Abstract
The resistance of green microalga Chlorella sorokiniana to the effect of certain toxicants (sodium dodecyl sulfate, formaldehyde, metribuzin) in the presence of activated sludge and the fungus Penicillium ochrochloron Biourge was studied using chlorophyll fluorescence methods. Detoxification of these substances increased under combined application of microalgae and activated sludge. Addition of activated sludge increased the photosynthetic activity of microalgae in the presence of sodium dodecyl sulfate (50 mg/L) and the herbicide metribuzin (0.01 and 0.05 mg/L). This was manifested in high values of the maximum quantum yield of photosystem II (FV/FM). Addition of the fungus P. ochrochloron revealed a decreasing toxic effect of the herbicide metribuzin at concentrations of 0.01 and 0.05 mg/L on the microalgae activity. Thus, the addition of activated sludge and the fungus P. ochrochloron to C. sorokiniana culture can be recommended in wastewater treatment technologies using microalgae, and chlorophyll fluorescence parameters (FV/FM) can be effectively used as indicators of the physiological state of microalgae in bioreactors under industrial conditions.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewater treatment using microalgae in photobioreactors (PBR) has been known since the 1960s. Currently, such photobioreactors for urban wastewater treatment operate as modern systems with various technical solutions at all stages of biomass production [1, 2]. Since then, various PBR systems of both open and closed type have been developed. However, both types of reactors cannot be considered monoculture reactors with constant quality of influent substrate.
Despite obvious advantages of using microalgae in wastewater treatment technologies, microalgae are very sensitive to the composition of influent wastewater, and their growth can be inhibited by various pollutants. At the same time, wastewater treatment efficiency depends on the microalgae growth rate and the amount of algal biomass.
Composition of domestic wastewater may alter due to new organic pollutants, and microalgae systems must be able to reduce the concentrations of toxicants in the photobioreactor [1]. It has been shown that microalgae can remove not only mineral nitrogen and phosphorus, but also some organic pollutants [3]. While microalgae can be highly effective in removing certain contaminants, the unexpected influx of new toxicants into wastewater can damage microalgae cells and reduce the effectiveness of treatment. Therefore, it is necessary to control the potential impact of individual contaminants (e.g., organic compounds from household products, pesticides, antibiotics) on the activity of microalgae in the treatment process [1, 4]. To solve this problem, some researchers studied the effect of typical pollutants in model experiments with microalgae cultures and determined the maximum acceptable toxicant concentrations that affect microalgae performance in bioreactors [5,6,7,8]. The methods of modeling microalgae growth in both types of reactors are similar, since “microcosms” in the volume of laboratory flasks under constant stirring create conditions similar to those in industrial reactors.
A single species system is rarely capable of completely degrading organic xenobiotic contaminants. Therefore, the combination of microalgae and other suitable microorganisms can increase the efficiency in removing contaminants from wastewater [9]. Microbial communities consisting of photoautotrophs and heterotrophs can be useful in sustainable biological wastewater treatment. The effective use of these microbial communities depends on the selection of their constituent species for artificial biocenosis [10]. A significant aspect of the functioning of these mixed communities is the discrepancy in the growth rate of microalgae and bacteria, as well as microalgae and fungi. It is established that the doubling time of heterotrophic bacteria and fungi is approximately 2 h, while the doubling time of diverse microalgal species is 1.5 days on average [11, 12]. Consequently, during the initial 24-h period of a mixed culture, bacteria, and fungi can markedly outpace microalgae in terms of growth. Additionally, bacteria can rapidly adapt their metabolism to the pollutants present in the substrate.
Recently, a new technological process has been developed to improve wastewater treatment including the addition of activated sludge to the culture of microalgae [13]. This process is known as the microalgae-based activated sludge (MAAS) process. It provides high organic matter removal (75–90% of chemical organic demand (COD) removal) and high total nitrogen removal (40–50%). The photobioreactor with the MAAS process functions as a symbiotic algae-bacteria system with bacterial oxidation of organic matter, nitrification, and removal of nitrogen and phosphorus by algae [13].
It is known that bacteria, fungi, and microalgae always coexist in wastewater reactors for effective treatment. At the same time, fungi can neutralize the most dangerous organic toxicants. It was previously shown that Penicillium ochrochloron contributes to the treatment of textile wastewaters degrading synthetic diamino triphenylmethane dye—malachite green to the non-phytotoxic compounds para-benzyl-N,N-dimethylaniline and N,N-dimethylaniline hydrochloride with the participation of peroxidase [14]. The most active strains of this species were able to decompose a maximum of 75% of 50 mg/L pyrene at 22 °C within 28 days of incubation and use it as a carbon source [15]. Penicillium ochrochloron showed resistance to extremely high concentrations of heavy metals such as copper, zinc, manganese, and cadmium. Its copper uptake reached a constant level of about 1000 μg/kg dry mycelium [16].
