Abstract
The gold standard for diagnosis of Obstructive sleep apnea (OSA) is an overnight polysomnography (PSG). However, PSG is time consuming, labour intensive and expensive. In our country PSG is not available everywhere. Therefore, a simple and reliable method of identifying patients of OSA is important for its prompt diagnosis and treatment. This study looks at the efficacy of three questionnaires to serve as a screening test for the diagnosis of OSA in the Indian population. For the first time in India, a prospective study was conducted wherein patients with history of OSA underwent PSG and were asked to fill three questionnaires-Epworth Sleepiness Score (ESS), Berlin Questionnaire (BQ) and Stop Bang Questionnaire (SBQ). The scoring of these questionnaires were compared with the PSG results. SBQ had a high negative predictive value (NPV) and the probability of moderate and severe OSA steadily increases with higher SBQ scores. In comparison, ESS and BQ had low NPV. SBQ is a useful clinical tool to identify patients at high risk of OSA and can facilitate in the diagnosis of unrecognised OSA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnoea (OSA) is a chronic condition characterized by frequent episodes of upper airway collapse during sleep [1]. Its effect on nocturnal sleep quality and ensuing daytime fatigue and sleepiness are widely acknowledged. Despite the numerous advancements in understanding the pathogenesis and clinical consequences of the disorder, a majority of those affected remain undiagnosed. Individuals with OSA are rarely aware of difficult breathing, even upon awakening. It is often recognized by others who observe the individual during episodes or is suspected because of its effects on the body. The symptoms may be present for years or even decades without diagnosis, during which the individual may become conditioned to daytime sleepiness and fatigue.
Up to 80% of men and 93% of women with moderate severe sleep apnoea are undiagnosed and a large proportion of surgical patients with OSA remain unrecognized [2]. Identifying OSA preoperatively helps to reduce postoperative complications. The proper identification and management of patients with continuous positive airway pressure (CPAP) appears to nullify the risk of OSA, prevent postoperative exacerbation of OSA, and may even improve patient’s health well beyond the pre-operative period [3]. Given the adverse consequences associated with untreated OSA, prompt diagnosis and treatment of unrecognised OSA is critical.
The gold standard for diagnosis of OSA is polysomnography (PSG) which is an objective test [4, 5]. PSG is a non-invasive technique that involves overnight monitoring of several physiological variables including electroencephalography EEG), electrocardiography (ECG), eye movements, muscle tone as well as respiratory effort, airflow and oxygen saturation.
The use of a brief and precise screening tool can assist general practitioners, surgeons, or sleep specialists in the early detection of OSA. This is especially needed in resource-poor countries where PSG is unavailable. A screening questionnaire helps to identify patients with undiagnosed OSA. The Epworth Sleepiness Scale (ESS), Berlin questionnaire (BQ) and STOP BANG questionnaire (SBQ) are some of the questionnaires used for screening. We conducted a study where we determined the severity of OSA using the BQ, SBQ and ESS. This was compared to the results obtained by PSG. This data was used to assess the accuracy levels of BQ, SBQ and ESS as a screening modality for the Indian population.
Methodology
Study design: A prospective study.
Study period: November 2018 to January 2020.
Study population: Patients coming in the ENT Outpatient department or admitted at K.E.M. Hospital, Pune.
Inclusion criteria: All patients above 18 years with history suggestive of sleep apnoea and/or snoring.
Exclusion criteria: All patients below the age of 18 years.
Method of measurement of outcome of interest:
Subjective Analysis
Every patient was given a patient information sheet and consent for participation. After confirming participation, a detailed history regarding symptoms, onset, duration and progression along with height, weight and other parameters were noted. Patients were given a set of questionnaires comprising of the ESS, Berlin and SBQ. These submitted questionnaires were scored and patients were divided into those with a high or low risk of OSA.
Objective Analysis
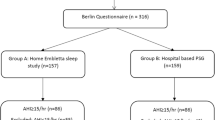
All patients above 18 years with history suggestive of sleep apnoea and/or snoring referred from ENT surgeon, chest physician or general physician were reviewed by a chest physician prior for their sleep study. Detail history was taken to identify the symptom and assessment of co-morbidities. After explanation of the procedure patients without co-morbidities were offered a choice of a hospital or portable home sleep study.
