Abstract
Multi-component white cast irons in which microstructures consist of multiple types of hard eutectic carbides embedded in a strong matrix have been developed for hot work roll materials. Their hard eutectic carbides contribute to improving the abrasive wear resistance of roll materials. The basic alloy composition is 5 wt% of Cr, Mo, W, V each and 2 wt% of C and Co. In this work, the effect of V content on heat treatment behavior of multi-component white cast irons with basic alloy composition was investigated. The V content of prepared cast irons varied from 2.01 to 6.64 wt%. After annealing, the specimens were hardened from 1050 °C and 1100 °C by fan air cooling and subsequently tempered between 350 and 600 °C. The results indicated that regardless of austenitizing temperature, the hardness in the as-hardened state increased to the maximum value and then decreased as V content rose. The volume fraction of retained austenite in the as-hardened state (Vγ-As) decreased greatly with a rise of V content at both austenitizing temperatures. In the tempered state, the hardness curves showed secondary hardening due to the precipitation of carbides and the transformation of decomposed austenite to martensite. The Vγ value began to decrease greatly at 450-500 °C tempering and then decreased gradually as the tempering temperature was elevated. The maximum tempered hardness (HTmax) was obtained in each specimen tempered between 500 and 520 °C for 1050 °C and 500–600 °C for 1100 °C austenitizing where the Vγ ranged from 1 to 15%. The highest HTmax, values near 1000 HV30 were obtained at 4 wt%V for 1050 °C and 5 wt%V for 1100 °C austenitizing, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of steel manufacturing technology is closely connected to that of the rolling machine, rolling technology and the roll itself. It is known that the development of roll materials can allow high productivity and provide high-grade products using a large-scale rolling machine with high-speed and high-load performances. Above all, the role of hot work rolls is very important and the roll itself must be equipped with excellent resistance to abrasive wear, surface roughening and heat. The wear resistance is related to hardness and strength at high temperatures of materials, while the surface roughness is influenced by peeling or banding, sticking and nonuniform wearing.1 Such properties necessary for the hot work rolls were formerly improved by alloy designing and heat treatment.
The mainstream of conventional hot work roll materials employed high chromium cast iron at earlier stands and graphite containing Ni-hard cast iron at later stands.1,2 However, the new type of rolls made of multi-component white cast iron, which have been developed for a long period by our group, has proved to have remarkably excellent performance compared with those made of high chromium cast iron.3 Since then, they have spread throughout many countries in the world. Presently, high chromium rolls have mostly been replaced by rolls made of multi-component white cast iron which is contributing to the improvement of the service life of rolls, productivity of steel and the cost performance.1,2,3
Multi-component white cast iron contains basically 5 wt% (hereafter wt% is expressed by %) each of carbide forming elements of chromium (Cr), molybdenum (Mo), tungsten (W), vanadium (V) and around 2%C so that the carbon balance (Cbal) value of the cast iron can be close to 0%.4,5,6,7 Moreover, cobalt (Co) is added to increase the high temperature strength.8 The microstructure of cast iron consists of MC and M2C eutectic carbides and a hard matrix composed of tempered martensite, fine special carbides secondarily precipitated and some retained austenite. The eutectic carbides in MC and M2C types are mainly formed by V and Mo and/or W, respectively. Since a roll made of multi-component white cast iron has higher C content than one made of high-speed tool steel, the roll contains around 20 vol% eutectic carbides, and which therefore provide much better abrasive wear resistance during hot rolling.
V is the very important carbide former in multi-component white cast iron. During solidification, V is consumed to form MC eutectic carbide first and the residuals are distributed into the matrix. In heat treatment, on the other hand, the V in the matrix precipitates as secondary carbides with extremely high hardness together with those formed by other carbide forming elements. The morphology of eutectic MC carbide shows flaky and granular shapes in two-dimensional view but in three-dimensional view, it has a petal-like morphology of a flower and is interconnected.1,6,9,10 The V also forms thin oxide film of V2O5 on the roll surface during hot rolling and it is effective in providing resistance to surface roughening.11
Regarding to the multi-component white cast iron with basic alloy composition, the solidification,5,12 heat treatment behavior 6,7,8,13,14,15,16,17 and abrasive wear resistance 8,18,19 have already been reported by our research groups. To enhance further the work roll performance made of this material, the effects of varying single or double alloying elements in the basic alloy composition, specifically focusing on phase transformation, heat treatment, wear resistance and fracture toughness must be explored. In our previous studies, the effects of C6, Cr7 and Mo13,15, Cbal16 and Tungsten equivalent (%Weq = %W+2%Mo) 17 on heat treatment behavior have been clarified for multi-component white cast irons. It was pointed out that there existed an optimal amount of each alloying element that gave the maximum hardness after tempering, say, the highest value of 943 HV30 in the specimen with Mo. Certainly, the V can be also expected to display significant potential improving hardness, strength, and abrasive wear resistance because V forms VC carbide with higher hardness than Mo2C carbide. Despite such an expectation, however, there has not been found any article about the influence of V content on heat treatment characteristics in this type of cast iron.