One of the technical issues in wastewater treatment plants is continuous monitoring of microalgae activity, which determines the intensity and quality of treatment. Photosynthesis as the main process in the algal cell provides oxygen release, thereby ensuring the oxidation of pollutants in the medium by microalgae. The most promising way to solve this issue in photobioreactors is using modern methods of chlorophyll fluorescence to monitor photosynthetic processes in microalgae [2, 17, 18]. Chlorophyll fluorescence methods are non-invasive, rapid, highly sensitive, and a reliable tool to diagnose the state of microalgae cells in the presence of toxicants directly in bioreactors in real time [19, 20].
The choice of these toxicants in this study is due to the fact that they are frequently found in various concentrations in municipal and agricultural wastewaters. Sodium dodecyl sulfate (SDS) is the sodium salt of lauryl sulfuric acid, an anionic surfactant with bactericidal action. SDS is found in detergents and hygiene products, and therefore frequently occurs in domestic wastewater. SDS is able to inhibit growth rate and reduce the content of chlorophyll-a and carotenoids in microalgae [21, 22]. Formaldehyde is a naturally occurring organic compound. Its occurrence in domestic wastewater is mainly due to the transformation of other xenobiotics, viz. pharmaceuticals. Formaldehyde has a bactericidal effect; therefore, it can cause serious damage to biological water treatment systems. The mechanism of action on microalgae is based on the formation of covalent chemical bonds between proteins [23, 24]. Metribuzin is a widely used herbicide frequently found in agricultural runoff. This highly mobile herbicide has high solubility in water. The mechanism of its action on photoautotrophs is based on the inhibition of PSII activity [25].
In this work, we studied the resistance of the microalga C. sorokiniana to common wastewater contaminants (sodium dodecyl sulfate, formaldehyde, and metribuzin) in a pure culture of microalgae and in the presence of other hydrobionts (activated sludge and the fungus P. ochrochloron) using chlorophyll fluorescence methods Our findings demonstrate that activated sludge organisms and the fungus P. ochrochloron play a detoxifying role in the presence of contaminants in a photobioreactor with microalgae. The significance of this study lies in the demonstration that cultivation of microalgae with the addition of bacterial or fungal cultures (with absolute predominance of microalgae biomass in the created community) can be more resistant to changes in the composition of influent wastewater. In this case, the resulting biomass will have the properties of microalgae biomass and can be utilized by the same technological methods as microalgae biomass (biogas production, animal feed, production of organic fertilizers, etc.).
Materials and Methods
A strain of Chlorella sp. (Chlorophyta) isolated from the White Sea (Russia) and identified as C. sorokiniana (GenBank ID: KC678067) [20] was used in this work. The green microalga C. sorokiniana was cultured in a modified Tamiya medium with 70 mg N/L and 5 mg P/L at 25 °C and constant shaking (120 rpm) under 31 W m−2 fluorescent illumination and a photoperiod of 16/8 h (light/dark) in a KBW 400 growth chamber (Binder GmbH, Germany).
Toxicants
Sodium dodecyl sulfate (SDS) (C12H25NaSO4), formaldehyde (CH2O), and metribuzin (C8H14N4OS) were purchased in the Laboratory of the Government Chemist (LGC Ltd., UK).
Activated sludge was obtained from municipal wastewater treatment plants (Podolsk, Moscow region). The Podolsk treatment facilities carry out the treatment using nitri-denitrification. This type of technological scheme was chosen as having the greatest functional diversity of bacteria [26].
The following phyla are consistently represented in activated sludge: Acidobacteriota, Actinobacteriota, Bacteroidota, Bdellovibrionota, Campylobacterota, Chloroflexi, Cyanobacteria, Desulfobacterota, Elusimicrobiota, Firmicutes, Fusobacteriota, Myxococcota, Patescibacteria, Planctomycetota, Proteobacteria, Spirochaetota, Verrucomicrobiota. Activated sludge contains functional groups: nitrifiers, denitrifiers, dephosphorators and other groups responsible for the transformation of nutrients under conditions of simultaneous nitri-denitrification [27].
A sample of activated sludge was collected from the aerobic zone with a dose of sludge 3 g/L and added in a volume of 1 mL at a concentration corresponding to this dose of sludge.
Fungus strain №. 38 (Thailand), belonging to the species Penicillium ochrochloron Biourge, was obtained from the collection of pure cultures of microscopic fungi of the Department of Mycology, Moscow State University. This strain was chosen due to the fact that this species was regularly isolated from samples collected at different stages in wastewater treatment plants in Thailand and the Netherlands [28]. It is probably quite typical of wastewater from various regions. Furthermore, P. ochrochloron is quite resistant to various pollutants and is able to degrade some of them. It grows quite quickly and forms abundant small homogeneous conidia, from which it is convenient to prepare a uniform spore suspension for introduction into the photobioreactor.
The fungus culture was grown on Czapek’s agar medium in 90-mm diameter petri dishes for 10 days in a thermostat at 25 °C [29]. The spore suspension was prepared in sterilized distilled water and the concentration was adjusted to 106/mL, controlled in the Goryaev chamber.
