in BAV repair surgery involving suture annuloplasty (Lavon 2018). Liu et al. used a fully-coupled FSI model for WSS directional abnormalities in BAV aortas, assessing the impact of helical flow in the aorta on circumferential WSS in BAV and TAV patients (Liu 2018). Sadeghpour et al. used two-way FSI using steered adaptive meshing (SAM) for stenotic aortic valve hemodynamics, showing that fluid jet caused high blood stress due to incomplete aortic valve opening (Sadeghpour 2016). Olcay et al. studied disturbed hemodynamics due to a stenosed aortic jet using a two-way FSI model using a realizable k-ε turbulence model (Olcay 2018). Soifer et al. Used ALE-based two-way FSI model to study the impact of pathologic venous valves on downstream valves, investigating valve stiffening, retrograde flow, and failure on downstream vessels as well as the design of prosthetic valves (Soifer 2016). Thus, FSI is increasingly employed to investigate the effects and dynamics of congenital bicuspid aortic valves (BAV), representing the most prevalent congenital valve defect. This utilization of FSI aids in better understanding the characteristics and behavior of BAVs, leading to improved insights into their clinical implications (like deciding between valve repair or valve replacement).
Several numerical methods are proposed in this section. We have presented a table that compares the advantages and limitations of the various proposed numerical methods for solving the cardiac valve analysis problem (Table 3).
Overall, CFD-based cardiac valve analysis provides a thorough understanding of hemodynamic behaviour, valve function, and how valve pathology affects cardiovascular health. These findings have important practical implications for detecting valve disorders, improving treatment techniques, and developing novel medical therapies.
Prosthetic Valve Dynamics
The study of prosthetic heart valve dynamics is highly compatible with FSI because it involves the interaction between the valve structure and fluid flow behavior (as mentioned in Fig. 6). Among the various types of prosthetic valves, those constructed from a fiber-reinforced polymer matrix are the most commonly used (Hirschhorn et al. 2020).
FSI model utilized to study aortic valve implants, analyzing thinner tissues. This aids in detecting flow disruptions, leaflet irregularities, and their impact on cardiovascular function and valve durability. A Problem setup for different cases, valve thickness, and ventricular pulsation. B FSI study coupled with signal analysis procedure to obtain velocity profile, leaflet strain, and traction. C Leaflet displacement was analyzed using flutter-analysis methodology. (Reproduced from (Johnson et al. 2020)
Some of the FSI-based studies (including transcatheter aortic valve replacement) are presented in the following paragraph, which also reviews FSI studies on aortic root displacement, internal stresses in the case of stented and stent-less aortic valves as well as additional structural support for valves used near LV where it can experience high pressure.
Avanzini et al. used an ALE-based two-way FSI model to assess the impact of fiber orientation and leaflet matrix stiffness on prosthetic tri-leaflet heart valve opening. The transvalvular pressure gradient revealed that circumferential fibers and increased matrix stiffness reduced the effective orifice area of prosthetic tri-leaflet valves degrading the valve performance (Avanzini 2017). Gharaie et al. also used an ALE-based two-way FSI model for leaflet kinematics in polymeric aortic valves (Gharaie 2018). The results revealed a maximum of 15% discrepancy between in silico and in vitro findings for non-linear deformation of polymeric aortic valves. Lurahi et al. Conducted a comparative study using finite element-based structural mechanics and immersed boundary-based FSI model for aortic valve prosthesis (Luraghi 2018). The benchtop testing showed that FSI results for maximum orifice opening area were within 5% of benchtop results, while the FEA model deviated by 46.5%. Hedayat et al. published two studies that used ALE based both one-way and two-way FSI models in which the one-way FSI model led to an overestimation of velocity and shear stress distribution in the hinge area of bileaflet mechanical heart valves. It involved a comparative study on platelet activation between the hinge and bulk flow in bileaflet mechanical heart valves (BMHV). One study examined the effect of leaflet gap size on platelet activation, showing that a more significant gap increased total activation but improved washout ability due to higher flow velocity. Another study compared platelet activation during systole in bioprosthetic and mechanical heart valves, finding slightly higher activation in bioprosthetic valves early in systole but significantly higher activation at the end of systole (Hedayat 2017, 2018). These results encourage the development of bioprosthetic valves that mimic native valve dynamics. Abu Bakar et al. studied PIV based two-way FSI model of a Bi-leaflet artificial heart valve, comparing velocity and vorticities in bi leaflet heart valve, validated by particle image velocimetry (Abu Bakar 2018). Ghosh et al. used ALE based two-way FSI model with SST k-Ω turbulence model for a comparative study in terms of hemodynamics and structural mechanics behavior between the polymeric transcatheter and surgical aortic valve demonstrating an improved leaflet opening (42%) and flow (27%) as compared to the SAVR in case of the new TAVR design, that showed comparable WSS and mechanical stresses (Ghosh 2018). Borowski et al. used both FEA and ALE-based FSI models in relation to heart valve dynamics revealing that the FEA model simulated valve dynamics incorrectly, showing leaflets opening five times faster as compared to the FSI model and, in turn, highlighting the utility of FSI (Borowski 2018). Kandail et al. studied optimal deployment locations of annular and supra-annular Core Valves on aortic and coronary artery hemodynamics. It used a sub-grid geometry resolution (SGGR) based two-way FSI model. The numerical Simulation demonstrated the utility of SGGR in FSI since SPH-based FSI is unsuitable as the fluid cannot be assumed to be fully incompressible, leading to imprecise WSS distribution and cumbersome assignment of boundary conditions, while ALE is found unsuitable due to rigorous re-meshing requirement (Kandail 2018). Vahidkhah et al. used a two-way FSI model with a k-ε turbulence model to study supra-annular valve-in-valve placement reducing blood stasis on TAVR. Flow stasis, potentially resulting in leaflet thrombosis, was observed with intra-annular and supra-annular TAVR positioning. The intra-annular position restricts valve opening and creates stagnant areas that increase the risk of thrombosis (Vahidkhah 2017).
The ability of FSI to predict conditions that can lead to thromboembolism or flow stagnation will help clinicians to avert these risks even before they can occur. For instance, malfunctioning heart valves can lead to disturbed flow patterns that can form stagnant zones giving more time to platelets and clotting factors to interact and initiate clotting. Based on FSI simulations, the clinician can take a more informed decision whether to prescribe anti-coagulant medications or perform immediate surgical procedures to replace the valve.
Nestola et al. made a comparative study of stented and stent-less aortic valve bio-protheses in terms of aortic root stresses showing that stent-less valve configuration closely resembled the healthy case in terms of aortic root displacement and internal seen across three patient-specific geometries. They used an ALE-based two-way FSI model for this study (Nestola 2016). Wang et al. used both FEA and immersed boundary-based FSI models, thus comparing their utility in the modeling of bioprosthetic heart valves. Results showed that the FSI study yielded better results than the FEA model and revealed that suture density significantly influenced principle stress and shear stress, with contrasting trends (Wang 2017b). Sodhani et al. used IB based FSI model since ALE is unsuitable due to rigorous re-meshing requirements, and even though IB may require the finer mesh to capture the deformations, it is suitable for large displacement. The authors validated an FSI model for artificial textile-reinforced heart valves with in vitro tests. The FSI model achieved results within 9% of the benchtop results (which was an in-vitro test) during diastole replicating the in vitro testing of a scaffold-reinforced tissue-engineered heart valve (Sodhani 2018). Chen et al. also used an IB-based FSI model for aortic valves studying leaflet deformation and vortex flow structures (Chen and Luo 2018). Xu et al. used a hybrid ALE-immersogeometric FSI model for the development of a framework for designing patient-specific bio-prosthetic heart valves giving results for deformation of the aortic root, prosthetic valve as well as surrounding hemodynamics that is in good agreement with patient-specific MRI data (Xu 2018). Thus, FSI can help test the efficiency of tissue-engineered valves in-vivo that are usually required to sustain the high pressures if deployed in the left ventricular region. This knowledge can guide the design, development, and optimization of these valves, ultimately leading to improved clinical outcomes for patients with heart valve diseases. In this section, we mainly used a few FSI based numerical methods to explain the prosthetic valve dynamics as part of the Computational Fluid Dynamics (CFD) approaches. Table 4 summarises the advantages and limitations of the proposed numerical approaches.
Overall, CFD research of prosthetic valve dynamics provides useful information about the functional behaviour, hemodynamic performance, and durability of mechanical heart valves. These findings are critical for optimising prosthetic valve designs, improving clinical results, and increasing patient safety and quality of life.
Cardiovascular Stents
In contrast to other areas of cardiovascular engineering problems, the One-Way FSI technique is frequently utilized in stent studies. This is because fluid pressure distribution has minimal influence on stent deformation, allowing for the structural domain to be modeled first to create a stented geometry (Hirschhorn et al. 2020). This stented geometry can then be employed in subsequent CFD studies (as depicted in Fig. 7).
FSI studies of an idealized stented coronary artery system involving comparison with a rigid wall model to understand the effect of wall compliance under the influence of wall shear stress distribution (computed as time-averaged WSS distribution) for two different materials (CoCr and PLLA). Here, FSI is used to calculate WSS, which cannot be measured in vivo. (Reproduced from Chiastra et al. 2014)
In the context of cardiovascular stents, pulse wave propagation (PWP) plays a crucial role in the circulatory system. The elastic nature of large arteries facilitates blood flow by responding to the cyclic pressure generated by the left ventricle (LV). Stents are believed to contribute to a decrease in PWP. However, further research is needed to thoroughly investigate the effects of stenting on PWP (Hirschhorn et al. 2020). A systematic review of both stent optimization FSI studies and their impact on pulse wave propagation (PWP) is made in the next section.
