Abstract
In this research, the effect of tungsten equivalent (Weq) on hardness and volume fraction of retained austenite (Vγ) of heat-treated semi-multi-alloyed white cast irons was investigated. Cast irons with 5.0 to 6.6%Weq under 2%C, 3%Cr, 1%W and 5%V were prepared. After annealing, the test specimens were hardened from austenitizing temperatures at 1050 °C and 1100 °C by fan air cooling. The tempering was carried out between 400 and 600 °C. In as-hardened state, the hardness dropped progressively as the Weq rose. High austenitizing temperature provided low hardness. The Vγ increased continuously as the Weq increased, and more Vγ was obtained by hardening from 1100 °C. In tempered state, the hardness curves showed an evident secondary hardening due to the precipitation of secondary carbides and the transformation of retained austenite to martensite. The degree of secondary hardening increased with a rise of Weq value and austenitizing temperature. The maximum tempered hardness (HTmax) was obtained by tempering at 500 °C in all the specimens regardless of austenitizing temperature. The highest values of HTmax, 830 HV30, was obtained in the specimen with 5.0%Weq hardened from 1100 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alloyed white cast irons have been utilized for a long time as parts of machines in steel-making, mining and cement industries due to high hardness and excellent abrasive wear resistance.1,2 When minerals give heavy abrasive damage to the surface of such parts during operation, the most important properties required for wear resistance are high hardness and toughness of the materials. Therefore, the development of alloyed white cast iron having both these properties has been demanded. The multi-alloyed white cast iron containing several kinds of strong carbide forming elements such as chromium (Cr), molybdenum (Mo), tungsten (W) and vanadium (V) was developed for such purposes.3,4 Those elements combine with carbon (C) to form MC, M2C and/or M6C and M7C3 eutectic carbides which have very high levels of hardness and improve abrasive wear resistance.3,4,5,6 The rest of the alloying elements after they are consumed to form their eutectic carbides dissolve into the matrix and influence the matrix transformation. Particularly, it is advantageous that the alloying elements dissolved in the matrix promote the precipitation of secondary carbides during heat treatment. The basic chemical composition of multi-alloyed white cast iron is 5 wt% Cr, Mo, W, V, Co each and 2 wt% C3 (hereafter wt% is expressed by %).
Several papers reported that rolls made of multi-alloyed white cast iron showed higher performance than those made of high Cr and Ni-hard cast irons.3,4,5 However, the manufacturing process to make such parts with multi-alloyed white cast iron is quite difficult.4 This is because the segregation of eutectic carbides occurs during solidification when the rolls are made by centrifugal casting (CF). To overcome this problem, the kind and amount of extremely heavy alloying elements should be reduced to decrease the difference in densities among the crystallized carbides.The alloyed white cast iron with lower contents of heavy carbide forming elements than those of regular multi-alloyed white cast irons has been developed, and it was named as “semi-multi-alloyed white cast iron.”7
It is well known that Mo and W have strong ability to form carbides compared with Cr. They could form complex carbides of M2C and M6C with higher hardness than chromium carbide of M7C3 in high Cr cast iron.1,3,4,7,8,9,10 In addition, Mo and W in the matrix improve the hardenability of cast iron and promote the secondary hardening during tempering. In the industrial applications, the content of Mo and W is varied randomly responding to the purpose. Particularly, in the case of roll manufacturing by CF method, Mo and W are controlled independently to prevent the segregation of eutectic carbides during solidification. When chemical composition of the cast iron is designed, the dealing with W and Mo all together is rather convenient than that with W and Mo separately. Since W and Mo have similar crystal structure with similar lattice constant, they should take similar action in the phase transformation during heat treatment. Because atomic density of Mo is about half of W additionally,8 Mo should work in the transformation twice as much as W.8,9 Consequently, the total content of Mo and W can be expressed by so-called tungsten equivalent (Weq = %W + 2%Mo).9,10,11
Wu et al.10 reported that the type and morphology of eutectic carbides in multi-alloyed white cast iron were controlled by Weq value. The nodular MC carbides crystalized in the specimens with less than 11%Weq. In addition, the M2C carbides with lamellar shape appeared when the Weq value was less than 15%. Out of this region, the flaky MC carbide or platelike M2C were formed. Even in a small amount, Mo and W dissolve into matrix and improve the hardenability of the cast iron. Yokomizo et al.9 reported that the nose of pearlite in CCT diagram of multi-alloyed white cast iron shifted to long time side with raising the Weq value. It was reported by Kim et al.11 that the highest fracture toughness of the high speed steel rolls was obtained when the Weq was about 10–11%.