Chlorophyll fluorescence was measured using an Aquapen-C 100 fluorometer (Photon System Instruments, Czech Republic). The dark-adapted samples were illuminated with blue light (λ = 455 nm) at a photosynthetic photon flux density (PPFD) of 3000 μmol photons m−2 s−1 for 2 s. The FO and FV/FM parameters were obtained from the chlorophyll fluorescence induction curves. Photosynthetic activity was estimated by the maximum quantum yield in photosystem II (PSII) as \(\frac{\left({F}_{M}-{F}_{O}\right)}{{F}_{M}}=\frac{{F}_{V}}{{F}_{M}}\) [30].
The content of chlorophyll was measured spectrophotometrically in 90% acetone extracts according to [31] using a spectrophotometer based on a USB 2000 portable spectrometer (Ocean Optics, Inc., USA).
The growth rate of algae was estimated by the fluorescence signal Fo. The FO fluorescence level correlates with the content of chlorophyll-a in the cell [32]. Before measurements, the fluorometer was calibrated for Fo signal intensity and chlorophyll-a content using C. sorokiniana with different densities. Chlorophyll-a (mg/L) was converted to dry biomass (mg/L) according to [33].
The content of SDS was measured by the extraction-photometric method on a [34] Cintra 6 UV–Visible spectrophotometer (GBC Scientific Equipment Ltd., USA).
The content of formaldehyde was measured photometrically with acetylacetone reagent [35] on a Cintra 6 UV–Visible spectrophotometer (GBC Scientific Equipment Ltd., USA).
Metribuzin content was measured by gas chromatography (Agilent J&W Intuvo column) [36].
The mass content of nitrite ions (with Griess reagent) was measured on a Cintra 6 UV–Visible spectrophotometer (GBC Scientific Equipment Ltd., USA). The content of nitrate ions was measured using a Dionex ICS-2000 ion chromatography system (Dionex, USA).
Experimental Design
Aliquots of C. sorokiniana were used for experiments when the algal batch culture was in the exponential growth phase. Algal samples (of 0.5-L Erlenmeyer flasks) having 0.2 mg/L DW were exposed to 50 and 500 mg/L of SDS, 50 mg/L of formaldehyde, and 0.01 and 0.05 mg/L of metribuzin for 10 days under growth condition as described above. Activated sludge and fungus were added separately to algal samples with toxicants according to the scheme (Fig. 1). Algal samples without toxicants, activated sludge, and fungus were used as controls.
Data Processing and Statistics
Three biological replicates and three technical repetitions were used throughout the experiment. OriginPro 2018 software (OriginLab, USA) was used for data processing and analysis. All experiments were conducted in triplicate and error bars show standard deviations of three parallel samples. One-way analysis of variance (ANOVA) with post hoc Dunnett test for multiple comparisons was performed to analyze significant differences using Statistica 10. Statistical significance was accepted at a probability of p < 0.05.
Results
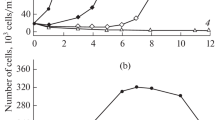
Figure 2 shows the growth rate and photosynthetic activity of C. sorokiniana under control conditions and in the presence of SDS and activated sludge. The growth rate in the control samples had a normal pattern. The growth curve demonstrates a lag phase in the first 3 days after placing in the photobioreactor, followed by an increase in the growth rate of microalgae in the following days. As we showed earlier, the transition of the photosynthetic apparatus of microalgae into an active state (increase in FV/FM) caused a sharp increase in the growth rate [37]. Photosynthetic activity of microalgae according to FV/FM increased on the 3rd day and then slightly decreased. Decreased photosynthetic activity is usually associated with the depletion of nutrients in the medium [38]. In the presence of SDS (50 mg/L), a decrease in FV/FM parameter was observed on day 3; however, this quantity was recovered on day 5. The growth of C. sorokiniana in the presence of SDS (50 mg/L) was relatively slow. In the presence of activated sludge, a significant increase in the FV/FM parameter (FV/FM = 0.72) was observed in the culture of C. sorokiniana with SDS on the 3rd day compared to the control. This was also accompanied by an increase in the growth rate of microalgae. This indicates a favorable effect of activated sludge on the photosynthetic apparatus of microalgae in the presence of low concentrations of SDS. The addition of activated sludge to the photobioreactor also increased the SDS removal rate in the microalgae medium (Fig. 2A).
High SDS concentrations of 500 mg/L, which might occur in case of accidents at wastewater treatment plants [39], inhibited the activity and growth rate of microalgae (Fig. 3). The addition of activated sludge did not restore these parameters at high SDS concentrations. However, the addition of activated sludge under these conditions stimulated SDS decomposition (Fig. 3B). A decrease in SDS concentration occurred in the first days of the experiment, the concentration of SDS decreased from 500 mg/L to values less than 0.2 mg/L.