In this research, therefore, specimens with V content from 2.01 to 6.64% under the constant alloy content of 5% were prepared so that MC carbides could precipitate, and the transformation behavior of matrix during heat treatment was investigated. Then, the relationships between V content and characteristics such as phase transformation, precipitation of secondary carbide, hardness, and volume fraction of retained austenite (Vγ) were clarified. It is convinced that the findings will contribute to improving the quality of multi-component white cast iron, and thus for work roll performance as well.
Experimental Procedures
Preparation of Test Specimens
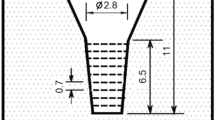
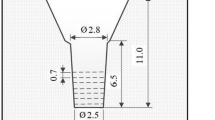
Various kinds of charge materials such as mild steel, pig iron, ferro-alloy and pure metal were used to prepare the specimens. The charge materials were melted in a 15 kg capacity high frequency induction furnace with alumina lining. The melt was superheated up to 1550 °C. After holding for 10 min, the melt was poured at 1500 °C into the CO2 bonded sand molds illustrated in Figure 1. To prevent the melt from cooling from the top, the riser was immediately covered with an exothermic powder just after pouring. The chemical compositions of specimens are shown in Table 1.
Heat Treatment Procedure
The heat treatment processes are shown schematically in Figure 2. The round bar ingots were coated with an anti-oxidation coat (C-PRO 202, Japan) to prevent the decarburization and annealed at 850 °C for 5 h to homogenize the micro-segregation. The annealed ingots were sectioned by a wire-cutting machine to obtain a disk-shaped test piece with 0.7 cm thickness. The test pieces were austenitized at 1050 °C and 1100 °C for 1 h in Ar atmosphere and then hardened by fan air cooling (FAC). Tempering was carried out at 350–600 °C for 3.33 h (200 min).
Observation of Microstructure and Measurement of Hardness
General microstructure of the specimen was observed by optical microscope (OM). The test piece was mirror-polished and etched by Murakami’s reagent to distinguish the types of carbides and Vilella’s reagent to reveal the matrix structure. The constituent phases in matrix microstructure were observed by scanning electron microscope (SEM) at high magnifications. Hardness was measured using a Vickers hardness tester with a load of 294.2 N (30 kgf). The measurement was made randomly at five points, and the average value was adopted.
Measurement of Volume Fraction of Retained Austenite (Vγ)
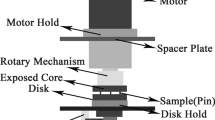
Volume fraction of retained austenite (Vγ) in the test specimen was quantitatively measured using the X-ray diffraction (XRD) method which was developed for high alloyed white cast iron.20 A specialized goniometer with a rotating and swinging sample stage was introduced to cancel the effect of preferred orientation of crystals. The X-ray source was a Mo-Kα beam with the wavelength of 0.007 nm which was filtered by Zr foil. The polished test piece was scanned from 24 to 44° of 2θ. The peaks of α(200) and α(220) for ferrite or martensite and γ(220) and γ(311) for austenite were selected for calculation of the Vγ value. The reason why the γ(211) peak was not selected is that a strong peak of M7C3 carbide overlapped close to it. The area of each peak was measured individually by an image analyzer. The calculations of Vγ were carried out from the ratio of integrated areas with combinations of α200 versus γ311, α200 versus Σγ(220+311), and Σα(200+220) versus. γ311 peaks, respectively. The values obtained from three calculations were averaged.
Results and Discussion
Effect of V Content on Microstructure of Specimens
As-Cast State
The as-cast microstructures of test specimens were made by OM revealing the eutectic carbides with Murakami reagent and the matrix structure with Vilella’s reagent as etchants, respectively, are shown in Figure 3a–f. Specimens No. 1 to No. 4 showed hypoeutectic structures consisting of primary austenite (γP), (γ+MC), (γ+M2C) and (γ+M7C3) eutectics for specimen No. 1; γP, (γ+MC) and (γ+M2C) eutectics for No. 2 to No. 4. Specimen No. 5 was a hypereutectic structure with primary MC carbide (MC)p, (γ+MC) and (γ+M2C) eutectics. The matrix consisted of bainite, martensite, austenite and some secondary carbides as shown in Figure 3f by a representative specimen No. 3.