Putra et al. used a one-way FSI model for a comparative study on different strut cross-section shapes to minimize the low wall shear stress distribution near the stent using surrogate-based optimization. One-way FSI was employed in a stent optimization study, utilizing FEA for stent deployment and CFD for fluid effects and vessel deformation to minimize low WSS area resulting in improved stent design and hemodynamics (Putra 2017). Simao et al. published two significant works using a one-way FSI model to compare two different stent configurations. Obtained numerical results in the form of WSS were assessed for flow disturbances in multiple stent designs to demonstrate the effectiveness of the one-way FSI model with the goal of minimizing detrimental effects like restenosis on the blood vessel wall (Simão 2016, 2017). Lui et al. studied the degradation mechanism in the simulated human micro-stress environment for magnesium alloy stent. The numerical results obtained for WSS for the degradable stent are integrated into a benchtop model to simulate the degradation process (Liu 2017). Frecentese et al. studied wave propagation in stented arteries using FSI. The two-way FSI results for PWP in stented arteries, testing various configurations and designs, were assessed for the occurrence of wave reflection. Stent geometry impacted stop-band location, with crosslinked coils increasing stop-bands and multiple stents reducing them. The obtained numerical results shed light on vessel response in terms of deformation to stenting and stent orientation for PWP improvement (Frecentese 2018). Papathanasiou et al. also used the 1D FSI model to study wave transmission and reflection in stented arteries. The FSI results assessed stenting’s impact on PWP with one and two stent configurations. Single-stent-induced wave reflection only in pathologic conditions, while the two-stent design could cause wave reflection in normal physiological conditions (Papathanasiou 2017). Thus, FSI simulations play a crucial role in stent design by enabling engineers and medical professionals to understand how stents interact with blood flow and how different design parameters influence their performance. However, further study the impact of stenting on PWP is necessary to get better insights.
In this section, we consider one-way and two-way FSI simulations to assess the performance of stents in blood vessels. The advantages and limitations of using a one-way FSI and two-way FSI approach for cardiovascular stent design analysis are summarized in Table 5.
In conclusion, CFD-based analysis of cardiovascular stents gives useful information on their hemodynamic effects, thrombogenicity, and potential for improving clinical outcomes in patients with coronary artery disease or other vascular disorders. These findings help to improve stent design and treatment techniques for cardiovascular disease management.
Hemodynamic Analysis of Cardiovascular Devices
FSI is utilized to simulate the vascular response of mechanical circulatory assist devices. One-way FSI studies are common for rigid impeller blades, as they do not deform under fluid pressure. Two-way FSI is used for pulsatile pneumatic VADs, where structural deformation is integral to the device’s mechanism (Liao 2018a; Hirschhorn et al. 2020). The following section provides an overview of FSI-based simulation studies for ventricular-assisted devices from both device design and operating condition standpoints.
Caimi et al. used two-way FSI for 12 cc Penn State pediatric pneumatic pulsatile VAD for the development of a virtual benchmark FSI model covering the interplay between pneumatic actuation on the diaphragm and blood chamber with the potential to optimize device design (Caimi 2017). Obidowski et al. used the IB-based FSI model as a method to identify areas of blood stagnation due to pulsatile VADs. The obtained numerical results in terms of velocity profile were used to discern stasis due to pulsatile VAD, which is validated using PIV measurements (Obidowski 2018). Alizadeh et al. also employed a two-way FSI model on AVICENA VAD to study aortic valve force and power generation. In this study, stress profiles were used to investigate the impact of the VAD and the aortic valve. Six sets of displacement curves and one set of boundary conditions were used to identify operating conditions that reduced wall shear stress on the aortic valve and thus enhanced power generation. Rahmani et al. published a series of works studying a prototype VAD based on AVICENA (Alizadeh 2018). In the first study, they determined an optimal set (a total of 12 displacements & 3 sets of boundary conditions studied) of boundary conditions to maximize power generation while minimizing aortic valve stress in the case of healthy, hypertensive, and hypotensive patients (Rahmani 2018a).
In addition, different parameters like potential materials, their thicknesses & choice of either continuous or pulsatile flow were explored using FSI to determine optimal novel balloon-based VADs encircling the aorta (Rahmani 2018b, c). Bakir et al. used fully coupled FSI for a biventricular cardiac model of LVAD that simulated heart failure with a continuous flow LVAD. It demonstrated a shift in the LV pressure–volume curve, cessation of aortic ejection at high rotational speeds, reduced LV volume, and collapse. The model assists in developing LVAD control mechanisms to prevent LV collapse (Ahmad Bakir et al. 2018).
Bhat et al. studied outflow graft size on flow in the aortic arch due to continuous flow pumps (Heartmate II and Heartware VADs) using ALE-based two-way FSI model for comparative analysis of outflow angle and diameter for the two VADs informing the parameters while designing patient-specific VADs (Bhat 2017). Chiu et al. used a one-way FSI with the k-ε turbulence model on Heartmate II and Heart Assist 5 to compare thromboresistance between two VADs. Optimal configuration with respect to inflow cannula angle, outflow, VAD orientation, and graft angle were studied (Chiu 2014). Liao et al. used one-way FSI on Heartware HVAD to study the effect of rotary blood pump speed modulation on thrombosis. It determined optimal speed modulation from constant, sinusoidal, and counter-copulation to reduce thrombotic potential and enhance the pulsatility index. Accordingly, the population was found to be superior (Liao 2018b). Liao et al. used a multiscale model involving CFD, lumped parameter network (LPN), and FEM on Heartware HVAD for sensitivity analysis of cannula length. It involved analysis of inflow cannula length with respect to apical thickness to reduce thrombotic potential and residence time. A larger cannula length to apical thickness ratio was found to be preferable (Liao 2018a). Sonntag et al. used one-way FSI on EVAHEART 2 VAD. The optimal length of the inflow cannula (double cuff tipless type) was determined with the help of patient-specific lump parameters and FSI studies (Sonntag 2019). Zhang et al. used an ALE-based two-way FSI model to study intraventricular flow under full and partial support of BJUT-II VAD during heart failure by analyzing WSS and oscillatory shear index (OSI) (Zhang 2017). Luraghi et al. made a comparative study using both structural Finite-element (FE) and ALE-based two-way FSI models on Carmat totally implantable artificial heart (TAH) assessing the LV including displacements of aortic and mitral valves. FSI-predicted geometric orifice area (GOA) showed a deviation of only 5% from experimental results while FE based GOA showed deviation of almost 46.5% (Luraghi 2017). Wappenschmidt et al. used IB-based FSI for the investigation of intraventricular dynamics in rotary piston blood pumps.
The FSI model (fitted to particle image velocimetry measurements) was used to perform an analysis of various aspects of a hybrid rotary piston pump that cannot be studied easily by PIV (Wappenschmidt 2016). Thus FSI studies are helping optimise VADs with respect to enhancing the pulsatility index, reducing thrombotic potential and residence time, and increasing washout by assessing WSS and oscillatory shear index (OSI). It can help clinicians in the development of safer, more efficient, and patient-specific VAD solutions.
In this section, we consider one-way FSI, Two-way FSI and ALE-based two-way FSI methods to understand the hemodynamic analysis of the cardiovascular devices. The advantages and limitations of the proposed numerical methods are summarized in below Table 6. Overall, hemodynamic analysis within the cardiovascular device using CFD approach gives useful information about hemodynamic behaviour, vascular function, and the pathophysiology of vascular disorders. These findings are critical for enhancing our understanding of cardiovascular health, guiding therapeutic decisions, and creating tailored therapies to prevent and treat vascular diseases.
Blood Flow Modeling in Normal and Diseased Vessels (Atherosclerosis and Stenosis)
In the study, FSI is utilized in terms of atherosclerosis due to the impact of atherosclerotic plaques on hemodynamics and their role in plaque initiation, growth, and possible rupture (Hirschhorn et al. 2020). CFD-based simulations based on microfluidic models in the context of thrombosis modeling are presented in Fig. 8.