Changes in the concentration of toxicants in the medium with microalgae C. sorokiniana and activated sludge during cultivation. 1 – in the presence of microalgae; 2 – in the presence of microalgae and activated sludge. A and B are SDS at 50 mg/L and 500 mg/L, respectively; C is formaldehyde at 50 mg/L
Formaldehyde at 50 mg/L, added as an additional contaminant, led to inhibition of growth and activity of the microalgae culture (Fig. 4). The addition of activated sludge did not restore the activity and viability of microalgae. Meanwhile, the decomposition of formaldehyde in the mixed culture (microalgae with activated sludge) occurred much faster, on the third day, than in the pure culture of microalgae (Fig. 3C).
Metribuzin at 0.1 mg/L resulted in complete inhibition of microalgae C. sorokiniana, which was manifested in zero values of photosynthetic activity (FV/FM) and cell growth rate (Fig. 5). This herbicide at 0.01 and 0.05 mg/L did not lead to the cell death of the microalgae. Figure 5 shows the growth rate and FV/FM of C. sorokiniana in the presence of the herbicide at 0.01 and 0.05 mg/L in combination with activated sludge or the fungus P. ochrochloron. Under control conditions, high photosynthetic activity was reached on day 2, whereas a slight decrease in FV/FM was observed on day 4 of cultivation due to nutrient depletion in the medium.
In the presence of metribuzin at 0.01 and 0.05 mg/L, FV/FM values decreased (Fig. 5), which is consistent with the effect of this herbicide on photosynthetic processes [40, 41]. Moreover, a significant decrease in the growth rate was observed under these conditions. The addition of activated sludge partly neutralized the effect of the herbicide at 0.01 mg/L. In this case, positive culture growth was observed with sufficiently high photosynthetic activity, indicated by FV/FM. In the case of metribuzin at 0.05 mg/L, the addition of activated sludge also caused the recovery of microalgae activity, although to a lesser extent.
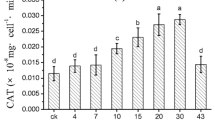
Our experiments revealed that it is also possible to reduce herbicide toxicity to microalgae by adding the fungus Penicillium ochrochloron Biourge to the photobioreactors. An increase in the FV/FM parameter was noted in the sample with the fungus P. ochrochloron and metribuzin at 0.01 and 0.05 mg/L (Fig. 5). Data on the decrease in metribuzin concentration in the experiments with microalgae and in combination with activated sludge or the fungus P. ochrochloron are given in the table. In all cases with microalgae, the decrease in metribuzin concentration ranged from 10 to 29% over 11 days. Significant decreases were observed with metribuzin at 0.01 mg/L in the presence of activated sludge (27%) or with the fungus P. ochrochloron (29%) (Table 1).
Discussion
Microalgae are capable of mixotrophic growth and are often used for wastewater treatment since organic matter can be removed with simultaneous biomass production [42]. In this work, we used a culture of the mixotrophic green alga C. sorokiniana, isolated previously from the White Sea (Russia). This strain was successfully tested in distillery wastewater treatment experiments, indicating virtually complete deodorizationand removal of most inorganic nutrients and organic matter, as well as algae biomass production [20].
In the current study, we investigated the resistance of the green microalga C. sorokiniana to some toxicants (sodium dodecyl sulfate, formaldehyde, metribuzin) in the presence of activated sludge and the fungus P. ochrochloron using chlorophyll fluorescence methods.
Photosynthetic activity of microalgae was determined by the fluorescence parameter FV/FM, which indicates the maximum quantum yield of PSII operation associated with water photolysis and oxygen release [30].
In our experiments, the microalga C. sorokiniana demonstrated high photosynthetic activity in the bioreactor, which is important for the process of wastewater treatment since oxygen released by photosynthesis is essential for the oxidation of pollutants [43]. The experiments showed that SDS at concentration of 50 mg/L, which often occurs in municipal wastewater, inhibits the photosynthetic activity of microalgae. Addition of activated sludge to microalgae culture restores the FV/FM and increases the biomass growth.
It was previously shown that activated sludge does not change its bacterial structure in the presence of SDS at concentrations less than 100 mg/L [44]. Our experiments also showed that a mixed community in the presence of low concentrations of SDS can function even more intensively than without toxicants. Moreover, activated sludge in the presence of microalgae accelerates SDS detoxification at low concentrations.
High concentrations of SDS (500 mg/L) irreversibly inhibited the photosynthetic activity and growth rate of microalgae. The negative effect of SDS at concentrations higher than 100 mg/L on pure cultures of microalgae was noted earlier [39]. Experiments with SDS at 500 mg/L revealed that activated sludge does not recover the activity of microalgae since at such concentrations activated sludge does not retain its own bacterial structure [44]. The structure of activated sludge at such concentrations changes drastically, the most common groups of bacteria in activated sludge are eliminated and replaced by dominant genera, for example, the genus Aeromonas [44]. New dominants are resistant to high concentrations of the pollutant and are able to use it as a substrate. In this experiment, the addition of activated sludge accelerated SDS detoxification even at high concentrations. Moreover, in this case, we recorded denitrification activity in the photobioreactor, confirmed by our data on the recording of nitrate and total nitrogen. We showed a decrease in nitrate and total nitrogen in the photobioreactor (Appendix). The application of SDS as a substrate for denitrifying bacteria was also reported earlier [45].