In the research concerning the regular or basic multi-alloyed white cast irons, the systematic studies on solidification sequence,10,12 heat treatment and wear behavior13,14,15,16,17,18,19 were carried out and have already been made public. However, research on semi-multi-alloyed white cast iron is quite limited. In previous work,7 the role of carbon balance (Cbal) on heat treatment behavior of semi-multi-alloyed white cast iron was clarified. Since the Weq has influence on solidification structure, phase transformation and hardenability of the multi-alloyed white cast iron,9,10,11 it should affect the heat treatment behavior as well. Up to now, however, there was not reported concerning the effect of Weq on heat treatment behavior of semi-multi-alloyed white cast iron. In this study, therefore, the effect of Weq on heat treatment behaviors, i.e., variation of macro-hardness, micro-hardness and volume fraction of retained austenite (Vγ) during heat treatment, were investigated using semi-multi-alloyed white cast irons with 5.0 to 6.6%Weq.
Experimental Procedures
Preparation of Test Specimens
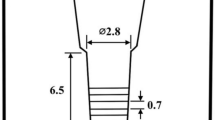
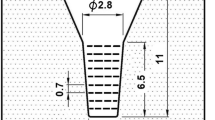
The charge materials consisting of mild steel, pig iron, ferro-alloys and pure metals were melted and superheated to 1580 °C. After being held at that temperature for 10 min, the melt was poured between 1490 and 1520 °C into preheated CO2 bonded sand molds in a round bar shape as shown in Figure 1. The substantial cavity size of the mold was 2.5 cm in diameter and 6.5 cm in length, together with sufficient riser. After pouring, the surface of the riser was covered immediately with dry exothermic powder to prevent the melt from fast cooling and oxidation. The chemical compositions, Weq and Cbal of test specimens are presented in Table 1.
Heat Treatment Procedures
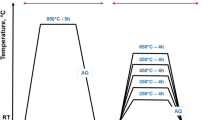
The round bar specimens were coated with an anti-oxidation solution and annealed at 950 °C for 5 h in an electric furnace. The annealed specimens were sectioned using a wire-cutting machine to obtain disk-shape test pieces with 0.7 cm in thickness. The specimens were austenitized at 1050 and 1100 °C in a vacuum furnace for 1 h and quenched by fan air cooling (FAC). The hardened specimens were tempered at 400–600 °C for 3.33 h with 50 °C intervals and cooled in still air.
Investigation of Microstructure
The microstructure was observed using an optical microscope (OM) and scanning electron microscope (SEM). The test pieces were polished using emery paper and buffered with 0.1 μm alumina powder. The microstructure of specimens was revealed using Murakami’s and Villella’s etchants.
Measurement of Hardness and Volume Fraction of Retained Austenite (Vγ)
The macro-hardness was measured using a Vickers hardness tester with a load of 30 kg and micro-hardness using a micro-Vickers hardness tester with a load of 100 g. The measurement was performed in five random locations, and the values were averaged.
The Vγ was measured using an X-ray diffraction method developed for alloyed white cast iron.2,7,14,15,16,17,18 The goniometer with special sample stage enabled the test piece to rotate and swing simultaneously so as to eliminate the effect of preferred crystal orientation of austenite. Mo–Kα characteristic line with a wavelength of 0.0711 nm was used as a source of X-ray beam. The scanning range was from 24° to 44° by 2θ. As an example, a x-ray diffraction pattern of specimen No. 3 hardened from 1050 °C with 41%Vγ is shown in Figure 2. For quantitative calculation, the (200)α and (220)α planes for ferrite or martensite as well as (220)γ and (311)γ planes for austenite were selected. The integrated area of the inner side of each peak was measured using an image analyzer. The calculation of Vγ was done for three combinations of peaks (220)α-(311)γ, (200)α-Σ(220,311)γ and Σ(200,220)α-(311)γ, and the average value was adopted.