A level 2 sleep study was performed at patients’ home. A level 2 sleep study refers to an unattended PSG study where no medical staff is present.
Alice PDX which is a type 2 sleep diagnostic device was used for this study. The basic channel set measures oral-nasal airflow and pressure via cannula and thermistor, respiratory efforts via abdominal and chest belts and arterial oxygen saturation level via the pulse oximeter (% SPO2 and pulse rate). The device also detects body position. In addition to the basic channel set indicators, the Alice PDX contains sensors for the recording of EEG, ECG and EOG.
The PSG studies were scored by an experienced chest physician according to the rules of American Association of Sleep Medicine.
Studies with more than 6 h of sleep and the key channels i.e., Respiratory bands, EEG, airflow and pulse oximetry present for almost 90% of sleep time were accepted as valid studies.
The results of the questionnaires, ESS, Berlin questionnaires (BQ) and STOP BANG questionnaires (SBQ) were compared with the Apnoea Hypopnea index (AHI). The accuracy rate, PPV (positive predictive value) and NPV (negative predictive value) of each questionnaire was calculated.
Sample size was calculated to be 94 using formula \({\text{n}} = {\text{z}}^{2} \frac{{{\text{pq}}}}{{\left( {me} \right)^{2} }}\).
Results and Statistical Analysis
Table 1 shows the comparison of the ESS with the AHI calculated by PSG. The assessment of the incidence of risk by the ESS compares significantly with the AHI for the prediction of OSA (P value < 0.01). However, the relatively lower Cohen Kappa value of 0.219 indicates none to slight level of agreement between ESS and AHI. The sensitivity, specificity, PPV, NPV and accuracy of ESS for detecting OSA against AHI as a gold standard was 76.7%, 100.0%, 100.0%, 16.0% and 77.7% respectively (Table 4).
Table 2 shows the comparison of the BQ with the AHI.
The results show a significant association with the AHI for the prediction of OSA (P value < 0.01). However, a Cohen Kappa value of 0.190 indicates none to slight level of agreement between BQ and AHI. The sensitivity, specificity, PPV, NPV and accuracy of BQ for detecting OSA against AHI as a gold standard was 73.3%, 100.0%, 100.0%, 14.3% and 74.5% respectively (Table 4).
Table 3 compares the SBQ with AHI. The results show a significant association with the AHI for the prediction of OSA (P value < 0.05). The lower to moderate Cohen Kappa value of 0.293 indicates a fair level of agreement between SBQ and AHI. The sensitivity, specificity, PPV, NPV and accuracy of SBQ for detecting OSA against AHI as a gold standard was 93.3%, 50.0%, 97.7%, 25.0% and 91.5% respectively (Table 4).
Discussion
Diagnosing OSA is clinically relevant because untreated OSA has been associated with increased morbidity and mortality. Given the relatively high prevalence of undiagnosed and untreated OSA and its associated cardiovascular, respiratory and neurocognitive morbidities [5, 6], a simple and effective OSA screening tool is essential. This approach is especially important for surgeries as often there is insufficient time to complete a preoperative assessment of OSA with the standard diagnostic approach.