To clarify the solidification sequence of each specimen, a quasi-binary phase diagram of M(Fe–5%Cr–5%W–5%Mo–2%Co–2%C)-V alloy was obtained using THERMO-CALC software and the result is shown in Figure 4. The V content at L→(γ+MC) eutectic point was around 5.4%, and therefore, it is understood that the specimens of No. 1 to No. 4 all had hypoeutectic composition and No. 5 (with the highest V content of 6.64%) was a hypereutectic composition where MC carbide crystallizes as a primary phase.21 Here, the following solidification sequence of each specimen can be predicted from the phase diagram in Figure 4.

To clarify how V content affects the fraction of crystallized phases from the liquid, the area fractions of each phase were quantitatively measured by an image analysis software and the results are shown in Figure 5. As the V content increased, the area fraction of primary austenite (γP) decreased significantly, while that of the (γ+MC) eutectic increased notably. This suggests that the presence of V promotes the formation of the (γ+MC) eutectic. However, the area fractions of (γ + M2C) eutectic did not vary significantly with changes in V content. Additionally, the (γ+M7C3) eutectic co-existed in the 2%V specimen, but for specimens with V content exceeding 2%, the eutectics were predominantly (γ+MC) and (γ+M2C). These results prove that the V content beyond 2% inhibited the formation of (γ+M7C3) eutectic and instead promoted the crystallization of (γ+MC) eutectic.
As-Hardened State
It is well known that matrix structure is widely changed by heat treatment. The microstructures of as-hardened specimens were observed by SEM focusing on the matrix, and the microphotographs of representative specimens with 2.01%, 3.00% and 4.98%V are shown in Figure 6. It can be seen that the secondary carbides (Sc) exist in a whole matrix, and simultaneously, martensite (M) and retained austenite (γR) coexist with secondary carbides. The size and the number of secondary carbides differ depending on the V content of the specimen and the austenitizing temperature. It is clear that in 1050 °C austenitizing, the carbides in the 2%V specimen were very fine and increased in size and number as V content rose. In 1100 °C austenitizing, the number and size of the carbides are reduced, but a quantitative difference of V content could not be seen. This can be explained as the higher austenitizing temperature making the solubility of V, C and other elements in the matrix expand and results in less precipitation of carbides during the hardening process. On the other hand, the retained austenite seems to decrease with an increase in V content. This is because higher V content in the austenite combined more with C and it raised the Ms temperature. Then, the retained austenite was decreased.
Relationship Between Hardness, Volume Fraction of Retained Austenite (Vγ) and V Content
As-Hardened State
It is believed that the eutectic carbides change little during heat treatment due to their high thermal stability3,4,10. Therefore, the variation of hardness depends on the phase transformation of the matrix during heat treatment. Generally, the hardness increases according to the amount of hard martensite and decreases with a rise of soft retained austenite. It is also known that the hardness of martensite rises as the concentration of dissolved C increases.
The relationship between hardness, volume fraction of retained austenite in the as-hardened state (Vγ-As) and V content is shown in Figure 7. The hardness rose to the maximum value and then decreased regardless of the austenitizing temperature. The highest hardness of 907 HV30 and 870 HV30 was obtained at about 5%V for 1050 °C and 6%V for 1100 °C austenitizing, respectively. This indicates that there is mutual dependence between V content and austenitizing temperature for hardness. The reason for the increase in hardness in the former stage is due to the fact that a rise of V content promotes the precipitation of secondary carbides. Resultantly, the C concentration in austenite is reduced to raise the Ms temperature during austenitization. As a result, more martensite is formed, and at the same time, the amount of retained austenite is decreased. An increase in the amount of (γ+MC) eutectic with raising the V content is one reason. The hardness lowers over the maximum value because an excessive precipitation of secondary carbides reduces C concentration in austenite, and then, the hardness of martensite itself is decreased. This suggests that the reduction in hardness caused by the presence of low-carbon martensite gave more significant effect than the increase in hardness resulting from the increase in eutectic structures. It was also found that a low austenitizing temperature provided higher hardness than high austenitizing temperature at the same V content. The reason for this can be explained by that the low austenitizing temperature produces less retained austenite because of lower concentrations of C and V in austenite compared with those of high austenitizing temperature. Here, it is found that the Vγ-As value at which the highest hardness can be obtained was 15% at 1050 °C and 12% at 1100 °C austenitizing, respectively. These results indicate that when the V content of specimen increases beyond 5% which more MC carbides formed, it becomes necessary to raise the austenitizing temperature to maintain the required C and V concentration in austenite, ensuring the optimal balance between retained austenite and high-hardness martensite in quenching at the same holding time.