Inhibition of microalgae activity was also revealed for formaldehyde, which is another common pollutant in municipal wastewater. This toxicant at 50 mg/L led to irreversible inhibition of growth and photosynthetic activity even in the presence of activated sludge. Meanwhile, in the absence of algae growth, formaldehyde detoxification was observed in both photobioreactors; however, it was more noticeable in the presence of activated sludge. A similar detoxification of formaldehyde was also previously observed [46]. The mechanisms of such detoxification, most likely related to bacterial processes, require further research.
The water-soluble herbicide metribuzin is frequently found in agricultural wastewater. Metribuzin is known to inhibit PSII by preventing plastoquinone binding and blocking electron transport in the Hill reaction [25]. Moreover, metribuzin is believed to cause chlorophyll photodamage [47]. Metribuzin at 0.1 mg/L led to complete inhibition of photosynthetic activity and growth rate of microalgae. Apparently, the 0.1 mg/L concentration of this herbicide can be considered the maximum allowable for reactors with microalgae. In the presence of metribuzin at 0.01 and 0.05 mg/L, the activity of microalgae decreased, which is consistent with the effect of this herbicide on photosynthesis [25]. The addition of activated sludge made it possible to neutralize the effect of this herbicide on microalgae at 0.01 mg/L. At a concentration of 0.05 mg/L, the addition of activated sludge also led to the restoration of microalgae activity, although to a lesser extent. Herbicide detoxification also increased in the presence of activated sludge.
It is known that P. ochrochloron Biourge no. 38 (Thailand) is widely used for wastewater treatment facilities in various regions [14]. According to the literature, P. ochrochloron is quite resistant to various pollutants and is able to degrade some of them [14,15,16]. Our experiments showed that toxicity of the herbicide for microalgae can be reduced by adding the fungus P. ochrochloron. In our case, the efficiency of herbicide removal in the reactors (Table 1) increased significantly after adding both activated sludge and fungus to the microalgae culture.
The technological process of microalgae cultivation in a photobioreactor requires systems to monitor the state of microalgae [48] and control the cultivation process [19]. Our data showed that the maximum quantum yield of PSII (FV/FM) can be effectively employed in systems for monitoring microalgae condition and controlling the cultivation process in a photobioreactor. This is consistent with the studies by other authors [19, 48]. Registration of photosynthetic activity by chlorophyll fluorescence parameters allows non-invasive monitoring of microalgae viability during water treatment in the cultivation process. In particular, many works note that the quantum yield of PSII (FV/FM) depends on the concentration of nutrients in the medium [38]. Depletion of nutrients, such as nitrogen or phosphorus, leads to suppression of PSII functioning, which is manifested by changes in chlorophyll fluorescence parameters and growth processes [49]. This means that the depletion of nitrogen- and phosphorus-containing substances in the photobioreactor can be controlled by chlorophyll fluorescence methods. Currently, there are small-sized fluorometric devices that not only provide continuous monitoring of the content and condition of microalgae for a long time, but also transmit the obtained information in a user-friendly form [18].
Conclusion
This study showed that the toxicants we tested at selected concentrations have deleterious effects on the growth rate and photosynthetic activity of microalgae. Toxicants such as formaldehyde at 50 mg/L and SDS at 500 mg/L resulted in a dramatic decrease in biomass and photosynthesis. Addition of activated sludge increased (p biomass and photosynthetic activity of microalgae in the presence of SDS (50 mg/L) and the herbicide metribuzin (0.01 and 0.05 mg/L). Application of another detoxifying agent such as fungus P. ochrochloron in microalgae culture revealed a decreasing toxic effect of the herbicide metribuzin at 0.01 and 0.05 mg/L on microalgae activity.
The biomass ratio in all experimental reactors was significantly in favor of microalgae biomass. Given the difference in growth rate and the difficulty of directly accounting for the biomass of bacteria and fungi during the experiment, it is difficult to estimate the resulting ratio by the end of the experiment. However, observation of photosynthetic activity and the appearance of the experimental reactors clearly indicate that this predominance of microalgae biomass was maintained. This implies that the quality of the resulting and total biomass growth corresponds to the microalgae biomass, while the resistance to pollutants has significantly increased.
The results obtained are relevant for practitioners who manage wastewater treatment and post-treatment using photobioreactors with microalgae.
Data Availability
Data is provided within the manuscript.
