Abstract
Various demographic and clinical predictors attributing to the severity of obstructive sleep apnea (OSA) in Indian subjects have not been extensively studied. Therefore, we aimed to study the various demographic, clinical and other predictors, if any, to corroborate the severity of OSA in the Indian population. We also validated the Epworth Sleepiness Scale (ESS) in the South Indian population, thereby assessing its utility in measuring excessive day time sleepiness (EDS) in this population which has not hitherto been performed. We recruited 145 consecutive subjects with polysomnography (PSG)-proven OSA attending our Comprehensive Sleep Disorder Clinic. Their demographic and clinical details were extracted using a standard proforma and EDS was assessed using ESS. We observed that EDS assessed by ESS positively correlated with the severity of OSA, and increasing body mass index (BMI) but not with age. We also noted that men were more prone to severe OSA than females. When individual questions of ESS were analyzed, only 3 out of 8 were able to strongly differentiate mild-to-moderate OSA from the severe group. We concluded that, severity of OSA correlated well with ESS, but not with age and BMI in Indian population and ESS could be a good indicator to assess the severity of EDS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemiological studies have suggested a high prevalence of undiagnosed sleep disordered breathing (SDB) in middle-aged population [1,2,3,4]. Few such studies from India have shown similar results with prevalence of obstructive sleep apnea (OSA) (AHI > 5/h) ranging from 3.5 to 13.7% and OSA along with excessive daytime somnolence (EDS) ranging from 1.7 to 3.6% [5, 6].
SDB is characterized by airway collapse leading to varying consequences of increased upper airway resistance manifested as loud snoring, hypopneas and apneas with desaturations in sleep [1]. Overnight supervised polysomnography (PSG) is the gold standard for diagnosing OSA [7]. However, the cost factor and limited availability of trained sleep technologists in India prohibits its timely use in all patients with suspected SDB. Demographic and clinical variables like male gender, habitual snoring and obesity are known to have consistent associations with OSA in population-based studies across the world, while EDS has been found to have a variable association [8]. EDS is a serious consequence of SDB, which imposes a substantial burden on the quality of life of the sufferer. It can be measured using different scales, of which, the most widely used one is Epworth Sleepiness Score (ESS) with a cut-off values ≥11 considered as critical [10]. Therefore, it is important to examine the severity and the predictors of OSA using a low-cost methodology in an Indian population to detect problems related to the SDB. In this study, we sought to examine the association between EDS, gender, age and BMI with the severity of OSA in a South Indian cohort. We also analyzed the responses to individual questions in ESS on the severity of OSA.
Materials and methods
Consecutive patients attending our sleep clinic with PSG-proven OSA (AHI > 5 events/h) were recruited for the study. The study was conducted at the Comprehensive Centre for Sleep Disorders, Department of Neurology, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, a tertiary referral center for neurological and cardiovascular disorders in South India from 2009 to 2011. After obtaining the informed consent, the demographic details, height, weight and body mass index (BMI) were collected using a structured proforma. Obesity was defined as per International Obesity task force (IOTF) Asia Pacific perspective as BMI ≥25 kg/m2 [2, 9]. All the patients had their EDS assessed using a vernacular translation of Epworth Sleepiness Scale (ESS) and a score >11 was taken as significant [10]. All subjects were screened for depression and any other psychiatric illness which can also cause EDS, by a psychiatrist with special expertise in treating sleep disorders. All patients who were on any sedatives or sleep-inducing drugs, disorders like hypothyroidism or any neurologic or other non-neurologic disorder which could account for EDS were excluded after a thorough evaluation.