In contrast, the Vγ value decreased remarkably with an increase in the V content regardless of austenitizing temperatures. This relationship can be understood by inverse thinking to the behavior of hardness. Since the C contents of specimens are almost the same, the amount of precipitated secondary carbides in austenite during austenitization is determined by the V content. Therefore, the C concentration in austenite decreased as the V content increased. As a result, the Ms temperature increased22 and the Vγ value diminished. The high austenitizing temperature provides a greater Vγ value than occurs across the whole range of V content. Here, the relation of hardness vs Vγ value in the as-hardened state (Vγ-As) is shown in Figure 8. The hardness increases for a while with increasing of Vγ-As value up to 15% and 7.5% for 1050 °C and 1100 °C austenitizing, respectively, and after that, it decreases as the Vγ-As value rises. The increase in hardness despite increasing Vγ-As value is due to the precipitation of secondary carbides and the increase in hardness of martensite itself. The decreasing hardness over the maximum points is because of an increase in the retained austenite and the reduction of martensite.
Based on the results described above, it is realized that the changes in hardness and Vγ resulting from variation of V content are more notable compared with the effects of other alloying elements obtained in the previous works.6,7,13,15,16,17 The MC carbides crystallized precedently in solidification are closely related to the hardness and volume fraction of retained austenite after hardening, i.e., the presence of MC carbides increases hardness and decreases concentration of C in the matrix. The reduction of C lowers the stability of solidified austenite and facilitates the transformation to martensite during hardening. Despite the positive effects on the hardness, the maximum hardness in as-hardened state is lower than that of the same type of cast iron with Mo.13,15 It is because more retained austenite formed in the hardening of the cast iron with V.
Tempered State
The specimens hardened from two austenitizing temperatures of 1050 °C and 1100 °C were tempered at 50 °C intervals between 350 and 600 °C. The relationship between hardness, Vγ value and tempering temperature is shown in Figure 9a–e for each specimen. The hardness and Vγ in the as-hardened state are added to diagrams for comparison. In each specimen, the hardness dropped greatly from the as-hardened state even at 350 °C tempering. This could be the first stage in tempering of martensite. As the temperature rose to around 400-450 °C, the hardness increased a little or more depending on the V content of the specimen.
After that, it continued to rise remarkably to the maximum value and then dropped steeply with an increase in the temperature. In other words, the tempered hardness curve shows an evident secondary hardening in the same way as previous studies6,7,13,15,16,17 which is due to the precipitation of secondary carbides and the transformation of martensite from the residual austenite in as-hardened state. The reason why the hardness lowers after arriving at the maximum is because of the coarsening of fine secondary carbides by merging with each other and into nearby eutectic carbides.
In addition, it is possible that the decomposition of still remaining austenite to pearlite or ferrite occurs. Hashimoto et al.23 investigated the type of precipitated carbides in the matrix of a basic multi-component white cast iron using XRD and TEM analysis. They reported that the precipitated carbides in heat-treated states were MC, M6C and M7C3 types. The maximum tempered hardness (HTmax) was obtained at 500–525 °C for 1050 °C and 500–600 °C for 1100 °C austenitizing, respectively. It was found that in the specimens with V content less than 4%, hardening from low temperature provided a higher HTmax than that from higher temperature.
The discrepancy in HTmax might be attributed to the smaller amount of retained austenite in specimens hardened from low austenitizing temperature. Here, most of retained austenite would challenge to transform fully into martensite during tempering, and therefore, it affects the HTmax as well. However, the opposite results were found in the specimens with V content more than 5%. This trend corresponds well with the relation of hardness vs. V content in Figure 7; that is, a higher V content is needed to achieve the same value of hardness by higher hardening temperature.
It is reasonable that the hardness is significantly affected by the Vγ content in the matrix and the reduction of Vγ value can contribute to an increase in the hardness. In the relationship between the Vγ value and tempering temperature shown in Figure 9, the Vγ value markedly decreased when the tempering temperature became greater than 450 °C to 500 °C. Above 520 °C, the Vγ values of all the specimens hardened from 1050 °C dropped below 10%. In case of hardening from 1100 °C, however, a large amount of austenite remained in the specimens with V content less than 4%. This is because the V content was not enough to raise the Ms temperature by combining with C. In such a case, double or triple tempering must be introduced to lower the Vγ value for applying the material to the hot work roll.