CFD-based simulation of mimicking microfluidic models of thrombosis to study the hemodynamics in case of atherosclerotic plaque and eccentric stenosis due to device insertion. Results show the wall shear stress distribution as well as strain rate. It presents CFD as a tool to gain rheological insights into thrombosis and biomechanical platelet aggregation. (Reproduced from Zhao et al. 2021)
Chhai et al. studied the responsible factors for atherosclerotic plaque rupture using ALE based FSI model. The results showed increased maximum cap stress (the mechanical stress exerted on the fibrous cap-like structure made of connective tissues on an atherosclerotic plaque), WSS, and rupture risk with growing plaque asymmetry (Chhai 2017). Tang et al. made an MRI-PET/CT-based FSI study on the effect of cap inflammation on plaque cap. The FSI model simulated inflammation-induced plaque weakening and observed significant strain under thin caps and hypertension conditions (Tang 2016). He et al. analyzed hemodynamics and wall shear stress distribution in the atherosclerotic arteries using ALE based FSI model, revealing early atherosclerosis effects on nearby vessels. This involved lower WSS and higher cyclic strain in arteries neighboring atherosclerotic vessels, thus emphasizing the importance of early diagnosis (He 2017). Kafi et al. studied blood flow in atherosclerotic arteries, comparing Newtonian and non-Newtonian fluid formulations in atherosclerotic arteries, highlighting the effect of inclusion of wall rigidity parameter in stress distribution (Kafi 2017). Bahrami et al. made a comparative analysis of hemodynamics in normal and hypertensive conditions in left coronary bifurcation studying atherosclerosis initiation using a non-Newtonian flow in both normal and hypertensive blood pressure in an idealized geometry using an ALE-based two-way FSI model (Bahrami 2018). Kallekar et al. evaluated the impact of wall compliance on the deformation during flow through stenosed coronary artery indicating that the FSI studies for atherosclerosis depend on the wall model used (Rigid, Linear Elastic, neo-Hookean, Mooney-Rivlin, and Holzapfel material models were compared under steady state and pulsatile conditions) using one-way FSI model (Kallekar 2017). Nejad et al. also studied pulsatile blood flow in stenosed arteries for different parameters like a viscoelastic and elastic wall, atherosclerosis, and high hematocrit. They used an ALE-based two-way FSI model (Nejad 2017). Pereira et al. used the IB FSI model as a multiscale hemorheological model for an atherosclerosis study using the particulate model for validation against existing literature results (Pereira 2016). In addition, Gholipour et al. used a two-way FSI model to study atherosclerotic coronary arteries for the effect of different factors like stenosis levels, pulsatile blood flow, plaque properties, realistic vessel, coronary artery bifurcation, and heart motion studied in relation to stenotic blood flow (Gholipour 2018a, b). Javadzadegan et al. studied the effect of freedom of movement of coronary arteries for modeling coronary arteries in the presence of stenosis. The results indicated that increased freedom of movement affected WSS at lower stenosis levels and got restricted as the stenosis level increased (as many as 25 coronary artery models with different support points were studied) (Javadzadegan 2016). Meza et al. also studied the fully coupled FSI model, which showed that the plaque decreased wall tensile strain, and the incorporation of myocardial contraction (the outside blood vessel wall was subjected to the pressure induced by myocardial contraction) significantly influenced periodic flow velocity and shear stress locations. LAD coronary artery with 70% stenosis (including transient blood flow system, cyclic artery binding, and heart muscle contraction) was also simulated in this study (Meza et al. 2018). Pinho et al. made an FSI and statistical-based study of the effect of the left coronary artery’s geometric parameters on atherosclerosis. These variable parameters included arterial cross-sectional area, length, branch & septum angles, artery curvature, and tortuosity on flow in LAD (Pinho 2018). Kaewbumrung et al. studied turbulent flow phenomena in coronary arteries affected by stenosis, generating fluid flow, pressure, and WSS profiles thus, analyzing the degree of stenosis in coronary arteries (Kaewbumrung 2017). In another study, Pinho et al. used FSI for patient-specific stenosed carotid artery bifurcation, making a comparison of arterial compliance on WSS in both normal and diseased vessels (Pinho 2017). In summary, studying the distribution of Wall Shear Stress (WSS) is crucial for modeling atherosclerosis because it provides insights into how blood flow patterns influence endothelial health, plaque formation, and the overall development of the disease. By understanding the relationship between WSS distribution and atherosclerosis, researchers can develop more accurate models and potentially identify new strategies for prevention and treatment.
To summarise, in this section, proposed numerical methods based Fluid–Structure interaction problem provide unique insights into blood flow modelling in normal and diseased vasculature, allowing for non-invasive inquiry, quantitative analysis, virtual experimentation, personalised therapy, and understanding of disease causes. However, they have constraints in terms of simplified geometry and boundary conditions, model validation, computational resources, fluid–structure interaction, and parameter sensitivity, all of which must be carefully addressed to assure the simulation results correctness and reliability.
Thrombosis Modeling and Risk Assessment
Thrombus formation is a complex process influenced by various biochemical factors, including the interaction of proteins and enzymes within the coagulation cascade, abnormal blood flow conditions, and interactions with foreign surfaces (which can be implanted devices like artificial heart valves or stents). The causes of thrombosis are multifaceted, involving intricate molecular mechanisms and external elements that contribute to the formation of blood clots (Blum et al. 2021).
So, computational modeling including protein reactions provides a valuable tool for comprehending the intricate nature of thrombus formation, encompassing diverse mathematical models that address thrombosis at various spatial and temporal scales (as depicted in Fig. 9). In this section, we will discuss in detail the attempts made on this front.
CFD-based study to predict rupture risk in intracranial berry aneurysm from the @neurIST project [142]. A Meshed 3D structure, B Pressure distribution and C Wall shear stress distribution that can provide insights to predict the possibility of rupture. (Reproduced from Morris et al. 2016)
Chong et al. used a two-way FSI model for thrombosis for type B aortic dissection investigating the effect of flap motion and wall compliance on false lumen thrombosis (FLT), discovering a linear relationship between thrombus growth and maximum flap displacement for varying degrees of flap rigidity (Chong et al. 2022). Fang et al. studied thrombus formation in patients with atrial fibrillation employing FSI in combination with the discrete phase method (DPM). The study detailed the effects of different left atrial appendage (LAA) locations with respect to the left atrium (LA) in the case of patients with atrial fibrillation to evaluate the thrombosis risk (Fang et al. 2021). Bluestein et al. conducted FSI modeling demonstrating that the inclusion of intraluminal thrombus in stress analysis of abdominal aortic aneurysm led to better accuracy in predicting rupture risk (Bluestein et al. 2009). Barrett et al. used the FSI model coupled with biochemical interactions to study subclinical leaflet thrombosis due to aortic valve replacement with a bioprosthetic valve that is capable of modeling adhesion to the surface of moving leaflets and pulsatile pressures (Barrett et al. 2023). Qiu et al. studied the impact of the presence and thickness of intraluminal thrombus on abdominal aortic aneurysm from a hemodynamic point of view, indicating that recirculation and low WSS may lead to local rupture or promote the growth of ILTs (Qiu et al. 2019). Taylor et al. used a CFD-based model for device-induced thrombosis to generate results in a fluid flow profile that controls thrombus progression in regions of separated flow due to cardiovascular devices. The model is verified using in vitro blood flow data (Taylor et al. 2016). Using CFD, Blum et al. developed a novel computationally efficient accelerated thrombosis model for rotary blood pumps (Blum et al. 2021).
In this section, we mostly use two way FSI model to understand thrombosis modeling and risk assessment. Here, we discuss about the advantages and limitations of two way FSI model in understanding the thrombosis modeling and risk assessment by capturing the interplay between the blood flow dynamics and the mechanical response of blood vessels and thrombi, which is listed in Table 7. Overall, we conclude that the CFD is a valuable tool for tackling thrombosis modeling by allowing researchers to simulate blood flow dynamics, wall shear stress distribution, thrombus formation and the effects of interventions. It aids in understanding the complex relationship between fluid dynamics and thrombosis, contributing to improved prevention, diagnosis, and treatment strategies for this critical cardiovascular condition.
Drug Delivery in Vasculature
Nanoparticles can be effectively used for targeted drug delivery overcoming the shortcomings of traditional drug delivery treatments that are not efficient and can have undesired side-effects on healthy cells (Kleinstreuer 2014). Though there exists techniques like active guidance of nanoparticles towards cell receptors using external magnetic field, it is greatly affected due to coupling between fluid drag forces and magnetic forces in diseased arteries (Hewlin et al. 2019). One of the recent developments uses the flow profile in stenosed arteries which acts on mechanoresponsive microparticles that release the nanoparticles carrying drug once exposed to a certain level of shear rate (Shadden and Hendabadi 2013). In this case, computation fluid mechanics can be used as an effective tool to discern various parameters that affect drug delivery in atherosclerosis having artery plaques (Khoury et al. 2020).
In summary, we conclude that the CFD modeling is increasingly used to simulate drug delivery in the vasculature, offering insights into drug distribution, transport mechanisms and the impact of various factors on therapeutic efficacy. It can be personalized using patient-specific data, such as medical imaging, hemodynamic parameters and drug pharmacokinetics.
Overall Limitations and Challenges of CFD in Cardiovascular Engineering
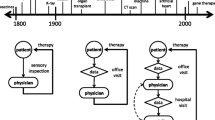
The conventional user groups of CFD/FSI models in cardiovascular medicine are depicted in Fig. 10. The level of detail in the medical image segmentation directly affects the accuracy of CFD simulation. While patient-specific boundary conditions and parameters are essential for accuracy, the model should still be able to generalize well to handle new cases or unseen variations. A model that can account for a broader range of anatomical differences will be more robust and applicable in a clinical setting. The accuracy of CFD simulations in medical applications is a multidimensional challenge that requires a combination of high-quality input data, well-designed models, and continuous advancements in imaging and computational techniques (Barber and Hose 2005).
Different user groups of CFD in medicine along with their respective research objective. Almost all of these groups construct complex cardiovascular models that require clinical data in terms of geometry and parametrization to prescribe suitable boundary conditions (Morris et al. 2016)
Many physiological metrics that are crucial for accurate CFD simulations might be difficult to measure directly in vivo. For example, microcirculatory resistance significantly affects coronary blood flow, but its measurement is not always straightforward. Sensitivity analysis in terms of mesh independence study and uncertainty quantification techniques can help in assessing the impact of different parameters on the numerical simulation outputs as well as balancing computational resources against the accuracy required (Brown et al. 2012b), but this comes with inherent limitations and may require experimental validation (Donders et al. 2015).
Another challenge is related to the development of industry standards. While CE marking and FDA directives (in European Union and the USA) provide a level of regulatory oversight for diagnostic software, they often focus on general safety and effectiveness rather than specific technical performance criteria. The FDA can drive the adoption of accurate and validated diagnostic software in medical applications by establishing rigorous benchmarking standards. It can also learn from successful implementations in other industries like aviation, wherein CAD (computer-aided design) is adopted over traditional physical testing by chalking out clear performance standards (Stewart et al. 2013).