References
Al-Jabri H, Das P, Khan S, Thaher M, AbdulQuadir M (2020) Treatment of wastewaters by microalgae and the potential applications of the produced biomass—a review. Water 13(1):27. https://doi.org/10.3390/w13010027
Shchegolkova N, Shurshin K, Pogosyan S, Voronova E, Matorin D, Karyakin D (2018) Microalgae cultivation for wastewater treatment and biogas production at Moscow wastewater treatment plant. Water Sci Technol 78(1):69–80. https://doi.org/10.2166/wst.2018.088
Sutherland DL, Ralph PJ (2019) Microalgal bioremediation of emerging contaminants: Opportunities and challenges. Water Res 164, 114921. https://doi.org/10.1016/j.watres.2019.114921
Hena S, Gutierrez L, Croué JP (2021) Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: a review. J Hazard Mater 403:124041. https://doi.org/10.1016/j.jhazmat.2020.124041
Guo Q, Zhan Y, Li Y, Hong N, Guan Y, Zhang Z et al (2020) Investigating toxicity of urban road deposited sediments using Chinese hamster ovary cells and Chlorella Pyrenoidosa. Chemosphere 245:125634. https://doi.org/10.1016/j.chemosphere.2019.125634
Kothari R, Pandey A, Ahmad S, Singh HM, Pathak VV, Tyagi VV, et al (2022) Utilization of Chlorella pyrenoidosa for remediation of common effluent treatment plant wastewater in coupling with Co-relational study: an experimental approach. Bull Environ Contam Toxicol 1–11. https://doi.org/10.1007/s00128-021-03292-7
Kurade MB, Kim JR, Govindwar SP, Jeon BH (2016) Insights into microalgae mediated biodegradation of diazinon by Chlorella sorokiniana: microalgal tolerance to xenobiotic pollutants and metabolism. Algal Res 20:126–134. https://doi.org/10.1016/j.algal.2016.10.003
Wang S, Poon K, Cai Z (2018) Removal and metabolism of triclosan by three different microalgal species in aquatic environment. J Hazard Mater 342:643–650. https://doi.org/10.1016/j.jhazmat.2017.09.004
Semple KT, Cain RB, Schmidt S (1999) Biodegradation of aromatic compounds by microalgae. FEMS Microbiol Lett 170(2):291–300. https://doi.org/10.1111/j.1574-6968.1999.tb13386.x
Scognamiglio V, Giardi MT, Zappi D, Touloupakis E, Antonacci A (2021) Photoautotrophs–bacteria co-cultures: advances, challenges and applications. Materials 14(11):3027. https://doi.org/10.3390/ma14113027
Maltceva OA (2017) Morphometric characteristic of cells microalgae Dunaliella viridis in batch culture (In Russian). Vopr Sovrem Algol 1:13
Gabrielyan DA, Sinetova MA, Gabrielyan AK, Bobrovnikova LA, Bedbenov VS, Starikov AY, Zorina AA, Gabel BV, Los DA (2023) Laboratory system for intensive cultivation of microalgae and cyanobacteria. (In Russian). Fiziol Rast 70(2):202–213. https://doi.org/10.31857/S0015330322600486
Schwede S, Anbalagan A, Krustok I, Lindberg CF, Nehrenheim E (2016) Evaluation of the microalgae-based activated sludge (MAAS) process for municipal wastewater treatment on pilot scale. In: Proceedings of the IWA world water congress; Brisbane, Australia. International Water Association. https://www.diva-portal.org/smash/get/diva2:1059419/FULLTEXT01.pdf
Shedbalkar U, Jadhav JP (2011) Detoxification of malachite green and textile industrial effluent by Penicillium ochrochloron. Biotechnol Bioprocess Eng 16:196–204. https://doi.org/10.1007/s12257-010-0069-0
Saraswathy A, Hallberg R (2005) Mycelial pellet formation by Penicillium ochrochloron species due to exposure to pyrene. Microbiol Res 160(4):375–383. https://doi.org/10.1016/j.micres.2005.03.001
Fukami M, Yamazaki S, Toda S (1983) Distribution of copper in the cells of heavy metal tolerant fungus, Penicillium ochrochloron, cultured in concentrated copper medium. Agric Biol Chem 47(6):1367–1369. https://doi.org/10.1080/00021369.1983.10863892
Havlik I, Lindner P, Scheper T, Reardon KF (2013) On-line monitoring of large cultivations of microalgae and cyanobacteria. Trends Biotechnol 31(7):406–414. https://doi.org/10.1016/j.tibtech.2013.04.005
Antal T, Konyukhov I, Volgusheva A, Plyusnina T, Khruschev S, Kukarskikh G et al (2018) Chlorophyll fluorescence induction and relaxation system for the continuous monitoring of photosynthetic capacity in photobioreactors. Physiol Plant 165(3):476–486. https://doi.org/10.1111/ppl.12693
Masojídek J, Vonshak A, Torzillo G (2010) Chlorophyll fluorescence applications in microalgal mass cultures. In: Chlorophyll a fluorescence in aquatic sciences: methods and applications. Dordrecht: Springer Netherlands 277–292. https://doi.org/10.1007/978-90-481-9268-7_13