Experimental Results and Discussions
Microstructure of Specimens
In order to investigate the microstructure on the whole surface of the specimen, the as-hardened specimens were etched by Murakami’s reagent and observed by OM. The microphotographs of representative specimens are shown in Figure 3. It is found that all the specimens showed hypoeutectic composition consisting of primary austenite dendrite (γp) and eutectic structure (γ + eutectic carbide). The type and morphology of eutectic carbides in multi-alloyed white cast iron were clarified by Wu et al.10 and Hashimoto et al.12 They reported that the morphology of MC carbide was nodular, while the M2C carbide was platelike and fine lamellar. The MC and M2C carbides can be distinguished by etching with Murakami’s reagent, that is, the M2C carbide is colored by dark brown but MC carbide is not.1,7,16 Therefore, it is clear that the eutectics in Figure 3 were almost (γ + MC) type and some (γ + M2C) type. It is well known that Mo and W promote the formation of M2C carbide. In order to clarify this, the area fractions of (γ + M2C) eutectic phases were quantitatively measured by an image analysis software. It was found that the area fraction of (γ + M2C) eutectic increased orderly from 1.8% in 5.0%Weq specimen to 10.1% in 6.6% Weq specimen. It can be concluded that an increase in Weq value promotes the crystallization of (γ + M2C) eutectic.
It is believed that the hardening process has little effect on the eutectic carbide, but it greatly influences phase transformation of the matrix. During austenitizing, the austenite is destabilized by the precipitation of secondary carbides and unstabilized austenite transforms to martensite by quenching. To clarify the constituent of phases in the matrix after quenching, the specimens were etched by Villella’s etchant to reveal the matrix structure and they are shown in Figure 4. It was found that the matrix of each specimen composed of martensite (M), retained austenite (γR) and fine secondary carbides (CS). The secondary carbides and martensite transformed from destabilized austenite are obviously observed throughout the matrix of all specimens. It is evident that the secondary carbides precipitated during austenitizing and allowed the transformation of austenite to martensite during quenching to room temperature. From the SEM microphotographs, the secondary carbides seem to decrease in size with increasing value of Weq. However, the variation in amount by increasing Weq value is not clear. The retained austenite is greater in the specimen with high Weq value along with high austenitizing temperature. It can be explained that the solubilities of C and other alloying elements in austenite increase with a rise of austenitizing temperature. Resultantly, the Ms temperature is lowered, and more austenite remains after hardening.
Behavior of Hardness and Volume Fraction of Retained Austenite (Vγ) in As-hardened State
The effect of Weq on hardness and Vγ of hardened specimens is shown in Figure 5(a) and (b) respectively. The macro-hardness and micro-hardness showed similar behavior, that is, they decreased progressively as the value of Weq rose. In contrast to the hardness, the Vγ increased with an increase in Weq value. This indicates that Mo stabilizes austenite. It was found that a decrease in hardness with a rise of Weq value corresponds to an increase in the amount of Vγ value together with a reduction in martensite. In addition, an increase in the amount of (γ + M2C) eutectic which has lower hardness than (γ + MC) eutectic should be one reason for this result. At the same Weq value, the hardness of specimens hardened from 1100 °C austenitizing is lower overall but the Vγ is greater overall. This can be explained by the fact that the solubility of C, Mo and other alloying elements in austenite is increased as the austenitizing temperature increases. As these alloying elements dissolved more in austenite, the Ms temperature decreases and more retained austenite forms in the matrix. As a result, the hardness was lowered.