In addition, numerical implementation in cardiovascular engineering poses several challenges due to the complex nature of physiological flow, complex geometries and multiphysics interactions involved. Blood flow in cardiovascular systems often exhibit the complex flow phenomena, including turbulence, separation and recirculation where CFD-based FSI may not suffice and hence high fidelity numerical methods may be required such as Large Eddy Simulation (LES) or Direct Numerical Simulation (DNS) which demand significant computational resources. Another important challenge is based on the constitutive modeling of the blood flow, with the consideration of vessel wall elasticity, material non-linearity (i.e., linear elastic material and non-linear Hookean model) and the tissue damage. To account all these aspects, accurate constitutive models are needed that require validation. However, experimental validation in cardiovascular engineering can be challenging due to limitations in imaging techniques, accessibility to physiological data, and variation among patient populations. Furthermore, evaluating uncertainties related to model assumptions, input parameters, and numerical approximations is crucial for determining the robustness of simulation results.
Access to large volumes of clinical data remains a challenge for the advancement of computational models in healthcare. By addressing privacy concerns, fostering collaboration, and adhering to ethical principles, researchers can unlock the potential of clinical data for model development, validation, and, ultimately, for improving patient outcomes through enhanced clinical translation. VPH-Share project is one of the earliest initiatives in this regard but requires continued collaboration (Viceconti and Hunter 2016).
The adoption of in silico systems in medicine poses another challenge to the new generation of doctors and healthcare professionals who will now also require training in computational methods to understand the physical principles and limitations of these proposed technologies (Cates and Gallagher 2012; Hunter et al. 2013). Modeling and in silico systems have the potential to disrupt traditional approaches in various fields, including medicine. They may be perceived as threats by traditional manufacturers of biomedical prototypes. CFD models might not account for all relevant physiological factors, such as endothelial behavior, tissue deformation, and biochemical interactions, a limitation that can influence flow patterns and disease progression. At the same time, it is important that CFD results are analyzed critically without overestimating or underestimating any conclusions (Lee 2011). Most of the FSI/CFD-based applications exhibit major limitations in terms of geometry, parameterization, material properties, the computational expense of the FSI model, and lack of clinical data for validation.
In conclusion, the development of rigorous benchmarking standards, access to computational resources like cloud computing & clusters, and advancements in sharing of clinical data within ethical limits can help to address some of the current challenges in FSI and CFD. Addressing these numerical implementation challenges needs interdisciplinary collaboration among engineers, mathematicians, doctors, and experimentalists..
Summary and Future Landscape
FSI models are helping to gain insights into complex cardiovascular diseases not only at the macro-level (like aortic aneurysms and ventricular mechanics) but also at the cellular level (like endothelial cell mechanics, platelet activation, and aggregation) utilizing a complete range of electro-mechano-fluid coupling (Bucelli et al. 2023). CFD and FSI are aiding in different fields of cardiovascular pathophysiology like disease assessment and prediction (like atherosclerosis and stenosis), biomedical prototype development, and impact of device intervention (like optimization of bioprosthetic valves, stents, and VADs), complex hemodynamic flow (like microcirculation and endothelial shear stress gradients), and vessel-wall biomechanics (like stenosed carotid artery (Kumar et al. 2020)). These examples highlight the capability of FSI and CFD to improve clinical decisions.
There is ongoing research in different aspects like patient-specific therapeutics, image-guided modeling of therapies, and electrical analog, as well as coupled to lumped parameter models and cardiac MRI data to develop realistic parametrization. On the hand, the development of new numerical solvers and coupling algorithms is gaining importance. FSI models are now made robust using efficient implementation strategies like IB along with lattice Boltzmann methods for complex flows and robust structural approaches like smoothed point interpolation method (Gilmanov 2019). More emphasis is now laid on parallel processing rather than sequential stepping schemes to improve solving speed (Margenberg 2019). All these developments will help solve complex multi-physics problems encountered in cardiovascular physiology, like cardiac arrhythmias and pulmonary hypertension.
The ready availability of advanced and large computational services (like cloud computing) is aiding the development of fully-coupled FSI models. Two-way FSI models will become more ubiquitous in the medical field with progress in both CFD and FEM methodologies. Availability and ease of sharing of clinical data and industrial benchmarks will help in formulating better models and aid in validation, increasing confidence in the utility of these diagnostic tools. CFD and FSI are of significant interest to cardiovascular medicine since they can help estimate information that could not be obtained from other imaging or experimental techniques. However, it is important to establish their equivalence to traditional invasive measurements by demonstration in clinical trials (Department of Cardiology, Madre Teresa Hospital, Schiavonia, Italy et al. 2022).
Benchmark Models for Comparative Analysis
Validating numerical methods in vivo is notably difficult due to the challenges in controlling experiments and obtaining precise measurement data. However, various open-source repositories like vascular model have been developed to primarily enable use of FSI models within medical-device approval process (Wilson et al. 2013). The cardiac atlas project is yet another attempt for development of better segmentation technology and benchmark models in cardiovascular physiology (Fonseca et al. 2011). Additionally, specialised softwares are progressively developed for FSI models some which are CircAdapt, FEBio, FEniCs, IBAMR, OpenCARP etc. and can be possibly used for uncertainty quantification on multiple case-studies using reduced-order models in multi-fidelity strategies (Arts et al. 2005; Logg et al. 2012; Maas et al. 2012; Senter et al. 2021; Plank et al. 2021). Contribution from clinicians in terms of reliable values for parametrization and engineers for mathematical formulation and validation is required to make these tools beneficial to patients and healthcare providers.
Regulatory Status of CFD Simulations into Clinical Practices
The integration of computational fluid dynamics (CFD) simulations into clinical practices is an area of active research and development. While CFD-based FSI studies have shown great promise in providing valuable insights into various biomedical and clinical applications, its regulatory status for direct clinical use varies depending on the specific application, jurisdiction, and regulatory agency. For instance, they may not require regulatory approval if they are conducted for academic or investigational purposes only.
Development and optimization of medical devices, such as cardiovascular stents, heart valves, and drug delivery system require regulatory approval from agencies such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA) before the device can be marketed or used in clinical practice. It may be possible that CFD simulations may be integrated into clinical decision as support tools. The regulatory status of such tools can vary depending on the intended use and claims associated with the software. Regulatory agencies may require validation studies and evidence demonstrating the clinical utility and safety of these tools before they can be used in patient care. Regardless of the specific application, CFD simulations used in a clinical context must adhere to rigorous quality standards and validation requirements. Regulatory agencies may require documentation of the simulation methodology, verification and validation processes, and quality assurance measures.
Overall, while computational fluid dynamics (CFD) simulations hold great potential for enhancing clinical practices in various biomedical applications, their regulatory integration requires careful consideration of quality, validation, and regulatory requirements.
References
Abu Bakar H, Abas A, Hafizah Mokhtar N, Razak N, Najib Bin Abdul Hamid M (2018) Particle image velocimetry and finite volume method study of bi-leaflet artificial heart valve. J Appl Fluid Mech 11:1365–1375. https://doi.org/10.29252/jafm.11.05.28808