Patients underwent overnight PSG in the sleep laboratory using the BIO-LOGIC System (Heinen und Löwenstein, Bad Ems, Germany). Nocturnal PSG consisted of electroencephalography (EEG), electrooculography (EOG), submental and tibialis anterior electromyography (EMG), oral–nasal airflow measurement using nasal thermistor, chest and abdominal belts, snore microphone, plethysmography and oxygen saturation by pulse oximetry. The sleep-related respiratory parameters were manually analyzed by physicians (AR/SES/PA) specialized in sleep medicine, in accordance with the ICSD-2 guidelines [11]. “Obstructive apnea” was defined as the cessation of airflow through the nostrils and oral cavity for at least 10 s, with continued respiratory efforts. “Hypopnea” was defined as the reduction of airflow during sleep by at least 30% for 10 s or more, with a 4% decrease in oxygen saturation. The apnea-hypopnea index is the number of apneic or hypopneic episodes occurring during 1 h of sleep. Individuals with an apnea-hypopnea index (AHI) greater than 5 were defined as having OSA. An AHI of 5–15 indicated mild OSA, 16–30 moderate OSA and >30 severe OSA.
Statistical analysis
Statistical analysis was performed using SPSS package version 17 (SPSS Inc, Illinois, Chicago). Unpaired t test and Chi-square test were used to test observed differences in quantitative and categorical variables, respectively. The Kruskal–Wallis test was used to compare the ESS scores at various levels of severity of OSA. As all variable distributions were not normal, Spearman’s rank correlation coefficients were used to explore linear dependence between ranked quantitative variables. Variables were considered for multivariate modeling if p < 0.1 in univariate analysis. Binary logistic regression analysis was employed to test for independent association of selected variables with severe OSA. The adjusted odds ratios and 95% confidence intervals (OR, 95% CI) depicted are prevalence odds ratios and may accurately predict existence of risk rather than actual quantification of the risk. Since all the study subjects were patients, it is beyond the scope of this study to measure the sensitivity and specificity of ESS scores for diagnosis of OSA. However, a receiver operating characteristic (ROC) curve analysis was attempted to explore the ability of ESS scores at various levels to correctly predict severe OSA. Chi-square tests or Fisher exact tests were used to compare responses to each question of the ESS made by patients with severe OSA with corresponding responses of those with mild-to-moderate OSA.
Results
145 consecutive patients attending the sleep clinic, with PSG proved OSA of varying severity formed the cohort for our study. We had 47 patients with mild OSA, 40 with moderate OSA and 58 with severe OSA in our study. Our study cohort were middle-aged persons (51.7 ± 13.3 years), predominantly male population (M:F = 112:33). The overall prevalence of obesity was very high. The mean BMI was marginally higher in severe OSA group (29.3 ± 4.3) when compared with the mild and moderate group (28.1 ± 5.4), but it did not reach statistical significance (Table 1 ). However, ESS was higher in patients with OSA of all severity. Mean ESS scores increased with the severity of OSA and the association was statistically significant (p = 0.004).
Increasing age was significantly associated with higher BMI (p = 0.019), but not with higher ESS values (p = 0.19) or severity of OSA (p = 0.78). Though, we observed a positive correlation between BMI and ESS scores (p < 0.068), BMI and AHI (p < 0.084), it did not reach statistical significance (Table 2). On performing simple linear regression analysis, ESS score and AHI index were found to be linearly related with a R 2 = 0.083 (Fig. 1). While females were protected (adjusted OR 9.243, 95% CI 2.571–33.229), a unit increase of ESS score increased the risk of severe OSA (adjusted OR 1.088, 95% CI 1.011–1.171). Rather than a unit increase in BMI, being overweight/obese increased the risk of severe OSA (adjusted OR 3.063, 95% CI 1.264–7.421) (Table 3).
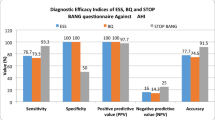
The receiver operating characteristic curve (ROC) curve analysis for correct prediction of severe OSA yielded an area under the curve of 0.632 (p = 0.008; 95% CI 0.54–0.73) and an ESS score of over 15.5 had 60.4% sensitivity and 60.9% specificity in predicting severe cases (Fig. 2). Table 4 shows the cut-off values of ESS and their respective sensitivity and specificity along with positive and negative predictive values.