The relationship between hardness and Vγ value was obtained for all the tempered specimens and is displayed in Figure 10. The hardness values are scattered widely between 660 and 1000 HV30 when Vγ value is less than 2% and they are comprehensively very high at Vγ value less than 10% in all the specimens. It can be understood that for the specimens with high hardness, the retained austenite was fully decomposed and transformed to martensite and for those with low hardness, the over-tempering of martensite and the coarsening of precipitated carbides could be the main reasons. As the Vγ value rises over 10%, the hardness lowered gradually with about the same range because of both the increase in soft retained austenite and the reduction of martensite. It can be said from the results that the Vγ value up to 15% is acceptable in order to obtain hardness greater than 800 HV30, which is required for rolls under the condition of plural tempering to decrease the Vγ value.
Correlation Between Maximum Tempered Hardness (H Tmax), Volume Fraction of Retained Austenite (Vγ) and V Content
In the hot work rolls, the amount of retained austenite must be limited to prevent the roll from cracking which can happen during operation by the strain-induced martensite and the following wear by spalling. Therefore, it is necessary to clarify how to obtain the maximum hardness with low Vγ value by tempering. The relationship between HTmax, Vγ at HTmax and V content is shown in Figure 11. Although the absolute values of hardness are different, the pattern resembles that of as-hardened state shown in Figure 7, i.e., the HTmax rises to the maximum value and then goes down and the Vγ values decrease gradually as the V content increases. In the tempered state, however, the highest value of HTmax near 1000 HV30 was obtained at about 4%V for 1050 °C and 5%V for 1100 °C austenitizing, respectively. The highest values of HTmax were much greater than those in the as-hardened state. Furthermore, their highest HTmax values are notably greater than the HTmax value of 946 HV30 13 obtained in the previous study of Mo addition. This indicates that secondary hardening by tempering is very effective to improve the hardness and the V content must be controlled; the greater the V content, the higher the austenitizing temperature. In the early stage of the hardness curve, the HTmax rises because of more hard tempered martensite and more precipitation of secondary carbides in the matrix in addition to an increase in the eutectic MC carbides. In the latter stage, the HTmax value decreases continuously because the much more secondary carbides precipitate by increasing V content and martensite with low C concentration or low hardness form in the as-hardened state, and such martensite cannot contribute to the secondary hardened by tempering.
The Vγ values at HTmax decreased gradually as the V content increased. At the same V content, the Vγ values at HTmax were high in the specimens hardened from 1100 °C. It can be understood that high austenitizing temperature encourages more retained austenite in as-hardened state. Under the same tempering time, the decomposition ratio of austenite or the precipitation ratio of secondary carbides was smaller in low tempering temperature and more austenite remained. When this type of material with V content less than 5% is applied to the hot work rolls, an extension of the tempering time or multiple tempering will be needed to decrease the retained austenite much more. In consideration of tempered hardness, the Vγ-As must be taken into account to obtain a target hardness. The HTmax values are connected to the Vγ-As and the relationship is shown in Figure 12. As the Vγ-As value increased to around 30%, the HTmax value of each specimen rose to near 1000 HV30 and then, decreased. This suggests that the amount of Vγ-As contributed largely to the secondary hardening caused by the precipitation of secondary carbides and the transformation of retained austenite to martensite. However, the HTmax value decreased when the Vγ-As rose over 30%. This also indicates that an excessive amount of austenite was left in the matrix even after tempering. In such a situation, the degree of decreasing the matrix hardness caused by an increase in the Vγ-As value overcomes that of raising the matrix hardness by the secondary hardening. In any case, a Vγ-As value of 5% to 60% provides a reliable indicator of the HTmax over 900 HV30 by tempering.
Here, a problem arises. As mentioned before, too large a value of Vγ in the roll is associated with a high risk of cracking during the operation; therefore, the Vγ values of hot work rolls were generally kept below 5%. In order for the tempered roll materials to hold the Vγ value as low as possible, the Vγ value must be controlled when hardening. It is understood from Figures 9 and 11 that the V content of the roll materials should be over 5% and the plural tempering must be carried out to reduce the Vγ value.
Relationship Between Tempering Temperature to Obtain the Maximum Tempered Hardness (H Tmax) and V Content
From Figure 9, the tempering temperature at the HTmax can be obtained and related to V content of the specimens. The relationship is shown in Figure 13. In the case of 1100 °C hardening, the tempering temperature to achieve HTmax falls gradually from 600 to 500 °C. This means that in hardening at high temperatures such as 1100 °C, a high temperature is necessary to obtain HTmax by tempering martensite and decomposing Vγ-As. The main reason for the fall in temperature could be a decrease of the Vγ-As value in response to the V content of specimens.
However, for 1050 °C hardening, despite the existence of a moderate amount of Vγ-As in each specimen, the tempering temperature for the same purpose is low and lowers slightly from 520 °C as the V content rises or Vγ-As value reduces. It is considered that this difference could arise from the stability of martensite and retained austenite in the as-hardened state, i.e., the stability of matrix hardened from 1100 °C is greater than that from 1050 °C austenitizing. Therefore, the lower temperature at around 520 °C is sufficient to achieve an HTmax over 6%V; the tempering temperatures are almost same in both the austenitizing temperatures because of lower Vγ-As values in hardening from both temperatures.