Aguado-Sierra J, Krishnamurthy A, Villongco C et al (2011) Patient-specific modeling of dyssynchronous heart failure: a case study. Prog Biophys Mol Biol 107:147–155. https://doi.org/10.1016/j.pbiomolbio.2011.06.014
Ahmad Bakir A, Al Abed A, Stevens MC et al (2018) A multiphysics biventricular cardiac model: simulations with a left-ventricular assist device. Front Physiol 9:1259. https://doi.org/10.3389/fphys.2018.01259
Alizadeh M, Rahmani S, Tehrani P (2018) Calculating the aortic valve force and generated power by a specific cardiac assist device (AVICENA) in different counterpulsation. J Braz Soc Mech Sci Eng 40:1–9. https://doi.org/10.1007/s40430-018-1208-3
Arbia G, Corsini C, Esmaily Moghadam M et al (2014) Numerical blood flow simulation in surgical corrections: what do we need for an accurate analysis? J Surg Res 186:44–55. https://doi.org/10.1016/j.jss.2013.07.037
Arefin S (2017) An investigation on the effects of the angles between the mitral and aortic orifice during diastolic period using FSI. Pathophysiol off J Int Soc Pathophysiol 24:133–153. https://doi.org/10.1016/j.pathophys.2017.03.002
Arts T, Delhaas T, Bovendeerd P et al (2005) Adaptation to mechanical load determines shape and properties of heart and circulation: the CircAdapt model. Am J Physiol Heart Circul Physiol 288:H1943–H1954. https://doi.org/10.1152/ajpheart.00444.2004
Astorino M, Hamers J, Shadden SC, Gerbeau J-F (2012) A robust and efficient valve model based on resistive immersed surfaces: resistive immersed surfaces for heart valves. Int J Numer Meth Biomed Eng 28:937–959. https://doi.org/10.1002/cnm.2474
Avanzini A (2017) Influence of leaflet’s matrix stiffness and fiber orientation on the opening dynamics of a prosthetic trileaflet heart valve. J Mech Med Biol 17:1750096. https://doi.org/10.1142/s0219519417500968
Bahrami S, Norouzi M (2018) A numerical study on hemodynamics in the left coronary bifurcation with normal and hypertension conditions. Biomech Model Mechanobiol 17:1785–1796. https://doi.org/10.1007/s10237-018-1056-1
Barber D, Hose D (2005) Automatic segmentation of medical images using image registration: diagnostic and simulation applications. J Med Eng Technol 29:53–63. https://doi.org/10.1080/03091900412331289889
Barrett A, Brown JA, Smith MA et al (2023) A model of fluid–structure and biochemical interactions for applications to subclinical leaflet thrombosis. Numer Methods Biomed Eng 39:e3700. https://doi.org/10.1002/cnm.3700
Benra F-K, Dohmen HJ, Pei J, Schuster S, Wan B (2011) A comparison of one-way and two-way coupling methods for numerical analysis of fluid-structure interactions. J Appl Math 2011:1–16. https://doi.org/10.1155/2011/853560
Bhat S, Jayakala M, Balakrishnan KR, Kumar RK (2017) Effect of outflow graft size on flow in the aortic arch and cerebral blood flow in continuous flow pumps: possible relevance to strokes. ASAIO J 63:144–149. https://doi.org/10.1097/mat.0000000000000507
Bluestein D, Dumont K, De Beule M et al (2009) Intraluminal thrombus and risk of rupture in patient specific abdominal aortic aneurysm—FSI modelling. Comput Methods Biomech Biomed Eng 12:73–81. https://doi.org/10.1080/10255840802176396
Blum C, Groß-Hardt S, Steinseifer U, Neidlin M (2021) An accelerated thrombosis model for computational fluid dynamics simulations in rotary blood pumps. Bioengineering 11:224
Borazjani I (2013) Fluid–structure interaction, immersed boundary-finite element method simulations of bio-prosthetic heart valves. Comput Methods Appl Mech Eng 257:103–116. https://doi.org/10.1016/j.cma.2013.01.010
Brown AG, Shi Y, Arndt A et al (2012a) Importance of realistic LVAD profiles for assisted aortic simulations: evaluation of optimal outflow anastomosis locations. Comput Methods Biomech Biomed Eng 15:669–680. https://doi.org/10.1080/10255842.2011.556628
Brown AG, Shi Y, Marzo A et al (2012b) Accuracy vs. computational time: translating aortic simulations to the clinic. J Biomech 45:516–523. https://doi.org/10.1016/j.jbiomech.2011.11.041
Bucelli M, Zingaro A, Africa PC, et al (2023) A mathematical model that integrates cardiac electrophysiology, mechanics, and fluid dynamics: application to the human left heart. Numer Methods Biomed Eng 39. https://doi.org/10.1002/cnm.3678
Caballero A, Mao W, McKay R, Primiano C, Hashim S, Sun W (2018) New insights into mitral heart valve prolapse after chordae rupture through fluid-structure interaction computational modeling. Sci Rep 8:17306–17306. https://doi.org/10.1038/s41598-018-35555-5
Philippe CKS (2016a) Aortic valve leaflet wall shear stress characterization revisited: impact of coronary flow. Comput Methods Biomech Biomed Eng 20:468–470. https://doi.org/10.1080/10255842.2016.1244266
Cao K, Sucosky P (2016b) Computational comparison of regional stress and deformation characteristics in tricuspid and bicuspid aortic valve leaflets. Int J Numer Methods Biomed Eng 33:e02798. https://doi.org/10.1002/cnm.2798
Cates CU, Gallagher AG (2012) The future of simulation technologies for complex cardiovascular procedures. Eur Heart J 33:2127–2134. https://doi.org/10.1093/eurheartj/ehs155
Chan BT, Lim E, Chee KH, Abu Osman NA (2013) Review on CFD simulation in heart with dilated cardiomyopathy and myocardial infarction. Comput Biol Med 43:377–385. https://doi.org/10.1016/j.compbiomed.2013.01.013
Chen D, Müller-Eschner M, Von Tengg-Kobligk H et al (2013) A patient-specific study of type-B aortic dissection: evaluation of true-false lumen blood exchange. BioMed Eng OnLine 12:65. https://doi.org/10.1186/1475-925X-12-65
Chen Y, Luo H (2018) A computational study of the three-dimensional fluid–structure interaction of aortic valve. J Fluids Struct 80:332–349. https://doi.org/10.1016/j.jfluidstructs.2018.04.009
Cheng Z, Juli C, Wood NB et al (2014) Predicting flow in aortic dissection: comparison of computational model with PC-MRI velocity measurements. Med Eng Phys 36:1176–1184. https://doi.org/10.1016/j.medengphy.2014.07.006
Chhai P, Lee JH, Rhee K (2017) Effects of longitudinal asymmetric distribution of a lipid core on plaque wall stress. J Biomech Sci Eng 12:16–00588. https://doi.org/10.1299/jbse.16-00588
Chiastra C, Migliavacca F, Martínez MÁ, Malvè M (2014) On the necessity of modelling fluid–structure interaction for stented coronary arteries. J Mech Behav Biomed Mater 34:217–230. https://doi.org/10.1016/j.jmbbm.2014.02.009
Chien S (2007) Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 292:H1209–H1224. https://doi.org/10.1152/ajpheart.01047.2006
Cho YI, Kensey KR (1991) Effects of the non-Newtonian viscosity of blood on flows in a diseased arterial vessel. Part 1: Steady flows. BIR 28:241–262. https://doi.org/10.3233/BIR-1991-283-415
Chong MY, Gu B, Armour CH et al (2022) An integrated fluid–structure interaction and thrombosis model for type B aortic dissection. Biomech Model Mechanobiol 21:261–275. https://doi.org/10.1007/s10237-021-01534-5
De Hart J, Peters GWM, Schreurs PJG, Baaijens FPT (2003) A three-dimensional computational analysis of fluid–structure interaction in the aortic valve. J Biomech 36:103–112. https://doi.org/10.1016/S0021-9290(02)00244-0
De Moura CA, Kubrusly CS (eds) (2013) The Courant–Friedrichs–Lewy (CFL) condition: 80 years after its discovery. Birkhäuser Boston, Boston
Deng L, Huang X, Zuo H, Zheng Y, Yang C, Song Y, Tang D (2018) Angle of attack between blood flow and mitral valve leaflets in hypertrophic obstructive cardiomyopathy: an in vivo multi-patient CT-based FSI study. Comput Model Eng Sci 116:115–125. https://doi.org/10.31614/cmes.2018.04076
Department of Cardiology, Madre Teresa Hospital, Schiavonia, Italy, Rigatelli G, Zuin M, et al (2022) Applications of computational fluid dynamics in cardiovascular disease. TTU J Biomed Sci 1:12–20. https://doi.org/10.53901/tjbs.2022.10.art02
Donders WP, Huberts W, Van De Vosse FN, Delhaas T (2015) Personalization of models with many model parameters: an efficient sensitivity analysis approach: efficient sensitivity analysis for large model personalization. Int J Numer Meth Biomed Eng 31:n/a-n/a. https://doi.org/10.1002/cnm.2727
Drewe CJ, Parker LP, Kelsey LJ, Norman P, Powell JT, Doyle BJ (2017) Haemodynamics and stresses in abdominal aortic aneurysms: a fluid–structure interaction study into the effect of proximal neck and iliac bifurcation angle. J Biomech 60:150–156. https://doi.org/10.1016/j.jbiomech.2017.06.029
Erhart P, Hyhlik-Dürr A, Geisbüsch P et al (2015) Finite element analysis in asymptomatic, symptomatic, and ruptured abdominal aortic aneurysms: in search of new rupture risk predictors. Eur J Vasc Endovasc Surg 49:239–245. https://doi.org/10.1016/j.ejvs.2014.11.010
Fang R, Li Y, Zhang Y et al (2021) Impact of left atrial appendage location on risk of thrombus formation in patients with atrial fibrillation. Biomech Model Mechanobiol 20:1431–1443. https://doi.org/10.1007/s10237-021-01454-4
Farag MB, Karmonik C, Rengier F, et al (2014) Review of recent results using computational fluid dynamics simulations in patients receiving mechanical assist devices for end-stage heart failure. Methodist DeBakey Cardiovasc J 10:185. https://doi.org/10.14797/mdcj-10-3-185