While analyzing responses to individual questions in ESS, the questions on dozing off while “sitting and reading”, “sitting inactive in a public place” and “sitting quietly after a lunch without alcohol” elicited significantly higher proportions of positive responses from subjects with severe OSA as compared to those with mild-to-moderate OSA. Ranked correlations of responses to individual questions and severity (mild, moderate or severe) of OSA were also done. The questions—“sitting and reading” (p < 0.001), “watching TV” (p = 0.002), “sitting quietly after a lunch without alcohol” (p = 0.035) and “in a car, while stopping for a few minutes in traffic” (p = 0.047) had statistically significant correlation coefficients. Neither the cross-tabulations nor the rank correlations revealed any significance for the questions “passenger in a car for an hour without a break”, and “lying down to rest in the afternoon when circumstances permit” as shown in Table 5.
Discussion
In this study, we analyzed the demographic and clinical predictors of the severity of OSA in South Indian patients. We had 145 subjects with OSA of varying severity, with comparable baseline characteristics. We observed that excessive day time sleepiness assessed by ESS was positively correlated with severity of OSA, and increasing BMI but not with age. We also noted that men were more prone to severe OSA than females. When individual questions of ESS were analyzed, only 3 out of 8 were able to strongly differentiate mild-to-moderate OSA from the severe group. Our patients’ age group is comparable to what is reported in various population-based studies across the globe [1, 4, 12]. Most of the Western and Indian studies have put a male-to-female prevalence ratio of 2–3.7:1, which is comparable to our patient cohort [1, 13]. However, some clinic-based studies have reported 8–10 times higher risk for OSA in males, which might be a referral bias [14].
We could not find an association between age and severity of OSA. Studies analyzing the impact of age on OSA and its severity have yielded mixed results across the world. A Swedish study found a higher risk of OSA in the fifth decade, while a British study found a less robust association of OSA with age [2, 15]. Bixler et al. reported that increasing age had a significant association with OSA, which has been found in other Asian studies as well [3, 16, 17]. Interestingly, the same authors found a decrease in severity of OSA with age [18]. Few Indian authors have reported an association between increasing age and OSA, but have not found any association with severity of OSA [19]. We found that mean age of our study population was in the early 50s. Less number of older subjects in our study group might have contributed to this result.
Our cohort of OSA comprising patients of all severity (mild to moderate versus severe) had a mean BMI falling in the range of obesity (as per Asian standards [20]) which had a positive correlation with age. However, we failed to find a positive correlation between increasing BMI and OSA severity. That is, even though obesity increases the risk of developing OSA, there was no linear relationship between increasing BMI and severity of OSA in our population.
All the Western and Asian studies have found an independent association between BMI and OSA. Udwadia et al. reported a positive correlation of increasing BMI with increasing severity of OSA [21]. A case control study from Delhi reported higher BMI in cases, but their sample size was small to correlate it with severity of OSA [22]. A community-based study from Delhi reported BMI >25 as a significant predictor of OSA [13]. But Sleep Heart Health Study reported a less robust association between severity of OSA and BMI [12]. They also observed that in older patients, body habitus, snoring and other clinical parameters were less effective in predicting OSA and its severity.
