Abstract
The Mio-Plio-Quaternary groundwater of Mchira-Teleghma suffers from an increasing rate of salinity especially in the northwestern part. To identify the reason for the water’s salinity and its aptitude for irrigation, physico-chemical analyses of 20 water samples, which were based on the different physical and chemical parameters (electric conductivity EC, pH, Ca2+, Mg2+, Na+, K+, HCO3−, Cl−, SO42−, NO3− and Sr2+), were carried out during the period of October 2015. This study showed disquieting anomalies of electric conductivity that reached the value of 4376.14 µS cm−1. The statistical analyses, the multivariate statistics: the principal component analysis, Q-mode cluster analyses, Sr2+/Ca2+ ratio and water type showed that the hydrochemistry of Mchira-Teleghma groundwater is controlled by the dissolution of carbonate rocks and the leaching of evaporite processes, which proved that these anomalies of the MPQ groundwater’s salinity of Mchira-Teleghma are mainly determined by the leaching of Triassic gypsum formations process. This hydrogeochemical process generates an unsuitable quality of water based on Wilcox’s and Water Quality Index’s methods, whereas Richard’s method classifies all water samples to C3S1 and C4S1 classes as they are recommended to be used with salt-tolerant species in well-drained and leached soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climate change has a significant impact on water resources, particularly in the Middle East and North Africa (MENA) region. These latter are generally characterized by the irregularity of the rainfall and the high prevailing temperatures that cause a strong evapotranspiration. In addition to the presence of evaporitic rocks, particularly the gypsum, in the North Africa region (Aubert 1975) that are leached by groundwater, water’s salinity is consequently increased and this can explain the deterioration of groundwater quality. In this context, several researches have been conducted on groundwater to assess the hydro-chemical processes, groundwater quality and water suitability for the irrigation of this region. They have shown that groundwater hydrochemistry depends on two important resources: natural and anthropogenic processes (Barkat et al. 2021; Bouteraa et al. 2019; Khedidja and Boudoukha 2015; Mihoubi 2009). They proved that the hydrochemistry of the studied groundwaters is mainly controlled by two primordial processes: the leaching of the evaporitic rocks and the dissolution of the carbonate massif. Taking into account the impact of the evaporation which synchronizes the two previous processes and contributes to the increase in the rate of mineralization, the aim of the present paper is to determine the Mchira-Teleghma groundwater mineralization origin, with thorough comprehension of the major processes that controlled this mineralization, and to evaluate the water quality for its appropriateness for irrigation purposes.
Geological and hydrogeological settings
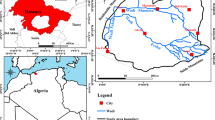
The plain of Mchira-Teleghma is an agricultural region located in the territory of Mila’s province, about 320 km from the capital Algiers. It is a part of the exoreic basin of Kebir Rhumel (Mebarki 2005) in north-eastern of Algeria. The plain of Mchira-Teleghma is a flat plain surrounded by different mountains with different altitudes, such as: DJ. Faham (1165 m), DJ. Teioualt (1285 m), DJ. Tadjerout (1011 m), DJ. Timetlas (1019 m), DJ. Feltene (1113 m), DJ. Grouz (1187 m) and DJ. Chettabbah (1263 m). The semi-arid climate prevails in this area with a precipitation of about 300–400 mm/year (Mebarki 1994). The plain is drained by several wadis with a less dense hydrographic network. The most important wadi is O. Rummel, which extends from the southwest to the northeast along the plain. The hydrogeological system of Mchira-Teleghma consists of mio-plio-quaternary MPQ aquifer and Jurassic–Cretaceous aquifer. The superficial unit of the mio-plio-quaternary is constituted from unconsolidated materials (gravel, silt, pebbles, rolled stones, rolled blocks and conglomerates), which form an alluvial tablecloth. The Jurassic–Cretaceous units are generally constituted of marls and carbonate massifs (limestone and/or dolomite rocks (Vila 1977b) forming karstic underground water. It is noted of the presence of Triassic evaporitic outcrops (clay and gypsum), which stand above the northern part and western borders of the study area (Vila 1977a) (Fig. 1). In order for establishing the piezometric map of MPQ groundwater (Fig. 2), the piezometric campaign was carried out in October 2015, where the water table depth ranges from 0.7 to 26.7 m (Table 1). The piezometric study shows that the flow of water generally occurs in the southwest to the northeast.