Since the eutectic carbide changes little during heat treatment, the change in macro-hardness is mainly determined by matrix hardness (micro-hardness) and the amount of eutectic carbides. The relationship between Vγ and micro-hardness of specimens is shown in Figure 6. The micro-hardness dropped in proportion to the Vγ value in the both austenitizing temperatures. The micro-hardness of specimens hardened from 1050 °C austenitizing is higher than that of specimens hardened from 1100 °C austenitizing. The highest micro-hardness is obtained at 28% Vγ in the hardening from 1050 °C to 32% Vγ in the hardening from 1100 °C austenitizing, respectively. Here, it can be said that an increase in the Weq value or the austenitizing temperature causes lower the hardness, because too much retained austenite is obtained in as-hardened state.
Behavior of Hardness and Volume Fraction of Retained Austenite (Vγ) in Tempered State
In alloyed white cast iron or tool steel, the Vγ in as-hardened state naturally decreases during tempering, because the retained austenite is destabilized by the precipitation of secondary carbides and the unstable austenite transforms to other phases during cooling. A study based on TEM analysis reported that the secondary carbides in heat-treated basic multi-alloyed white cast iron were MC, M6C and M7C3 types.20 It can be considered that such carbides could precipitate secondarily during tempering in semi-multi-alloyed white cast iron. Because of precipitation of secondary carbides, the concentration of C and Mo as well as other alloying elements in austenite decreases. The decrease in alloy concentration makes the Ms temperature rise and enables the residual austenite to transform into martensite during post cooling.
As examples, the relationships between macro-hardness, micro-hardness, Vγ and the tempering temperature of specimens are shown in Figure 7. The hardness and Vγ of as-hardened specimens are plotted in each diagram for the comparison. It was found that the secondary hardening was clearly observed in all of tempered hardness curves. This phenomenon occurs due to the precipitation of secondary carbides and martensite transformation from the residual austenite. The hardness dropped naturally from as-hardened state by tempering at 400 °C. Then, it increased to the maximum value as the temperature was elevated. After that, the hardness decreased remarkably when the tempering temperature became more than the temperature at which the maximum hardness was obtained. The reason for the decrease in hardness could be due to the coarsening of fine precipitated carbides and full tempering of martensite. In all of the specimens, the maximum tempered macro-hardness (HTmax) and matrix hardness (HTmax-M) were obtained by tempering around 500 °C, irrespective of austenitizing temperature. On the other side, the Vγ dropped greatly up to 500 °C and it continued to decrease gradually as the temperature increased. The Vγ value at HTmax was less than 6% in the case of hardening from 1050 °C and less than 17% in hardening from 1100 °C austenitizing, respectively.
Based on the results detailed above, it can be said that the hardness of a specimen depends on the eutectic carbide and the matrix structure. Since the hardness of eutectic carbide is not affected by tempering, the variation of hardness in tempering is greatly influenced by the phase transformation of the matrix. The relationship between macro-hardness, micro-hardness and Vγ value of all the specimens in tempered state is shown in Figure 8(a) and (b) for the case of hardening from 1050 °C and that from 1100 °C austenitizing, respectively. On the whole, the distribution of macro-hardness lies more in the location of higher hardness than that of micro-hardness.
In Figure 8, the hardness increased markedly to the maximum value at 2 to 3% Vγ and then decreased slowly as the Vγ value increased regardless of austenitizing temperatures. In the region with Vγ value less than 3%, the hardness varied over a wide range. The test pieces with low hardness were over-tempered. In the region of high hardness, the retained austenite transformed mostly to martensite. Over the maximum hardness, they decreased gradually with an increase in the Vγ value. This is because an excessively soft austenite remained after tempering, and it reduced the matrix hardness. The decreasing rates of hardness are nearly the same at both austenitizing temperatures. It may be said that the maximum tempered hardness in the 1100 °C austenitizing is a little higher than those in the 1050 °C austenitizing, because greater secondary hardening is obtained in the specimens hardened from higher austenitizing temperature, as shown in Figure 7.