Effect of V Content on Degree of Secondary Hardening (ΔHs)
As shown in Figure 9, it became clear that the tempered hardness curves clearly showed a secondary hardening in all of the specimens. It can be expected that the degree of secondary hardening (ΔHs), which is defined as the difference between the lowest and the highest hardness in the tempering curves, should be affected by the heat treatment condition and the V content. It is known that secondary hardening also occurs during tempering in high alloyed white cast irons, and it is considered that the main reason is due to the formation of hard tempered martensite and the precipitation of secondary carbides from retained austenite. In addition to this, at around 527 °C, nearly pure carbides with much higher hardness formed from martensite by the so-called “Carbide Reaction” as well as the martensite formation from residual austenite in the post-cooling. In the tempering process, it is said that the formation of special carbides by the carbide reaction contributes most to the achievement of HTmax.6,7,13,15,16,17,23,24,25,26 Here, the tempering process concentrating the transformation behavior on the carbide reactions is shown in Figure 14 which is easy to understand by illustrating in the form of a flow chart. From the chart, it can be noted that the precipitation of extremely hard MC carbides, which contribute to the increase in matrix hardness and the high HTmax value near 1000 HV30, occurs in high V specimens. In the results of specimens with Mo in the previous studies,13,15 relatively softer M2C or M6C carbides precipitate secondarily instead of hard MC carbides. It can be understood that an addition of V alloy should be taken priority to the multi-component white cast iron in achieving higher HTmax value.
The previous studies6,7,13,15,16,17 indicated that the alloying elements significantly affected the ΔHs values. For instance, an increase in C, Cbal and Weq values raised the ΔHs value, while a rise of Cr and Mo contents resulted in a decrease of the ΔHs value. To clarify the effect of V content on the secondary hardening, the ΔHs was obtained from each hardness curve, and the relationship between ∆Hs value and V content is shown in Figure 15. It is natural that the ΔHs values in the specimens hardened from 1100 °C are higher than those hardened from 1050 °C. It is due to the expansion of solubility of C and other alloying elements in austenite. The ∆Hs value decreased gradually as the V content rose to 5%V at 1050 °C and to 4%V at 1100 °C austenitizing, respectively. Then, they dropped steeply with the increase in V content. This behavior shows the same trend as the case of Cr 7 and Mo addition.13,15 It can be explained that the strong carbide formers like Mo, Cr and V are consumed to form their carbides and resultantly, the concentration of C and other alloying elements in the austenite lowers and in a result, the Vγ-As value is reduced. Furthermore, the reduction of γP, which is where secondary hardening occurs, with rising the V content contributes to the lowering of ∆Hs as well. So, it can be said that the critical Vγ-As values at which the ΔHs values begin to fall steeply are 5%V at 1050°C and 4%V at 1100 °C austenitizing. In order to show the effect of Vγ-As value on the ΔHs more clearly, the ΔHs values were related to their Vγ-As values and the relationship is displayed in Figure 16. The ΔHs value goes up remarkably in response to an increase in the Vγ-As value in both austenitizing temperatures. When compared at the same Vγ-As value, the ΔHs values hardened from high temperature of 1100°C were greater overall than those from low temperature of 1050 °C (Figure 15). It was clearly found from these relations that the higher hardening temperature provided a larger Vγ-As value, and as a result, the ΔHs value was increased.
Conclusions
The effect of V content on heat treatment behavior of multi-component white cast iron with basic alloy composition was investigated by varying V content from 2.01 to 6.64%. The heat treatment processes of annealing, hardening from two austenitizing temperatures of 1050 °C and 1100 °C and tempering between 350 and 600 °C were applied to the cast irons. The correlations among hardness, volume fraction of retained austenite (Vγ), heat treatment conditions and V content were clarified. The results are summarized as follows.
As-Cast State
-
1.
The as-cast microstructures of specimens with 2%V showed hypoeutectic structure consisting of primary austenite (γP), (γ+MC), (γ+M2C) and (γ+M7C3) eutectics and those with 3% to 5%V were also hypoeutectic structures consisting of primary austenite (γP), (γ+MC) and (γ+M2C) eutectics.
-
2.
The specimen with 6.64%V showed hypereutectic structure made up of primary MC carbide (MCP), (γ+MC) and (γ+M2C) eutectics.
-
3.