History and clinical findings lack sensitivity and specificity in diagnosing OSA. It predicts OSA in 50% of patients. Currently, overnight PSG is the gold standard for diagnosis of OSA [4]. PSG is a comprehensive test to study sleep and diagnose a variety of sleep disorders. It is a procedure that collect physiologic parameters during sleep. It involves EEG, EOG, pulse oximetry as well as airflow, respiratory effort to evaluate underlying causes of sleep disorders which include OSA, central sleep apnoea, sleep related hypoventilation etc. PSG can also be used to evaluate other sleep disorders including nocturnal seizures, narcolepsy, periodic limb disorder and REM related disorders. This study can be done in hospital under supervision of sleep technician or even at home to confirm a diagnosis of moderate to severe OSA in the absence of co-morbid medical conditions or other suspected sleep disorder. It is a non-invasive test. A variety of sensors and belts are taped to the body to record brain waves, eye movements, heart rate and breathing patterns overnight. The oxygen saturation is measured with pulse oximeter. Recordings of electroencephalogram, electrooculogram, electromyography, electrocardiogram, oximetry, airflow and breathing effort provide important data to evaluate the severity of disease [7]. OSA is diagnosed when the apnoea– hypopnea index (AHI), i.e., the total number of obstructive apneas and hypopneas per hour of sleep is more than 5. An apnoea is defined as the complete cessation of airflow for a minimum of 10 s [8, 9]. The definition of a hypopnea includes a reduction of airflow that is associated with either an oxygen desaturation (of at least 3% or 4%) or an arousal [8, 9]. The severity of OSA is graded according to commonly used clinical criteria as mild (AHI ≥ 5 but < 15), moderate (AHI ≥ 15 but < 30), or severe (AHI ≥ 30) [8]. However, the test is expensive, time consuming, difficult to access outside big cities and is always not well accepted by patients. There are various screening questionnaires which have been developed to enable early diagnosis of OSA [3].
An ideal screening tool for detecting OSA should consider sleep domains, feasibility and diagnostic accuracy. We used three questionnaires namely ESS, BQ and SBQ as a screening tool for detecting OSA in this study. These questionnaires involve questions related to sleep disturbances and can be answered easily either with a simple yes or no response or can be scored according to symptom severity. In this study we have tried to identify the best questionnaire for screening OSA in the Indian population.
The ESS is an eight-item questionnaire to measure daytime sleepiness. It has a four-point Likert response format (0–3), and the score ranges from 0 to 24. ESS score ≥ 10 indicates excessive daytime sleepiness and high risk for OSA [10]. BQ was developed in 1999 and includes three sections [6]. The first section is about snoring, the second section is about daytime fatigue and sleepiness and the last section is about medical history and anthropometric measures such as hypertension and BMI. If two or more categories were positive, the patient is considered to be of high risk for OSA [11].
The STOP-BANG questionnaire includes four subjective items (STOP: snoring, tiredness, observed apnoea, and high blood pressure) and four demographics items (Bang: body mass index [BMI], age, neck circumference, gender) [12]. A positive response to three or more items is categorized as high risk for OSA [13].
The BQ focuses on symptoms related to OSA and the ESS has been developed for assessing the level of excessive daytime sleepiness rather than OSA.
The content of the SBQ has been developed both according to the OSA-related symptoms (i.e., snoring, tiredness during the daytime, stopped breathing during sleep, and hypertension) and clinical characteristics (BMI, age, neck circumference, and gender). Concerning feasibility, the SBQ consists of four self-administered questions along with four items for demographic data and clinical characteristics collection (BMI, age, neck circumference, and sex). All responses are rated as yes or no [4]. Because the SBQ includes few questions and requires short administration time, a high response rate (91.2–91.5%) has been observed in previous studies.
In this study all 94 patients underwent PSG. Patients having symptoms like excessive sleepiness, daytime tiredness, disturbed sleep, snoring etc. were referred for PSG. All patients in the study were given ESS, BQ and SBQ to fill and their final results were compared.
Of the 94 cases studied, as per PSG reports, 4 (4.3%) were normal (AHI < 5), 14 (14.9%) had mild OSA (AHI 5–14), 29 (30.9%) had moderate OSA (AHI 15–29) and 47 (50.0%) had severe OSA (AHI > 30).
The ESS showed that out of 94 cases, 25 (26.6%) had low risk (ESS score < 10) and 69 (73.4%) had high risk (ESS score ≥ 10) of having OSA. It was shown that distribution of levels of ESS were significantly associated with overall AHI grading for the prediction of OSA (P value < 0.01) with relatively lower Cohen Kappa value of 0.219. Lower Kappa value indicates none to slight level of agreement between ESS and AHI. The sensitivity, specificity, PPV, NPV and accuracy of ESS for detecting OSA against AHI was found to be 76.7%, 100.0%, 100.0%, 16.0% and 77.7% respectively. Thus, our study revealed that ESS has low sensitivity in detecting different grades of OSA. This finding was consistent with the study carried out by Leticia Boeri et al. [7]. They concluded that ESS could detect normal and severe levels of apnoea, but is not able to detect mild and moderate levels. They also found out that ESS can be used in the follow-up of patients with OSA, but it cannot replace PSG because it does not detect all levels of apnoea.