It was noted previously that the hardness values varied over a wide range of Vγ less than 3%. It is believed that the hardness within this region was not influenced so much as a result of a small amount of Vγ but by other phases such as secondary carbides, martensite, pearlite or ferrite. Since the amount of each phase in the matrix could not be measured quantitatively, the micro-hardness, which is determined by the kind and amount of all the phases in matrix, and which shows the comprehensive hardness, was adopted for discussion. The relationship between micro-hardness and tempering temperature of specimens with less than 3%Vγ is re-arranged and shown in Figure 9. The micro-hardness decreased proportionally as the tempering temperature increased regardless of austenitizing temperatures. This evidence explains that the remarkable lowering of hardness is due to the coarsening of secondary carbides and transformation of austenite to pearlite or ferrite when the tempering temperature was elevated over 500 °C. It is noted that in the specimens tempered from 550 to 600 °C, the coarsening of carbides and other transformation were completed.
The relationship between Weq, maximum tempered hardness (HTmax and HTmax-M) and Vγ at the HTmax is shown in Figure 10. The HTmax and HTmax-M of specimens hardened from 1050 °C austenitizing increased a little as the Weq value rose. The highest values of 815 HV30 and 746 HV0.1 were obtained in the specimen with 6.6%Weq. In the case of hardening from 1100 °C austenitizing, the HTmax and HTmax-M changed little until 5.6% Weq and then decreased gradually with a rise of Weq value. The highest values of HTmax and HTmax-M, 830 HV30 and 766 HV0.1, were obtained in the specimen with 5.0% Weq. In contrast, the Vγ value in specimens with maximum tempered hardness did not change in the case of 1050 °C austenitizing but increased progressively in 1100 °C austenitizing. This proves that a certain amount of austenite still remains in the specimens with maximum hardness in this tempering condition. A longer tempering time will be needed to decompose all the retained austenite.
The HTmax in the case of 1050 °C austenitizing increased slightly, because the retained austenite was almost decomposed to martensite by tempering. The improvement in matrix hardness due to the secondary hardening could compensate for the reduction in hardness due to an increase in (γ + M2C) eutectic with lower hardness than (γ + MC) eutectic. In the case of 1100 °C austenitizing, however, the HTmax decreased over 5.6% Weq. This is explained by more austenite existing in as-hardened state and there being more residual austenite after tempering.
In order to prove the variation of the HTmax, the SEM observations of specimens with HTmax were carried out focusing on the matrix and resulting microphotographs are shown in Figure 11 for specimens with 5.6% and 6.6% Weq. The matrices of both specimens are made up by secondary carbides (Cs) and martensite (M) coexisting with some retained austenite (γR). The amount of secondary carbides as well as that of residual austenite differs in the specimens with different Weq value and austenitizing temperature. The fine secondary carbides and martensite are observed throughout the matrix in both specimens. In 1050 °C austenitizing, as the Weq value increased, the amount of secondary carbides and martensite increased and the residual austenite decreased. Since such precipitated special carbides have higher hardness, they should promote the secondary hardening significantly. In the 1100 °C austenitizing, a large amount of austenite was left in the matrix in as-hardened state and therefore, the residual austenite increased as the Weq went up.
From all the above results, it is certain that the Vγ in as-hardened state is closely related to the tempered hardness and secondary hardening of specimens. Therefore, the relationship between HTmax, HTmax-M and Vγ in as-hardened state was obtained and is shown in Figure 12. The hardness increased to around 35%Vγ and then, decreased gradually as the Vγ value increased. In the former stage, the retained austenite in the as-hardened state encouraged an increase in the HTmax. There, the retained austenite promoted secondary hardening of the matrix by tempering. In the latter stage, however, too much Vγ value in as-hardened state increased the residual austenite after tempering and resultantly, reduced the hardness as the Vγ increased accompanying an increase in the Weq value. It is found that Vγ values from 28 to 48% are needed to obtain HTmax over 800 HV30 and HTmax-M over 740 HV0.1, respectively.