Fedele M, Faggiano E, Dedè L, Quarteroni A (2017) A patient-specific aortic valve model based on moving resistive immersed implicit surfaces. Biomech Model Mechanobiol 16:1779–1803. https://doi.org/10.1007/s10237-017-0919-1
Feng L, Qi N, Gao H, Sun W, Vázquez M, Griffith BE, Luo X (2018) On the chordae structure and dynamic behaviour of the mitral valve. IMA J Appl Math 83:1066–1091. https://doi.org/10.1093/imamat/hxy035
Fonseca CG, Backhaus M, Bluemke DA et al (2011) The Cardiac Atlas Project—an imaging database for computational modeling and statistical atlases of the heart. Bioinformatics 27:2288–2295. https://doi.org/10.1093/bioinformatics/btr360
Frecentese S, Argani LP, Movchan A, Movchan NV, Carta G, Wall ML (2018) Waves and fluid–solid interaction in stented blood vessels. Proc Math Phys Eng Sci 474:20170670–20170670. https://doi.org/10.1098/rspa.2017.0670
Fung Y-C (1993) Biomechanics. Springer, New York
Gao H, Feng L, Qi N, Berry C, Griffith BE, Luo X (2017) A coupled mitral valve-left ventricle model with fluid–structure interaction. Med Eng Phys 47:128–136. https://doi.org/10.1016/j.medengphy.2017.06.042
Gasser TC, Nchimi A, Swedenborg J et al (2014) A novel strategy to translate the biomechanical rupture risk of abdominal aortic aneurysms to their equivalent diameter risk: method and retrospective validation. Eur J Vasc Endovasc Surg 47:288–295. https://doi.org/10.1016/j.ejvs.2013.12.018
Georgakarakos E, Ioannou CV, Papaharilaou Y et al (2011) Computational evaluation of aortic aneurysm rupture risk: what have we learned so far? J Endovasc Ther 18:214–225. https://doi.org/10.1583/10-3244.1
Gharaie SH, Mosadegh B, Morsi Y (2018) In vitro validation of a numerical simulation of leaflet kinematics in a polymeric aortic valve under physiological conditions. Cardiovasc Eng Technol 9:42–52. https://doi.org/10.1007/s13239-018-0340-7
Gholipour A, Ghayesh MH, Zander AC (2018) Nonlinear biomechanics of bifurcated atherosclerotic coronary arteries. Int J Eng Sci 133:60–83. https://doi.org/10.1016/j.ijengsci.2018.08.003
Gholipour A, Ghayesh MH, Zander AC, Mahajan R (2018) Three-dimensional biomechanics of coronary arteries. Int J Eng Sci 130:93–114. https://doi.org/10.1016/j.ijengsci.2018.03.002
Ghosh R, Marom G, Rotman OM, Slepian MJ, Prabhakar S, Horner M, Bluestein D (2018) Comparative fluid–structure interaction analysis of polymeric transcatheter and surgical aortic valves’ hemodynamics and structural mechanics. J Biomech Eng 140:121002. https://doi.org/10.1115/1.4040600
Gilmanov A, Barker A, Stolarski HK, Sotiropoulos F (2019) Image-guided fluid–structure interaction simulation of transvalvular hemodynamics: quantifying the effects of varying aortic valve leaflet thickness. Fluids 4:119. https://doi.org/10.3390/fluids4030119
Gilmanov A, Stolarski H, Sotiropoulos F (2016) Non-linear rotation-free shell finite-element models for aortic heart valves. J Biomech 50:56–62. https://doi.org/10.1016/j.jbiomech.2016.11.031
Govindarajan V, Kolanjiyil A, Johnson NP et al (2022) Improving transcatheter aortic valve interventional predictability via fluid–structure interaction modelling using patient-specific anatomy. R Soc Open Sci 9:211694. https://doi.org/10.1098/rsos.211694
Govindarajan V, Mousel J, Udaykumar HS et al (2018) Synergy between diastolic mitral valve function and left ventricular flow aids in valve closure and blood transport during systole. Sci Rep 8:6187. https://doi.org/10.1038/s41598-018-24469-x
Griffith BE, Patankar NA (2019) Immersed methods for fluid–structure interaction. Ann Rev Fluid Mech 52:421–448. https://doi.org/10.1146/annurev-fluid-010719-060228
He F, Hua L, Gao L (2017) Computational analysis of blood flow and wall mechanics in a model of early atherosclerotic artery. J Mech Sci Technol 31:1015–1020. https://doi.org/10.1007/s12206-017-0154-9
Hedayat M, Asgharzadeh H, Borazjani I (2017) Platelet activation of mechanical versus bioprosthetic heart valves during systole. J Biomech 56:111–116. https://doi.org/10.1016/j.jbiomech.2017.03.002
Iman HMB (2018) Comparison of platelet activation through hinge vs bulk flow in bileaflet mechanical heart valves. J Biomech 83:280–290. https://doi.org/10.1016/j.jbiomech.2018.12.003
Hewlin RL, Ciero A, Kizito JP (2019) Development of a two-way coupled Eulerian–Lagrangian computational magnetic nanoparticle targeting model for pulsatile flow in a patient-specific diseased left carotid bifurcation artery. Cardiovasc Eng Tech 10:299–313. https://doi.org/10.1007/s13239-019-00411-8
Hirschhorn M, Tchantchaleishvili V, Stevens R et al (2020) Fluid–structure interaction modeling in cardiovascular medicine—a systematic review 2017–2019. Med Eng Phys 78:1–13. https://doi.org/10.1016/j.medengphy.2020.01.008
Hou G, Wang J, Layton AT (2012) Numerical methods for fluid-structure interaction—a review. Commun Comput Phys 12:337–377. https://doi.org/10.4208/cicp.291210.290411s
Hunter P, Chapman T, Coveney PV et al (2013) A vision and strategy for the virtual physiological human: 2012 update. Interface Focus 3:20130004. https://doi.org/10.1098/rsfs.2013.0004
Jahed M, Ghalichi F, Farhoudi M (2018) Fluid–structure interaction of patient-specific circle of Willis with aneurysm: investigation of hemodynamic parameters. Biomed Mater Eng 29:357–368. https://doi.org/10.3233/bme-181732
Javadzadegan A, Yong ASC, Chang M, Ng MKC, Behnia M, Kritharides L (2016) Haemodynamic assessment of human coronary arteries is affected by degree of freedom of artery movement. Comput Methods Biomech Biomed Eng 20:260–272. https://doi.org/10.1080/10255842.2016.1215439
Jiménez JM, Davies PF (2009) Hemodynamically driven stent strut design. Ann Biomed Eng 37:1483–1494. https://doi.org/10.1007/s10439-009-9719-9
Johnson EL, Wu MCH, Xu F et al (2020) Thinner biological tissues induce leaflet flutter in aortic heart valve replacements. Proc Natl Acad Sci USA 117:19007–19016. https://doi.org/10.1073/pnas.2002821117
Kaewbumrung M, Wiwatanapataphee B, Orankitjaroen S, Siriapisith T (2017) Numerical simulation of turbulent blood flow in the system of coronary arteries with stenosis. J Biometr Biostat 8:1–13. https://doi.org/10.4172/2155-6180.1000344
Kafi O, Khatib NE, Tiago J, Sequeira A (2017) Numerical simulations of a 3D fluid-structure interaction model for blood flow in an atherosclerotic artery. Math Biosci Eng 14:179–193. https://doi.org/10.3934/mbe.2017012
Kallekar L, Viswanath C, Anand M (2017) Effect of wall flexibility on the deformation during flow in a stenosed coronary artery. Fluids 2:16. https://doi.org/10.3390/fluids2020016
Kandail HS, Trivedi SD, Shaikh A, Bajwa T, O’Hair D, Jahangir A, LaDisa JF (2018) Impact of annular and supra-annular CoreValve deployment locations on aortic and coronary artery hemodynamics. J Mech Behav Biomed Mater 86:131–142. https://doi.org/10.1016/j.jmbbm.2018.06.032
Karmonik C, Müller-Eschner M, Partovi S et al (2013) Computational fluid dynamics investigation of chronic aortic dissection hemodynamics versus normal aorta. Vasc Endovascular Surg 47:625–631. https://doi.org/10.1177/1538574413503561
Khalafvand SS, Xu F, Westenberg JJM, Gijsen FJH, Kenjeres S (2018) Intraventricular blood flow with a fully dynamic mitral valve model. Comput Biol Med 104:197–204. https://doi.org/10.1016/j.compbiomed.2018.11.024
Kheyfets VO, Rios L, Smith T et al (2015) Patient-specific computational modeling of blood flow in the pulmonary arterial circulation. Comput Methods Programs Biomed 120:88–101. https://doi.org/10.1016/j.cmpb.2015.04.005
Khodaei S, Fatouraee N, Nabaei M (2017) Numerical simulation of mitral valve prolapse considering the effect of left ventricle. Math Biosci 285:75–80. https://doi.org/10.1016/j.mbs.2016.12.008
Khodaei S, Henstock A, Sadeghi R et al (2021) Personalized intervention cardiology with transcatheter aortic valve replacement made possible with a non-invasive monitoring and diagnostic framework. Sci Rep 11:10888. https://doi.org/10.1038/s41598-021-85500-2
Khoury M, Epshtein M, Zidan H et al (2020) Mapping deposition of particles in reconstructed models of human arteries. J Control Release 318:78–85. https://doi.org/10.1016/j.jconrel.2019.12.004
Kleinstreuer C (2014) Drug-targeting methodologies with applications: a review. WJCC 2:742. https://doi.org/10.12998/wjcc.v2.i12.742
Kumar N, Khader SMA, Pai R et al (2020) Fluid structure interaction study of stenosed carotid artery considering the effects of blood pressure. Int J Eng Sci 154:103341. https://doi.org/10.1016/j.ijengsci.2020.103341
LaDisa JF, LarsE O, Molthen RC et al (2005) Alterations in wall shear stress predict sites of neointimal hyperplasia after stent implantation in rabbit iliac arteries. Am J Physiol Heart Circ Physiol 288:H2465–H2475. https://doi.org/10.1152/ajpheart.01107.2004
Lavon K, Halevi R, Marom G, Zekry SB, Hamdan A, Schäfers HJ, Raanani E, Haj-Ali R (2018) Fluid–structure interaction models of bicuspid aortic valves: the effects of nonfused cusp angles. J Biomech Eng 140:031010. https://doi.org/10.1115/1.4038329
Lee B-K (2011) Computational fluid dynamics in cardiovascular disease. Korean Circ J 41:423. https://doi.org/10.4070/kcj.2011.41.8.423