Solovchenko A, Pogosyan S, Chivkunova O, Selyakh I, Semenova L, Voronova E, Scherbakov P, Konyukhov I, Chekanov K, Kirpichnikov M, Lobakova E (2014) Phycoremediation of alcohol distillery wastewater with a novel Chlorella sorokiniana strain cultivated in a photobioreactor monitored on-line via chlorophyll fluorescence. Algal Res 6:234–241. https://doi.org/10.1016/j.algal.2014.01.002
Markina ZV (2010) Effects of sodium dodecyl sulfate on the growth dynamics and physiological state of the microalga Dunaliella salina (Chlorophyta). Russ J Mar Biol 36:191–194. https://doi.org/10.1134/S1063074010030041
Taghavijeloudar M, Park J, Hashemi S, Han M (2019) The effects of surfactants (sodium dodecyl sulfate, triton X-100 and cetyl trimethyl ammonium bromide) on the dewaterability of microalgae biomass using pressure filtration. Bioresour Technol 273:565–572. https://doi.org/10.1016/j.biortech.2018.11.062
Peng WX, Yue X, Chen H, Ma NL, Quan Z, Yu Q et al (2022) A review of plants formaldehyde metabolism: implications for hazardous emissions and phytoremediation. J Hazard Mater 436:129304. https://doi.org/10.1016/j.jhazmat.2022.129304
Yoshida K, Ishii H, Ishihara Y, Saito H, Okada Y (2009) Bioremediation potential of formaldehyde by the marine microalga Nannochloropsis oculata ST-3 strain. Appl Biochem Biotechnol 157:321–328. https://doi.org/10.1007/s12010-008-8314-0
Buman RA, Gealy DR, Fuerst EP (1992) Relationship between temperature and triazinone herbicide activity: I. Herbicide binding to thylakoid membranes. Pestic Biochem Physiol 43(1):22–28. https://doi.org/10.1016/0048-3575(92)90015-R
Gupta RK, Poddar BJ, Nakhate SP, Chavan AR, Singh AK, Purohit HJ et al (2022) Role of heterotrophic nitrifiers and aerobic denitrifiers in simultaneous nitrification and denitrification process: a nonconventional nitrogen removal pathway in wastewater treatment. Lett Appl Microbiol 74(2):159–184
Shchegolkova NM, Krasnov GS, Belova AA, Dmitriev AA, Kharitonov SL, Klimina KM, Melnikova NV, Kudryavtseva AV (2016) Microbial community structure of activated sludge in treatment plants with different wastewater compositions. Front Microbiol 7:90. https://doi.org/10.3389/fmicb.2016.00090
Kharitonov S, Shchegolkova N, Alexandrova A, Saynchuk A, Michel P, Maciejewski K et al (2022) Taxonomic diversity of fungi and bacteria in Azoé-NP® vertical flow constructed wetlands. Water 14(5):698. https://doi.org/10.3390/w14050698
Crous PW, Verkley GJM, Groenewald JZ, Samson RA (2009) Fungal biodiversity. CBS laboratory manual series 1. Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the Chlorophyll a fluorescence transient. Chlorophyll a Fluorescence: a Signature Photosynth 19:321–362. https://doi.org/10.1007/978-1-4020-3218-9_12
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382
Matorin DN, Antal TK, Ostrowska M, Rubin AB, Ficek D, Majchrowski R (2004) Chlorophyll fluorimetry as a method for studying light absorption by photosynthetic pigments in marine algae. Oceanologia 46(4)
Ayed HBAB, Taidi B, Ayadi H, Pareau D, Stambouli M (2016) Magnesium uptake by the green microalga Chlorella sorokiniana in batch cultures. J Microbiol Biotechnol 26(3):503–510. https://doi.org/10.4014/jmb.1507.07039
EPA-NERL (1978) Method 425.1: Surfactants by colorimetry. Cincinnati, OH: National exposure research laboratory, office of research and development, U.S. Environmental Protection Agency. Report No. EPA-600/4–78–027. https://www.nemi.gov/methods/method_summary/5266/
US Environmental protection agency (1998) Method 1667 Revision A. Formaldehyde, isobutyraldehyde, and furfural by derivatization followed by high performance liquid chromatography. Washington, DC: U.S. Environmental protection agency. https://www.epa.gov/sites/default/files/2015-09/documents/method_1667a_1998.pdf
ASTM International (2002) Method D5475–93 (2002): Standard test method for nitrogen- and phosphorus-containing pesticides in water by gas chromatography with a nitrogen-phosphorus detector. West conshohocken, PA. ASTM International. https://cdn.standards.iteh.ai/samples/17060/37a04a8a4a0346bcbc722f4976c4939d/ASTM-D5475-93-2002-.pdf
Mosharova IV, Il’inskii VV, Matorin DN, Mosharov SA, Akulova AY, Protopopov FF (2015) Monitoring of the Moskva River water using microbiological parameters and chlorophyll a fluorescence. Microbiology 84:811–821. https://doi.org/10.1134/S0026261715060065
Falkowski PG, Raven JA (2013) Aquatic photosynthesis. Princeton University Press