The area fraction of primary austenite (γP) decreased but that of (γ+MC) eutectic increased with an increase in the V content. The area fraction of (γ+M2C) eutectic was not affected by the addition of V. When V content was increased beyond 2%, the formation of the (γ+M7C3) eutectic was inhibited.
As-Hardened State
-
1.
In the as-hardened state, the microstructures of all specimens composed of martensite, retained austenite and precipitated secondary carbides. The amount of secondary carbides increased as the V content rose.
-
2.
The hardness increased to the maximum value at 5%V in the case of 1050 °C and 6%V in 1100 °C austenitizing, respectively, and then decreased as V content increased, regardless of austenitizing temperature.
-
3.
The Vγ value decreased progressively with increased V content. At the same V content, the higher austenitizing temperature provided a larger amount of Vγ.
-
4.
The highest hardness of 907 HV30 at 1050 °C and 870 HV30 at 1100 °C austenitizing was obtained, where the Vγ values in the as-hardened state (Vγ-As) were about 15% and 12%, respectively.
Tempered State
-
1.
The hardness curves showed an obvious secondary hardening in all the specimens. The Vγ began to reduce markedly when the tempering temperature rose over 450 °C to 500 °C.
-
2.
The maximum tempered hardness (HTmax) was obtained at 500-520 °C tempering in 1050 °C and 500-600 °C tempering in 1100 °C austenitizing where the Vγ value ranged from 1 to 15%. The highest values of HTmax near 1000 HV30 were obtained at 4%V for 1050 °C and 5%V for 1100 °C austenitizing, respectively.
-
3.
The specimen with 30%Vγ in the as-hardened state (Vγ-As) provided the highest value of HTmax.
-
4.
The degree of secondary hardening (ΔHs) decreased with an increase in the V content, but secondary hardening was promoted by increasing the austenitizing temperature in the whole V content.
-
5.
From the viewpoints applying the multi-component white cast iron to hot work rolls, the materials satisfying the requirement of hardness over 950 HV30 would be accomplished by containing the V from 3.5 to 6.0% in the basic multi-component white cast iron, hardening from 1050 to 1100 °C and tempering at 500 °C to 550 °C.
References
Y. Matsubara, N. Sasaguri, and M. Hashimoto, The history and development of cast rolls for hot working mill, in The 4th Asian Foundry Congress-Australia, (1996), pp. 251-261
G. Laird, R. Gundlach, K. Rohrig, Abrasion-Resistance Cast Iron Handbook (American Foundry Society, USA, 2000)
M. Hashimoto, Development of multi-component white cast iron (HSS) rolls and rolling technology in steel rolling, in Abrasion Wear Resistant Alloyed White Cast Iron for Rolling and Pulverizing Mills, (2008), pp. 1-23
H.-Q. Wu, M. Hashimoto, N. Sasaguri, Y. Matsubara, Solidification sequence of multi-component white cast iron. JJFS 68, 637–643 (1996)
H.Q. Wu, N. Sasaguri, Y. Matsubara, M. Hashimoto, Solidification of multi-alloyed white cast iron: type and morphology of carbides. AFS Trans. 104, 103–108 (1996)
W. Khanitnantharak, M. Hashimoto, K. Shimizu, K. Yamamoto, N. Sasaguri, Y. Matsubara, Effects of carbon and heat treatment on the hardness and austenite content of a multi-component white cast iron. AFS Trans. 117, 435–444 (2009)
J. Opapaiboon, P. Sricharoenchai, S. Inthidech, Y. Matsubara, Effect of carbon content on heat treatment behavior of multi-alloyed white cast iron for abrasive wear resistance. Mater. Trans. 56, 720–725 (2015). https://doi.org/10.2320/matertrans.M2015001
N. Sasaguri, K. Yamamoto, Y. Yokomizo, K. Shimizu, Y. Matsubara, Influence of cobalt content on heat treatment behavior and abrasive wear characteristics of multi-component white cast iron. JJFS 82, 667–673 (2010). https://doi.org/10.11279/jfes.82.667
V. Raghavan, Phase Diagrams of Ternary Iron Alloys (ASM International Indian Institute of Metals, Kolkata, 1987), pp.111–125
M. Boccalini Jr., Overview: high speed steels for hot rolling mill rolls, in Abrasion Wear Resistant Alloyed White Cast Iron for Rolling and Pulverizing Mills, (2011), pp. 123-142
L. Hao, T. Li, Z. Xie, Q. Duan, G. Zhang, The oxidation behaviors of indefinite chill roll and high speed steel materials. Metals 10, 1095 (2020). https://doi.org/10.3390/met10081095