Lee J, Smith NP (2012) The multi-scale modelling of coronary blood flow. Ann Biomed Eng 40:2399–2413. https://doi.org/10.1007/s10439-012-0583-7
Lee S-W, Steinman DA (2007) On the relative importance of rheology for image-based CFD models of the carotid bifurcation. J Biomech Eng 129:273–278. https://doi.org/10.1115/1.2540836
Liao S, Neidlin M, Li Z, Simpson B, Gregory SD (2018) Ventricular flow dynamics with varying LVAD inflow cannula lengths: in-silico evaluation in a multiscale model. J Biomech 72:106–115. https://doi.org/10.1016/j.jbiomech.2018.02.038
Liao S, Wu EL, Neidlin M, Li Z, Simpson B, Gregory SD (2018) The influence of rotary blood pump speed modulation on the risk of intraventricular thrombosis. Artif Organs 42:943–953. https://doi.org/10.1111/aor.13330
Liu D, Hu S, Yin X, Liu J, Jia Z, Qinglin L (2017) Degradation mechanism of magnesium alloy stent under simulated human micro-stress environment. Mater Sci Eng C Mater Biol Appl 84:263–270. https://doi.org/10.1016/j.msec.2017.12.001
Liu J, Shar JA, Sucosky P (2018) Wall shear stress directional abnormalities in BAV aortas: toward a new hemodynamic predictor of aortopathy? Front Physiol 9:993–993. https://doi.org/10.3389/fphys.2018.00993
Logg A, Mardal K-A, Wells G (eds) (2012) Automated solution of differential equations by the finite element method: the FEniCS book. Springer, Heidelberg
Lungu A, Wild JM, Capener D et al (2014) MRI model-based non-invasive differential diagnosis in pulmonary hypertension. J Biomech 47:2941–2947. https://doi.org/10.1016/j.jbiomech.2014.07.024
Maas SA, Ellis BJ, Ateshian GA, Weiss JA (2012) FEBio: finite elements for biomechanics. J Biomech Eng 134:011005. https://doi.org/10.1115/1.4005694
Mao W, Caballero A, McKay RG, Primiano C, Sun W (2017) Fully-coupled fluid–structure interaction simulation of the aortic and mitral valves in a realistic 3D left ventricle model. PLoS ONE 12:e0184729. https://doi.org/10.1371/journal.pone.0184729
Margenberg N, Richter T (2019) Parallel time-stepping for fluid–structure interactions. arXiv: Numerical Analysis NA:NA–NA
Mendez V, Di Giuseppe M, Pasta S (2018) Comparison of hemodynamic and structural indices of ascending thoracic aortic aneurysm as predicted by 2-way FSI, CFD rigid wall simulation and patient-specific displacement-based FEA. Comput Biol Med 100:221–229. https://doi.org/10.1016/j.compbiomed.2018.07.013
Meza D, Rubenstein DA, Yin W (2018) A fluid–structure interaction model of the left coronary artery. J Biomech Eng 140:121006. https://doi.org/10.1115/1.4040776
Mishra A, Wong A, Zhang W et al (2008) Improved interactive medical image segmentation using Enhanced Intelligent Scissors (EIS). In: 2008 30th Annual international conference of the IEEE engineering in medicine and biology society. IEEE, Vancouver, BC, pp 3083–3086
Mohammadi H, Cartier R, Mongrain R (2017a) Fiber-reinforced computational model of the aortic root incorporating thoracic aorta and coronary structures. Biomech Model Mechanobiol 17:263–283. https://doi.org/10.1007/s10237-017-0959-6
Mohammadi H, Cartier R, Mongrain R (2017b) The impact of the aortic valve impairment on the distant coronary arteries hemodynamics: a fluid–structure interaction study. Med Biol Eng Comput 55:1859–1872. https://doi.org/10.1007/s11517-017-1636-8
Mohammadi H, Cartier R, Mongrain R (2016) 3D physiological model of the aortic valve incorporating small coronary arteries. Int J Numer Methods Biomed Eng 33:e2829. https://doi.org/10.1002/cnm.2829
Molony DS, Kavanagh EG, Madhavan P et al (2010) A computational study of the magnitude and direction of migration forces in patient-specific abdominal aortic aneurysm stent-grafts. Eur J Vasc Endovasc Surg 40:332–339. https://doi.org/10.1016/j.ejvs.2010.06.001
Morlacchi S, Migliavacca F (2013) Modeling stented coronary arteries: where we are, where to go. Ann Biomed Eng 41:1428–1444. https://doi.org/10.1007/s10439-012-0681-6
Morris PD, Narracott A, Von Tengg-Kobligk H et al (2016) Computational fluid dynamics modelling in cardiovascular medicine. Heart 102:18–28. https://doi.org/10.1136/heartjnl-2015-308044
Morris PD, Ryan D, Morton AC et al (2013) Virtual fractional flow reserve from coronary angiography: modeling the significance of coronary lesions. JACC Cardiovasc Interv 6:149–157. https://doi.org/10.1016/j.jcin.2012.08.024
Morris PD, Van De Vosse FN, Lawford PV et al (2015) “Virtual” (computed) fractional flow reserve. Cardiovasc Interv 8:1009–1017. https://doi.org/10.1016/j.jcin.2015.04.006
Nejad AATZ, Cheraghali D, Shahbani-Zahiri A, Norouzi M (2017) Pulsatile flow of non-Newtonian blood fluid inside stenosed arteries: investigating the effects of viscoelastic and elastic walls, arteriosclerosis, and polycythemia diseases. Comput Methods Programs Biomed 154:109–122. https://doi.org/10.1016/j.cmpb.2017.11.016
Neofytou P, Tsangaris S (2006) Flow effects of blood constitutive equations in 3D models of vascular anomalies. Int J Numer Meth Fluids 51:489–510. https://doi.org/10.1002/fld.1124
Niederer SA, Plank G, Chinchapatnam P et al (2011) Length-dependent tension in the failing heart and the efficacy of cardiac resynchronization therapy. Cardiovasc Res 89:336–343. https://doi.org/10.1093/cvr/cvq318
Nørgaard BL, Leipsic J, Gaur S et al (2014) Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease. J Am Coll Cardiol 63:1145–1155. https://doi.org/10.1016/j.jacc.2013.11.043
Obidowski D, Reorowicz P, Witkowski D, Sobczak K, Jóźwik K (2018) Methods for determination of stagnation in pneumatic ventricular assist devices. Int J Artif Organs 41:653–663. https://doi.org/10.1177/0391398818790204
Olcay AB, Amindari A, Kirkkopru K, Yalcin HC (2018) Characterization of disturbed hemodynamics due to stenosed aortic jets with a Lagrangian coherent structures technique. J Appl Fluid Mech 11:375–384
Papathanasiou TK, Alexander M, Bigoni D (2017) Wave reflection and transmission in multiply stented blood vessels. Proc Math Phys Eng Sci 473:20170015. https://doi.org/10.1098/rspa.2017.0015
Patankar SV, Spalding DB (1983) A calculation procedure for heat, mass and momentum transfer in three-dimensional parabolic flows. In: Numerical prediction of flow, heat transfer, turbulence and combustion. Elsevier, pp 54–73
Peach TW, Ngoepe M, Spranger K et al (2014) Personalizing flow-diverter intervention for cerebral aneurysms: from computational hemodynamics to biochemical modeling: personalizing flow-diverter intervention for cerebral aneurysms. Int J Numer Meth Biomed Eng 30:1387–1407. https://doi.org/10.1002/cnm.2663
Pei J, Benra F-K, Dohmen HJ (2012) Application of different strategies of partitioned fluid–structure interaction simulation for a single-blade pump impeller. Proc Inst Mech Eng Part E J Proc Mech Eng 226:297–308. https://doi.org/10.1177/0954408911432974
Peiró J, Sherwin S (2005) Finite difference, finite element and finite volume methods for partial differential equations. In: Yip S (ed) Handbook of materials modeling. Springer, Netherlands, pp 2415–2446
Pennati G (2013) Computational fluid dynamics models and congenital heart diseases. Front Pediatr 1:4. https://doi.org/10.3389/fped.2013.00004
Pereira GC (2016) A multiscale haemorheological computer-based model of atherosclerosis: an in-depth investigation of erythrocytes-driven flow characteristics in atheroma development. J Biomed Eng Med Dev 1:1–16. https://doi.org/10.4172/2475-7586.1000119
Pieper S, Halle M, Kikinis R (2004) 3D Slicer. In: 2004 2nd IEEE international symposium on biomedical imaging: macro to nano (IEEE Cat No. 04EX821). IEEE, Arlington, VA, USA, pp 632–635
Pinho N, Bento M, Sousa LC, Pinto S, Castro CF, António CAC, Azevedo E (2017) Patient-specific study of a stenosed carotid artery bifurcation using fluid–structure interactive simulation. VipIMAGE 2017:495–503
Pinho N, Castro CF, António CAC, Bettencourt N, Sousa LC, Pinto SIS (2018) Correlation between geometric parameters of the left coronary artery and hemodynamic descriptors of atherosclerosis: FSI and statistical study. Med Biol Eng Comput 57:715–729. https://doi.org/10.1007/s11517-018-1904-2
Pittaccio S, Migliavacca F, Dubini G et al (2005) On the use of computational models for the quantitative assessment of surgery in congenital heart disease. Anadolu Kardiyol Derg 5:202–209
Plank G, Loewe A, Neic A et al (2021) The openCARP simulation environment for cardiac electrophysiology. Comput Methods Programs Biomed 208:106223. https://doi.org/10.1016/j.cmpb.2021.106223
Putra NK, Palar PS, Anzai H, Shimoyama K, Ohta M (2017) Comparative study between different strut’s cross section shape on minimizing low wall shear stress along stent vicinity via surrogate-based optimization. In: Advances in structural and multidisciplinary optimization, pp 2097–2109
Qiu Y, Wang Y, Fan Y et al (2019) Role of intraluminal thrombus in abdominal aortic aneurysm ruptures: a hemodynamic point of view. Med Phys 46:4263–4275. https://doi.org/10.1002/mp.13658
Qureshi MU, Vaughan GDA, Sainsbury C et al (2014) Numerical simulation of blood flow and pressure drop in the pulmonary arterial and venous circulation. Biomech Model Mechanobiol 13:1137–1154. https://doi.org/10.1007/s10237-014-0563-y