As shown in Figure 7, the tempered hardness curves showed more or less a secondary hardening depending on the Weq value and austenitizing temperature. Additionally, the HTmax and HTmax-M were obtained at 500 °C tempering in all the specimens. It is believed that the secondary hardening occurring in alloyed white cast iron is dominated by two mechanisms: the martensite transformation from retained austenite and the carbide reaction of martensite. The precipitation of secondary carbides in residual austenite can be added. This is explained for the carbide reaction that martensite is formed by hardening supersaturates C, Mo and other alloying elements. Such elements cause the precipitation of special secondary carbides with extremely high hardness during tempering. The carbide reaction during tempering in semi-multi-alloyed white cast iron can be explained as follows:7
The precipitation of such special carbides by carbide reaction promoted the secondary hardening and improved the matrix hardness efficiently.
In order to clarify how the Weq value affects the secondary hardening, the degree of secondary hardening (ΔHs), which is expressed by the difference in the hardness between maximum tempered hardness and the hardness where the secondary hardening begins, was introduced. The effect of Weq value on ΔHs is shown in Figure 13. It was found that the ΔHs increased in proportion to an increase in Weq value in each case of austenitizing temperature. The ΔHs values are more in 1100 °C austenitizing than those in 1050 °C austenitizing. From there results, it is clear that the ΔHs is closely related to the Vγ in as-hardened state. The relation of Vγ and ΔHs is shown in Figure 14. The ΔHs value increased proportionally to an increase in Vγ in as-hardened state regardless of austenitizing temperatures. This result agrees with Figure 11 that the HTmax value rises to around 35%Vγ as the Vγ increases. However, it is noted that too much Vγ value over 35% leads to lower the HTmax even if the amount of ΔHs is high. This is because the residual austenite at the HTmax increases with a rise of Weq value.
In all the experimental results, the HTmax of 830 HV30 was obtained. In order to get the higher HTmax under the same heat treatment condition, the alloy composition must be designed so as to attain a suitable amount of retained austenite in as-hardened state as well as the suitable type and amount of eutectics.
Conclusions
The effect of Weq on the heat treatment behavior of semi-multi-alloyed white cast iron was investigated. Cast irons with Weq values varying from 5.0 to 6.6% were produced, and the ingots were annealed. Annealed specimens were hardened from austenitizing temperatures of 1050 °C and 1100 °C by fan air cooling (FAC). Then, hardened specimens were tempered between 400 °C and 600 °C with 50 °C intervals. The results are summarized as follows:
As-Hardened State
-
1.
The microstructure of each specimen consisted of primary austenite dendrite and (γ + MC) and (γ + M2C) eutectics. The amount of (γ + M2C) eutectic increased with an increase in Weq value. The matrices in all the specimens were composed of secondary carbides (Cs), martensite (M) and retained austenite (γR).
-
2.
The hardness decreased continuously as the Weq value increased. Austenitizing at 1050 °C resulted in higher hardness than that at 1100 °C.
-
3.
The volume fraction of retained austenite (Vγ) increased gradually as Weq value rose. At the same Weq value, the higher austenitizing temperature produced a larger amount of Vγ.
Tempered State
-
1.
Secondary hardening was clearly obtained in all the tempered hardness curves. The maximum tempered macro-hardness (HTmax) and micro-hardness (HTmax-M) were obtained at 500 °C tempering. The 830 HV30 and 766 HV0.1 for respective largest HTmax and HTmax-M were obtained in the specimen with 5.0%Weq hardened from 1100 °C austenitizing. The Vγ values at the HTmax were less than 6% in 1050 °C and 17% in 1100 °C austenitizing.
-
2.
The HTmax and HTmax-M in 1050 °C austenitizing increased a little as the Weq rose. In 1100 °C austenitizing, however, they changed little until 5.6% Weq and then, decreased gradually. The Vγ value at the HTmax did not changed in 1050 °C austenitizing but increased gradually in 1100 °C austenitizing as the Weq value rose.
-
3.
The degree of secondary hardening (ΔHs) rose with an increase in both of Weq value and Vγ in as-hardened state. The ΔHs was more in the 1100 °C austenitizing than that in 1050 °C austenitizing.