Oda Y, Sakamoto M, Miyabara Y (2022) Colony formation in three species of the family Scenedesmaceae (Desmodesmus subspicatus, Scenedesmus acutus, Tetradesmus dimorphus) exposed to sodium dodecyl sulfate and its interference with grazing of Daphnia galeata. Arch Environ Contam Toxicol 1–11. https://doi.org/10.1007/s00244-021-00890-8
Almeida AC, Gomes T, Langford K, Thomas KV, Tollefsen KE (2019) Oxidative stress potential of the herbicides bifenox and metribuzin in the microalgae Chlamydomonas reinhardtii. Aquat Toxicol 210:117–128. https://doi.org/10.1016/j.aquatox.2019.02.021
Thomas MC, Flores F, Kaserzon S, Fisher R, Negri AP (2020) Toxicity of ten herbicides to the tropical marine microalgae Rhodomonas salina. Sci Rep 10(1):7612. https://doi.org/10.1038/s41598-020-64116-y
Cabanelas ITD, Ruiz J, Arbib Z, Chinalia FA, Garrido-Pérez C, Rogalla F et al (2013) Comparing the use of different domestic wastewaters for coupling microalgal production and nutrient removal. Bioresour Technol 131:429–436. https://doi.org/10.1016/j.biortech.2012.12.152
Guldhe A, Ansari FA, Singh P, Bux F (2017) Heterotrophic cultivation of microalgae using aquaculture wastewater: a biorefinery concept for biomass production and nutrient remediation. Ecol Eng 99:47–53. https://doi.org/10.1016/j.ecoleng.2016.11.013
Shchegolkova NM, Kharitonov SL, Astrkhanov ME, Krasnov GS (2020) Microbial community structure of active sludge in model experiments under the influence surfactant. Water Purif Water Treat Water Supply 9:16–22 (In Russian)
Paulo AMS, Plugge CM, García-Encina PA, Stams AJM (2013) Anaerobic degradation of sodium dodecyl sulfate (SDS) by denitrifying bacteria. Int Biodeter Biodegr 84:14–20. https://doi.org/10.1016/j.ibiod.2013.05.027
Yamada H, Kawamura A, Somiya I (1991) Decomposition and formation of formaldehyde during blue-green algal growth cycles. Int J Environ Stud 38(1):55–64. https://doi.org/10.1080/00207239108710649
Jones R (2005) The ecotoxicological effects of Photosystem II herbicides on corals. Mar Pollut Bull 51(5–7):495–506. https://doi.org/10.1016/j.marpolbul.2005.06.027
Obata M, Toda T, Taguchi S (2009) Using chlorophyll fluorescence to monitor yields of microalgal production. J Appl Phycol 21:315–319. https://doi.org/10.1007/s10811-008-9369-6
Suggett DJ, Prášil O (2010) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Borowitzka MA (ed) Dordrecht: Springer. 293–309. https://doi.org/10.1007/978-90-481-9268-7
Acknowledgements
The authors thank the management and staff of the Podolsk wastewater treatment plant for providing the activated sludge used in this research.
Funding
The work was carried out according to the state order to the WPI RAS (subject FMWZ-2022–0002 “Studying Geoenvironmental Processes in Terrestrial Hydrological Systems, the Formation of the Quality of Surface and Subsurface Water, the Problems of Water Resources Management and Water Use under Changing Climate and Anthropogenic Impacts”) and the state order to the Moscow State University (subject 122011800459–3 “Soil Biomarkers: Identification, Stability, Activity, and Potential Use for Monitoring “).
Author information
Authors and Affiliations
Contributions
Nataliya M. Shchegolkova: conceptualization and experimental design, supervision, review and editing. Daria A. Todorenko: data acquisition, data analysis and interpretation, original draft preparation. Dmitry N. Matorin: methodology, data analysis and interpretation, review and editing. Dmitry O. Karyakin: data analysis and interpretation, original draft preparation. Kirill N. Shmonin: data acquisition, data analysis and interpretation, original draft preparation. Rostislav A. Streletskii: experimental design, review and editing. Alina V. Aleksandrova: review and editing.
Corresponding author
Ethics declarations
Ethics Approval
All work complies with ethical standards.
Consent to Participate
The authors consent to their participation in the entire review process.
Consent for Publication
The authors give their permission to publish.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shchegolkova, N.M., Todorenko, D.A., Matorin, D.N. et al. Chlorophyll Fluorescence Parameters of Chlorella sorokiniana Exposed to Toxicants in the Presence of Activated Sludge and Fungus: Approaches to Wastewater Treatment. Water Conserv Sci Eng 9, 63 (2024). https://doi.org/10.1007/s41101-024-00295-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41101-024-00295-3