Ng et al. in 2019, found that the BQ was unreliable in predicting OSA as compared to PSG-AHI [14, 15]. Our study had similar findings, where distribution of levels of BQ was significantly associated with overall AHI grading for the prediction of OSA (P value < 0.01) with relatively lower Cohen Kappa value of 0.190. Lower Kappa value indicates none to slight level of agreement between BQ and AHI. The sensitivity, specificity, PPV, NPV and accuracy of BQ for detecting OSA against AHI was calculated to be 73.3%, 100.0%, 100.0%, 14.3% and 74.5% respectively. It shows BQ has equal specificity and PPV as ESS, but is less accurate. Although BQ is less sensitive than ESS in detecting OSA, it can be used to detect high and low risk patients of OSA.
Systematic meta-analysis done in 2015 by Mahesh Nagappan, Pu Liao & team confirms the high performance of the STOP-Bang questionnaire in the sleep clinic and surgical population for screening of OSA [12]. The higher the STOP-Bang score, the greater is the probability of moderate-to-severe OSA [16].
In our cohort 8 (8.5%) had Low risk (score 0–2), 30 (31.9%) had Intermediate risk (score 3–4) and 56 (59.6%) had High risk (score 5–8) as per the SBQ. This co-related significantly with overall the AHI grading or the prediction of OSA (P value < 0.05) with relatively lower to moderate Cohen Kappa value of 0.293. Lower to moderate Kappa value indicates fair level of agreement between SBQ and AHI. The sensitivity, specificity, PPV, NPV and accuracy of STOP BANG questionnaire for detecting OSA against AHI as a Gold standard was 93.3%, 50.0%, 97.7%, 25.0% and 91.5% respectively. Thus, SBQ is the most accurate amongst the three questionnaires. Also, the high negative predictive value of the STOP-Bang questionnaire indicated that it is more sensitive than the other two questionnaire.
Studies have shown that STOP-Bang questionnaire has been validated to be an excellent screening tool for OSA in sleep clinic and surgical population [16]. The probability of moderate and severe OSA steadily increases with higher STOP-Bang scores [16]. These characteristics make the STOP-Bang questionnaire a useful clinical tool to identify patients at high risk of OSA and can facilitate the diagnosis and treatment of unrecognised OSA. Furthermore, concerning diagnostic accuracy, we observed that the SBQ had excellent sensitivity for detecting OSA in different populations, however, the unsatisfactory specificity of this questionnaire limits its practicability [17].
In this study the specificity of BQ was more than SBQ which is consistent with the systematic review performed by Abrishami et al. [18]. They found out that BQ was the second and SBQ was the third most sensitive questionnaire in people without a history of sleep disorders. They concluded that SBQ had high quality methodological and reasonably accurate results [17].
In this study, when evaluating the predictive values of Berlin questionnaire, STOP-BANG and ESS to identify patients at risk for OSA, STOP BANG had the highest sensitivity, but BERLIN and ESS had the highest specificity and PPV. The NPV and accuracy of STOP-BANG was highest.
The STOP-Bang questionnaire in our study proved to be a concise, effective, and reliable OSA screening tool. It can facilitate efficient allocation of resources in both diagnosing and treating previously unrecognised OSA. The probability of moderate to severe OSA increases in direct proportion to the STOP-Bang score, which makes the questionnaire an easily used tool for identifying patients at high risk for OSA (Figs. 1, 2, 3, 4).
Conclusion
Despite numerous advancements in understanding of the pathogenesis and clinical consequences of OSA, a majority of those affected remain undiagnosed. History and clinical findings have shown to lack sensitivity and specificity in diagnosing OSA.
Polysomnography is the gold standard for diagnosis of OSA. However, this is not available easily in all parts of our country. A screening tool in the form of a questionnaire will enable early diagnosis and intervention. Although SBQ has low specificity, its accuracy is relatively higher and it also has higher NPV. Thus, it can be concluded that among the different questionnaires, namely ESS, BQ and SBQ, SBQ is concise, effective, more reliable and preferable questionnaire for screening of OSA in the Indian population.