Radaelli AG, Augsburger L, Cebral JR et al (2008) Reproducibility of haemodynamical simulations in a subject-specific stented aneurysm model—a report on the virtual intracranial stenting challenge 2007. J Biomech 41:2069–2081. https://doi.org/10.1016/j.jbiomech.2008.04.035
Rahmani S, Alizadeh M, Tehrani P, Navidbakhsh M (2018) Performance and biomechanical analysis of an intra-aortic cardiac assist device in different boundary conditions. J Mech Sci Technol 32:3995–4002. https://doi.org/10.1007/s12206-018-0750-3
Rahmani SN, Alizadeh M (2018) Investigation of a new prototype of multi-balloons LVAD using FSI. J Braz Soc Mech Sci Eng 40:1–15. https://doi.org/10.1007/s40430-017-0923-5
Rahmani S, Oveysi M, Heidari A, Navidbakhsh M, Alizadeh M (2018) Numerical modeling of a prototype cardiac assist device by implementing fluid–structure interaction. Artery Res 22:24–35. https://doi.org/10.1016/j.artres.2018.01.002
Sadeghpour F, Fatouraee N, Navidbakhsh M (2016) Haemodynamic of blood flow through stenotic aortic valve. J Med Eng Technol 41:108–114. https://doi.org/10.1080/03091902.2016.1226439
Schneiders JJ, Marquering HA, Van Ooij P et al (2015) Additional value of intra-aneurysmal hemodynamics in discriminating ruptured versus unruptured intracranial aneurysms. AJNR Am J Neuroradiol 36:1920–1926. https://doi.org/10.3174/ajnr.A4397
Senter DM, Douglas DR, Strickland WC et al (2021) A semi-automated finite difference mesh creation method for use with immersed boundary software IB2d and IBAMR. Bioinspir Biomim 16:016008. https://doi.org/10.1088/1748-3190/ababb0
Sermesant M, Chabiniok R, Chinchapatnam P et al (2012) Patient-specific electromechanical models of the heart for the prediction of pacing acute effects in CRT: a preliminary clinical validation. Med Image Anal 16:201–215. https://doi.org/10.1016/j.media.2011.07.003
Seshadhri S, Janiga G, Beuing O et al (2011) Impact of stents and flow diverters on hemodynamics in idealized aneurysm models. J Biomech Eng 133:071005. https://doi.org/10.1115/1.4004410
Shadden SC, Hendabadi S (2013) Potential fluid mechanic pathways of platelet activation. Biomech Model Mechanobiol 12:467–474. https://doi.org/10.1007/s10237-012-0417-4
Sigüenza J, Pott D, Mendez S et al (2018) Fluid–structure interaction of a pulsatile flow with an aortic valve model: a combined experimental and numerical study: fluid–structure interaction of a pulsatile flow with an aortic valve model: a combined experimental and numerical study. Int J Numer Meth Biomed Eng 34:e2945. https://doi.org/10.1002/cnm.2945
Simão M, Ferreira JM, Mora-Rodríguez J, Fragata J, Ramos HM (2016) Behaviour of two typical stents towards a new stent evolution. Med Biol Eng Comput 55:1019–1037. https://doi.org/10.1007/s11517-016-1574-x
Simão M, Ferreira JM, Mora-Rodríguez J, Ramos HM (2017) Structural analysis of two different stent configurations. Comput Methods Biomech Biomed Eng 20:869–883. https://doi.org/10.1080/10255842.2017.1306058
Simon HA, Ge L, Sotiropoulos F, Yoganathan AP (2010) Simulation of the three-dimensional hinge flow fields of a bileaflet mechanical heart valve under aortic conditions. Ann Biomed Eng 38:841–853. https://doi.org/10.1007/s10439-009-9857-0
Smith N, de Vecchi A, McCormick M et al (2011) euHeart: personalized and integrated cardiac care using patient-specific cardiovascular modelling. Interface Focus 1:349–364. https://doi.org/10.1098/rsfs.2010.0048
Soifer E, Weiss D, Marom G, Einav S (2016) The effect of pathologic venous valve on neighboring valves: fluid–structure interactions modeling. Med Biol Eng Comput 55:991–999. https://doi.org/10.1007/s11517-016-1575-9
Sonntag SJ, Lipinski E, Neidlin M, Hugenroth K, Benkowski R, Motomura T, Kaufmann TAS (2019) Virtual fitting and hemodynamic simulation of the EVAHEART 2 left ventricular assist device and double-cuff tipless inflow cannula. ASAIO J 65:698–706. https://doi.org/10.1097/mat.0000000000000867
Sotiropoulos F, Borazjani I (2009) A review of state-of-the-art numerical methods for simulating flow through mechanical heart valves. Med Biol Eng Comput 47:245–256. https://doi.org/10.1007/s11517-009-0438-z
Stewart SFC, Hariharan P, Paterson EG et al (2013) Results of FDA’s first interlaboratory computational study of a nozzle with a sudden contraction and conical diffuser. Cardiovasc Eng Tech 4:374–391. https://doi.org/10.1007/s13239-013-0166-2
Stupak E, Rimantas K, Kačeniauskas A, Starikovičius V, Maknickas A, Pacevič R, Staškūnienė M, Davidavicius G, Aidietis A (2017) The geometric model-based patient-specific simulations of turbulent aortic valve flows. Arch Mech 69:317–345
Jin S, Yang Y, Oshinski J, et al (2004) Flow patterns and wall shear stress distributions at atherosclerotic-prone sites in a human left coronary artery—an exploration using combined methods of CT and computational fluid dynamics. In: The 26th annual international conference of the IEEE engineering in medicine and biology society. IEEE, San Francisco, CA, USA, pp 3789–3791
Tang BT, Pickard SS, Chan FP et al (2012) Wall shear stress is decreased in the pulmonary arteries of patients with pulmonary arterial hypertension: an image-based, computational fluid dynamics study. Pulm Circ 2:470–476. https://doi.org/10.4103/2045-8932.105035
Tango AM, Salmonsmith J, Ducci A, Burriesci G (2018) Validation and extension of a fluid–structure interaction model of the healthy aortic valve. Cardiovasc Eng Technol 9:739–751. https://doi.org/10.1007/s13239-018-00391-1
Taylor JO, Meyer RS, Deutsch S, Manning KB (2016) Development of a computational model for macroscopic predictions of device-induced thrombosis. Biomech Model Mechanobiol 15:1713–1731. https://doi.org/10.1007/s10237-016-0793-2
Theodorakakos A, Gavaises M, Andriotis A et al (2008) Simulation of cardiac motion on non-Newtonian, pulsating flow development in the human left anterior descending coronary artery. Phys Med Biol 53:4875–4892. https://doi.org/10.1088/0031-9155/53/18/002
Tu S, Barbato E, Köszegi Z et al (2014) Fractional flow reserve calculation from 3-dimensional quantitative coronary angiography and TIMI frame count. JACC Cardiovasc Interv 7:768–777. https://doi.org/10.1016/j.jcin.2014.03.004
Vahidkhah KA, Ali N (2017) Supra-annular valve-in-valve implantation reduces blood stasis on the transcatheter aortic valve leaflets. J Biomech 58:114–122. https://doi.org/10.1016/j.jbiomech.2017.04.020
Versteeg HK, Malalasekera W (2007) An introduction to computational fluid dynamics: the finite volume method, 2nd edn. Pearson Education Ltd, Harlow, England, New York
Viceconti M, Hunter P (2016) The virtual physiological human: ten years after. Annu Rev Biomed Eng 18:103–123. https://doi.org/10.1146/annurev-bioeng-110915-114742
Wang ZC, Yuan Q, Zhu HW, Shen BS, Tang D (2017) Computational modeling for fluid–structure interaction of bioprosthetic heart valve with different suture density: comparison with dynamic structure simulation. Int J Pattern Recognit Artif Intell 31:1757007. https://doi.org/10.1142/s0218001417570075
Weese J, Groth A, Nickisch H et al (2013) Generating anatomical models of the heart and the aorta from medical images for personalized physiological simulations. Med Biol Eng Comput 51:1209–1219. https://doi.org/10.1007/s11517-012-1027-0
Wentzel JJ, Krams R, Schuurbiers JCH et al (2001) Relationship between neointimal thickness and shear stress after wallstent implantation in human coronary arteries. Circulation 103:1740–1745. https://doi.org/10.1161/01.CIR.103.13.1740
Wilson NM, Ortiz AK, Johnson AB (2013) The vascular model repository: a public resource of medical imaging data and blood flow simulation results. J Med Devices 7:040923. https://doi.org/10.1115/1.4025983
Yoganathan AP, Chandran KB, Sotiropoulos F (2005) Flow in prosthetic heart valves: state-of-the-art and future directions. Ann Biomed Eng 33:1689–1694. https://doi.org/10.1007/s10439-005-8759-z
Yushkevich PA, Gao Y, Gerig G (2016) ITK-SNAP: an interactive tool for semi-automatic segmentation of multi-modality biomedical images. In: 2016 38th Annual international conference of the IEEE engineering in medicine and biology society (EMBC). IEEE, Orlando, FL, USA, pp 3342–3345
Zhang Q, Gao B, Chang Y (2017) Computational analysis of intra-ventricular flow pattern under partial and full support of BJUT-II VAD. Med Sci Monitor 23:1043–1054. https://doi.org/10.12659/msm.900481
Zhao FXX (2013) An overview on interactive medical image segmentation. Ann BMVA 2013:1–22
Zhao YC, Vatankhah P, Goh T et al (2021) Hemodynamic analysis for stenosis microfluidic model of thrombosis with refined computational fluid dynamics simulation. Sci Rep 11:6875. https://doi.org/10.1038/s41598-021-86310-2
Acknowledgements
This work was supported by Sir J. C. Bose National Fellowship, Department of Science and Technology, Government of India (2020-2025).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dake, P.G., Mukherjee, J., Sahu, K.C. et al. Computational Fluid Dynamics in Cardiovascular Engineering: A Comprehensive Review. Trans Indian Natl. Acad. Eng. 9, 335–362 (2024). https://doi.org/10.1007/s41403-024-00478-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41403-024-00478-3