M. Hashimoto, O. Kubo, Y. Matsubara, Analysis of carbides in multi-component white cast iron for hot rolling mill rolls. ISIJ Int. 44, 372–380 (2004). https://doi.org/10.2355/isijinternational.44.372
T. Meebupha, S. Inthidech, P. Sricharoenchai, Y. Matsubara, Effect of molybdenum content on heat treatment behavior of multi-alloyed white cast iron. Mater. Trans. 58, 655–662 (2017). https://doi.org/10.2320/matertrans.M2016396
J. Opapaiboon, M.S.N. Ayudhaya, P. Sricharoenchai, S. Inthidech, Y. Matsubara, Effect of chromium content on heat treatment behavior of multi-alloyed white cast iron for abrasive wear resistance. Mater. Trans. 60, 346–354 (2019). https://doi.org/10.2320/matertrans.M2018318
J. Opapaiboon, S. Inthidech, P. Visuttipitukul, Y. Matsubara, Solidification structure and heat treatment behavior of multi-alloyed white cast iron with extensive molybdenum content for applying to hot work rolls. Int. Metalcast. 16, 2065–2078 (2022). https://doi.org/10.1007/s40962-021-00750-0
S. Inthidech, Y. Matsubara, Effects of carbon balance and heat treatment on hardness and volume fraction of retained austenite of semi-multi-alloyed white cast iron. Int. Metalcast. 14, 132–143 (2020). https://doi.org/10.1007/s40962-019-00343-y
S. Inthidech, K. Yamamoto, Y. Matsubara, Effect of tungsten equivalent on heat treatment behavior of semi-multi-alloyed white cast iron for abrasive wear resistance. Int. Metalcast. 15, 229–240 (2021). https://doi.org/10.1007/s40962-020-00449-8
J. Opapaiboon, M.S.N. Ayudhaya, P. Sricharoenchai, S. Inthidech, Y. Matsubara, Effect of chromium content on the three-body-type abrasive wear behavior of multi-alloyed white cast iron. J. Met. Mater. Miner. 28, 94–105 (2018)
S. Inthidech, J. Opapaiboon, K. Yamamoto, Y. Matsubara, Three-body-type abrasive wear behavior of multi-alloyed white cast iron with different carbon contents used for hot work rolls. ISIJ Int. 61, 2832–2843 (2021). https://doi.org/10.2355/isijinternational.ISIJINT-2021-035
C. Kim, X-ray method of measuring retained austenite in heat treated white cast irons. J. Heat. Treat. 1, 43–51 (1979). https://doi.org/10.1007/BF02833237
W. Leng, L. Xu, T. Jiang, X. Wang, X. Shi, S. Wei, M. Li, Carbide and matrix microstructure evolution of high-vanadium wear-resistance cast iron with high-silicon content during austempering. Int. Metalcast. (2022). https://doi.org/10.1007/s40962-022-00886-7
Y. Yokomizo, K. Yamamoto, N. Sasaguri, Y. Matsubara, Effect of chromium and vanadium contents on continuous cooling transformation behavior of multi-component white cast iron. KEM 457, 237–242 (2011). https://doi.org/10.4028/www.scientific.net/KEM.457.237
M. Hashimoto, O. Kubo, N. Sasaguri, Y. Matsubara, Analysis of precipitated carbides in matrix of multi-component white cast iron. JJFS 76, 205–211 (2004). https://doi.org/10.11279/jfes.76.205
S. Karagoz, R. Riedl, M.R. Gregg, H. Fischmeister, The role of M2C carbides in high speed steels. Sonderbd. Praktischen Metallogr. 14, 362–389 (1983)
Y. Sano, T. Hattori, M. Haga, Characteristics of high-carbon high speed steel rolls for hot strip mill. ISIJ Int. 32, 1194–1201 (1992). https://doi.org/10.2355/isijinternational.32.1194
W. Theisen, Design of wear resistant alloys against abrasion, in Abrasion Wear Resistant Alloyed White Cast Iron for Rolling and Pulverizing Mills, (2008), pp. 128-138
Acknowledgements
This research project was financially supported by Mahasarakham University. The authors would like to thank the Department of Metallurgical Engineering, Chulalongkorn University, Manufacturing and Materials Engineering Research Unit, Faculty of Engineering, Mahasarakham University, Thailand, and Cast metals laboratory of National Institute of Technology, Kurume College, Japan, for use of the experimental devices and helpful discussions. The authors appreciate the support of Dr. Adrian Roderick Plant, a native English speaker, for help with the English language.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Opapaiboon, J., Inthidech, S., Yamamoto, K. et al. Effect of V Content on Microstructure and Heat Treatment Behavior of Multi-component White Cast Iron used for Hot Work Roll Applications. Inter Metalcast 18, 2484–2498 (2024). https://doi.org/10.1007/s40962-023-01187-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-023-01187-3