Abbreviations
- AHI:
-
Apnoea Hypopnea Index
- BQ:
-
Berlin questionnaire
- CPAP:
-
Continuous positive airway pressure
- ESS:
-
Epworth Sleepiness Scale
- NPV:
-
Negative predictive value
- OSA:
-
Obstructive sleep apnoea
- PPV:
-
Positive predictive value
- PSG:
-
Polysomnography
References
Kim B, Lee EM, Chung YS, Kim WS, Lee SA (2015) The utility of three screening questionnaires for obstructive sleep apnea in a sleep clinic setting. Yonsei Med J 56(3):684–690
Jordan AS, McEvory RD (2003) Gender differences in sleep apnea: epidemiology, clinical aspects and pathological mechanisms. Sleep Med Rev 7(5):377–389
Mbata GC, Chukwuka JC (2012) Obstructive sleep apnea-hypopnea syndrome. Ann Med Health Sci Res 2(1):74–77
Chung F, Chau E, Yang Y, Liao P, Hall R, Mokhlesi B (2013) Serum bicarbonate level improves specificity of STOP-bang screening for obstructive sleep apnea. Chest 143(5):1284–1293
Chung F, Yang Y, Brown R, Liao P (2014) Alternative scoring models of STOP bang questionnaire improve specificity to detect undiagnosed OSA. J Clin Sleep Med 10(9):951–958
Kang K, Park KS, Kim JE, Kim SW, Kim YT, Kim JS, Lee HW (2013) Usefulness of the Berlin questionnaire to identify patients at high risk for obstructive sleep apnea: a population-based door-to-door study. Sleep Breath 17(2):803–810
Boari L, Cavalcanti CM, Bannwart S, Sofia O, Dolci J (2004) Evaluation of Epworth Sleepiness Scale in patients with obstructive sleep apnea-hypoapnea syndrome. Rev Bras Otorrinolaringol 70:722–723
Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J (2002) Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 6(2):49–54
Kapor V, Ackley D, Chowdhuri S et al (2017) Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Sleep Med 13(3):479–504
(2000) Obstructive sleep apnea, polysomnography, and split-night studies: consensus statement of the connecticut thoracic society and the Connecticut neurological society; Conn Med 64(8):465–468
Chiu HY, Chen PY, Chuang LP et al (2017) Diagnostic accuracy of the Berlin questionnaire, STOP BANG and Epworth sleepiness scale in detecting OSA: a bivariate meta-analysis. Sleep Med Rev 36:57–70
Nagappa M, Liao P, Wong J et al (2015) Validation of the STOP Bang questionnaire as a screening tool for OSA among different populations: a systematic review and meta-analysis. PLoS ONE 10(12):1371
Goyal M, Johnson J (2017) Obstructive sleep apnea diagnosis and management. Mo Med 114(2):120–124
Friedman M, Wilson MN, Pulver T et al (2010) Screening for obstructive sleep apnea/hypopnea syndrome: subjective and objective factors. Otolaryngol Head Neck Surg 142(4):531–535
Ng SS, Tam W, Chan TO et al (2019) Use of Berlin questionnaire in comparison to polysomnography and home sleep study in patients with obstructive sleep apnea. Respir Res 20(1):40
Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y (2012) High STOP Bang score indicates a high probability of obstructive sleep apnea. Br J Aneesh 108(5):768–775
Chung F, Yang Y, Liao P (2013) Predictive performance of the STOP-Bang score for identifying obstructive sleep apnea in obese patients. Obes Surg 23(12):2050–2057
Abrishami A, Khajehdehi A, Chung F (2010) A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anish 57(5):423–438
Acknowledgements
I would like to express my gratitude and appreciation to all those who gave me the possibility to complete this article. Special thanks are to Dr Smita Dhadge whose help has been substantial in writing this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nair, A.S., Vaze, V. & Vaid, N. Comparative Study of Subjective and Objective Analysis in Diagnosis of Obstructive Sleep Apnea. Indian J Otolaryngol Head Neck Surg 75 (Suppl 1), 715–722 (2023). https://doi.org/10.1007/s12070-022-03264-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-022-03264-1