We found that only 3 of the questions of ESS had very good independent discriminative power in differentiating severity of OSA between subjects. Previous studies have shown that the ESS is very effective in differentiating pure snorers from snores with OSA [23]. In a review published by Rosenthal et al. assessed the sensitivity of ESS in identification of OSA, and found fair discriminatory ability as a screening tool for OSA [24]. However, the overall ESS score in our study cohort had optimal discriminatory power to differentiate between mild-to-moderate and severe OSA. Rosenthal et al. also suggested a score ≥8 as a cut-off among clinical populations being screened for sleep disorders. Some other authors have also suggested a similar cut-off for detecting EDS in elderly subjects [25, 26]. Reason often cited is that >60% of subjects were not able to answer at least one question of ESS. Zhang et al. used a modified Chinese version of ESS where they omitted “question 8” on “driving” and included one more question—“sleepiness while working or studying in the late afternoon without post lunch nap” and found that it correlated well not only with OSA, but also could discriminate between OSA of variable severity [27]. Another study in Chinese population found that in patients with comparable BMI, OSA was more severe in men [17]. We also found a protective effect of female gender on OSA severity. Excessive daytime sleepiness is a serious consequence of SDB, imposes a substantial burden on the quality of life.
When ESS–AHI correlation was analyzed by several authors, conflicting results were noted. Many authors could not find a correlation between severity of OSA and ESS score [28, 29]. They found a better association with nocturnal hypoxemia rather than AHI to EDS severity. But few others found a very good correlation between EDS and higher AHI [30]. Few Indian studies have found good discriminative power of ESS for detecting OSA from controls [21, 22]. But they did not assess its ability to discriminate OSA of varying severity. Reddy et al. who published a community-based study on OSA could not find a correlation between OSA severity and EDS [13].
Our study on demographic and clinical predictors of OSA is not without limitations. We did not have an age- and gender-matched control group and hence could not assess sensitivity and specificity of ESS in predicting OSA. However, ESS was never intended as a screening tool for OSA in general population, as EDS has umpteen number of causes. We could not assess the impact of BMI on gender effects due to the weaker strength of female subjects in our study cohort with AHI > 30 (n = 3). We did not extract information on snoring and witnessed apneas which have been shown to have consistent association with severe OSA [4]. We did not study other polysomnography parameters like nocturnal hypoxemia which also has been reported to have an association with EDS [29]. Whether these clinical and polysomnographic parameters would have probably helped us predict OSA better in our population needs to be studied further.
Despite these limitations, our study is one of its kind from South India with a large sample size of 145 subjects. Moreover, all the participants had undergone PSG and we looked into predictors of severity of OSA based on PSG results and the validation of ESS in our local population were done. A similar validation of ESS to detect SDB in Hindi speaking persons in North India, 115 were assessed by the Hindi version of ESS and 95 further underwent PSG also concluded that the Hindi version of the ESS had a good internal consistency and a strong correlation with the English version [31]. We too found that ESS is indeed a useful tool to predict OSA severity in our population, despite the fact that only 3 individual questions of the 8-point scale had a statistically significant correlation with OSA severity. Even though majority of our patients were obese, we could not find a significant difference in BMI between mild-to-moderate versus severe OSA cohort. Female gender was protective against severe OSA. Each population should try to validate such universally accepted scales which are often followed blindly in one’s own population before one starts to apply it on our patients.
References
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5.
Gislason T, Almqvist M, Eriksson G, Taube A, Boman G. Prevalence of sleep apnea syndrome among Swedish men—an epidemiological study. J Clin Epidemiol. 1988;41:571–6.
Bixler EO, Vgontzas NA, Lin HM, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women—effects of gender. Am J Respir Crit Care Med. 2001;163:608–13.
Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea–hypopnea and related clinical features in a population-based sample of subjects aged 30–70 year. Am J Resp Crit Care Med. 2001;163:685–9.
Vijayan VK, Patial V. Prevalence of obstructive sleep apnea syndrome in Delhi, India. Chest J. 2006;130:92S.
Sharma SK, Kumpawat S, Banga A, Goel A. Prevalence and risk factors of obstructive sleep apnea syndrome in a population in Delhi, India. Chest J. 2006;130:149–56.
Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester: American Academy of Sleep Medicine; 2007.
Sharma SK, Ahluwalia G. Epidemiology of adult obstructive sleep apnea in India. Indian J Med Res. 2010;131:121–5.