References
G. Laird, R. Gundlach, K. Rohrig, Abrasion-Resistant Cast Iron Handbook (AFS, Schaumburg, 2000), pp. 203–204
S. Inthidech, P. Sricharoenchai, Y. Matsubara, Inter Metalcast. 6, 25–34 (2012)
Y. Matsubara, N. Sasaguri, M. Hashimoto, in Presented in Part at The 4th Asian Foundry Congress, Queensland, Australia (1996)
M. Hashimoto, S. Otomo, K. Yoshida, K. Kimura, R. Kurahashi, T. Kawakami, T. Kouga, ISIJ Int. 32, 1202–1210 (1992)
G. Gevelmann, W. Theisen, presented in part at The International Conference Abrasion 2011 (Abrasion Wear Resistant Alloyed White Cast Iron for Rolling and Pulverizing Mills), University of Liege, Belgium (2011)
M. Boccalini Jr., presented in part at The International Conference Abrasion 2011 (Abrasion Wear Resistant Alloyed White Cast Iron for Rolling and Pulverizing Mills), University of Liege, Belgium (2011)
S. Inthidech, Y. Matsubara, Inter Metalcast 14, 132–143 (2020)
A. M. Bayer, B. A. Becherer, T. Vasco, High Speed Tool Steels, ASM Handbook, vol. 16: Machining, (1989), pp 51–59
Y. Yokomizo, N. Sasaguri, K. Yamamoto, Y. Matsubara, J. JFS 82, 8–15 (2010)
H.-Q. Wu, N. Sasaguri, Y. Matsubara, M. Hashimoto, AFS Trans. 140, 103–108 (1996)
C.K. Kim, J.I. Park, S. Lee, Y.C. Kim, N.J. Kim, J.S. Yang, Metall. Mater. Trans. A 36A, 87–97 (2005)
M. Hashimoto, O. Kubo, Y. Matsubara, ISIJ Int. 44(2), 372–380 (2004)
W. S. Chang, Y. N. Pan, N. Sasaguri, Y. Matsubara, presented in part at The International Conference Abrasion 2008 (Abrasion Wear Resistant Alloyed White Cast Iron for Rolling and Pulverizing Mills), University of Trento, Italy (2008)
J. Opapaiboon, M. Supradist, P. Sricharoenchai, S. Inthidech, Y. Matsubara, Mater. Trans. 60(2), 346–354 (2019)
J. Opapaiboon, P. Sricharoenchai, S. Inthidech, Y. Matsubara, Mater. Trans. 56(5), 720–725 (2015)
T. Meebupha, S. Inthidech, P. Sricharoenchai, Y. Matsubara, Mater. Trans. 58(4), 655–662 (2017)
W. Khanitnantharak, M. Hashimoto, K. Shimizu, K. Yamamoto, N. Sasaguri, Y. Matsubara, AFS Trans. 117, 435–444 (2009)
N. Sasaguri, Y. Yokomizo, K. Yamamoto, Y. Matsubara, presented in part at The International Conference Abrasion 2011 (Abrasion Wear Resistant Alloyed White Cast Iron for Rolling and Pulverizing Mills), University of Liege, Belgium (2011)
Y. Matsubara, N. Sasaguri, K. Shimizu, S.K. Yu, Wear 250, 502–510 (2001)
M. Hashimoto, O. Kubo, N. Sasaguri, Y. Matsubara, J. JFS. 76(3), 205–211 (2004)
Acknowledgement
This research project was financially supported by the Faculty of Engineering, Mahasarakham University (Fiscal year 2019). The authors appreciated the Cast Metals Laboratory of National Institute of Technology-Kurume College and Faculty of Engineering Mahasarakham University for use of their experimental equipment. In addition, the authors appreciated the support of Dr. Adrian Roderick Plant, native English speaker, for help with English language.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Inthidech, S., Yamamoto, K. & Matsubara, Y. Effect of Tungsten Equivalent on Heat Treatment Behavior of Semi-multi-alloyed White Cast Iron for Abrasive Wear Resistance. Inter Metalcast 15, 229–240 (2021). https://doi.org/10.1007/s40962-020-00449-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-020-00449-8