WHO/IASO/IOTF. The Asia-Pacific perspective: redefining obesity and its treatment. Melbourne: Health Communications Australia; 2000.
Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5.
International Classification of Sleep Disorders. Diagnostic and coding manual. Westchester: American Academy of Sleep Medicine; 2005.
Young T, Shahar E, Nieto J, et al. Predictors of sleep disordered breathing in community dwelling adults the sleep heart health study. Arch Intern Med. 2002;162:893–900.
Reddy EV, Kadhiravan T, Mishra HK, Sreenivas V, Handa KK, Sinha S, et al. Prevalence and risk factors of obstructive sleep apnea among middle-aged urban Indians: a community-based study. Sleep Med. 2009;10:913–8.
Guilleminault C, Quera-Salva MA, Partinen M, Jamieson A. Women and the obstructive sleep apnea syndrome. Chest. 1988;93:104–9.
Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46:85–90.
Ip MS, Lam B, Lauder IJ, Tsang KW, Chung KF, Mok YW, Lam WK. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119:62–9.
Ip MS, Lam B, Tang LC, Lauder IJ, Ip TY, Lam WK. A community study of sleep-disordered breathing in middle-aged Chinese women in Hong Kong: prevalence and gender differences. Chest. 2004;125:127–34.
Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: i. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8.
Sharma SK, Reddy EV, Sharma A, Kadhiravan T, Mishra HK, Sreenivas V, et al. Prevalence and risk factors of syndromes in urban Indians. Sleep Med. 2010;11:562–8.
WHO Expert Consultation. Appropriate-body mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63.
Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged Urban Indian men. AJRCCM. 2004;169:168–73.
Pradeep Kumar VG, Bhatia M, Tripathi M, Srivastava AK, Jain S. Obstructive sleep apnoea: a case-control study. Neurol India. 2003;51:497–9.
Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea: the Epworth Sleepiness Scale. Chest. 1993;103:30–6.
Rosenthal LD, Dolan DC. The Epworth Sleepiness Scale in the identification of obstructive sleep apnea. J Nerv Ment Dis. 2008;196:429–31.
Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4578 elderly persons: the cardiovascular health study. Sleep. 1998;21:27–36.
Onen F, Moreau T, Gooneratne NS, Petit C, Falissard B, Onen SH. Limits of the Epworth Sleepiness Scale in older adults sleep breath. 2012;12:700–8.
Zhang JN, Peng B, Zhao TT, Xiang M, Fu W, Peng Y. Modification of the Epworth Sleepiness Scale in Central China. Qual Life Res. 2011;20:1721–6.
Sauter C, Asenbaum S, Popovic R, Bauer H, Lamm C, Klösch G, et al. Excessive daytime sleepiness in patients suffering from different levels of obstructive sleep apnoea syndrome. J Sleep Res. 2000;9:293–301.
Mediano O, Barcelo A, de la Pen˜a M, Gozal D, Agustı A, Barbe F. Daytime sleepiness and polysomnographic variables in sleep apnea patients. Eur Resp J. 2007;30:110–3.
Hesselbacher S, Subramanian S, Allen J, Surani S, Surani S. Body mass index gender, and ethnic variations alter the clinical implications of the Epworth Sleepiness Scale in patients with suspected obstructive sleep apnea. Open Respir Med J. 2012;6:20–7.
Kanabar K, Sharma SK, Sreenivas V, Biswas A, Soneja M. Validation of Hindi version of the Epworth Sleepiness Scale (ESS) at AIIMS, New Delhi in sleep-disordered breathing. Sleep Breath. 2016;. doi:10.1007/s11325-016-1344-x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakersʼ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Sreedharan, S.E., Vijayakumari, A.A., Agrawal, P. et al. Does obstructive sleep apnea correlate with Epworth Sleepiness Scale in an Indian population?. Sleep Biol. Rhythms 15, 89–95 (2017). https://doi.org/10.1007/s41105-016-0085-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41105-016-0085-3