Materials and methods
Sampling analysis
The collection of water samples is a delicate process in which the greatest care must be taken as the analytical results and interpretation that will be given rely on it. The sample should be homogeneous, representative and obtained without modifying the physical and chemical characteristics of water (dissolved gases, suspended matters, etc.) (Rodier 2009). The field investigation led us to choose twenty wells that are spread all over the area to cover the whole plain. These wells are primarily used for an agricultural purpose. This study is based on the physical and chemical analyses (EC, pH, Ca2+, Mg2+, Na+, K+, HCO3−, Cl−, SO42−, NO3− and Sr2+) of twenty samples that were taken during the dry period (October 2015) (Table 1). The conductivity was measured by conductivity meter BEVAC. The pH was measured by pH meter BEVAC. Sulfates and Nitrates were dosed by the spectrophotometer type DR2000 (HACH). Sodium, potassium, magnesium and strontium were dosed by flame spectrophotometry atomic absorption (PYE UNICAM PHILIPS). Calcium, Chloride and Bicarbonate were dosed by titrimetric methods. These chemical processes were performed in the Hydrogeology Laboratory of Constantine 1 University, Algeria.
Statistical analysis
To classify groundwater analyses and identify the major mechanisms influencing groundwater chemistry, on the basis of their physical and chemical characteristics, we used the linear correlation and multivariate statistical analysis (principal component analysis PCA and Q-mode cluster analysis Q-CA). In groundwater research, multivariate statistical analysis is a quantitative approach to groundwater classification allowing the grouping of groundwater samples and making correlations between samples and their chemical parameters (Kouadra et al. 2019). Many researchers have successfully used the multivariate statistical analysis tool to classify water samples (Ayadi et al. 2018; Belkhiri et al. 2011; Bouaicha et al. 2019; Foued et al. 2017; Khelif and Boudoukha 2018; Kouadra et al. 2019), because of its efficiency to determine the different hydro-chemical processes occurring in the studied groundwaters.
Base exchange (CAI-I and CAI-II)
The chloro-alkali Indices (CAI-I and CAI-II) are used to evaluate the influence of ion exchange process in water mineralization between Ca2+, Mg2+, Na+ and K+. Positive index suggests the exchange of Na+ and K+ in water with Ca2+ and Mg2+ in aquifer materials, whereas negative index CAI indicates the exchange of Na+ and K+ in aquifer materials with Ca2+ and Mg2+ in water. In our case, the aquifer materials responsible for the base exchange are: the clay minerals and organic substances (Humus). The chloro-alkali indices are calculated using the following equations: (1) and (2) (Schoeller 1959):
All the ions are expressed in meq/L.
Saturation Index (SI)
To determine the water–mineral state of studied groundwater, we use the Saturation Index. SI of a given mineral is calculated by the following Eq. (3):
IAP is the ion activity product and Ksp is the equilibrium constant.
There are three water–mineral states: undersaturated, in equilibrium and supersaturated. If SI is below 0, the water is undersaturated with respect to the mineral in question. An SI of 0 means water is in equilibrium with the mineral, whereas an SI is greater than 0 means a supersaturated state with respect to the mineral in question. PHREEQ-C was used to calculate the SI in our research.
Irrigation water quality criteria
In classifying irrigation water, it is assumed that the water will be used under average conditions with respect to soil texture, infiltration rate, drainage, quantity of water used, climate, and the crop’s salt of the crop (Wilcox 1955). The salts affect directly plant’s development and reduce the growth of crops and their production. The salts also affect the vegetation indirectly, particularly the sodium, by degrading the soil’s structure where it becomes compact (Aubert 1975). The sodium adsorption ratio (SAR), sodium percentage (Na%) and Water Quality Index (WQI) are used in this work to evaluate the Mchira-Teleghma groundwater suitability for irrigation purposes (Agoubi et al. 2016; Haritash et al. 2017; Ravikumar et al. 2011). The SAR of a soil solution is simply related to the adsorption of the sodium by the soil, this ratio is defined by the following Eq. (4) (Richards 1954). The percentage of sodium \(\left(\mathrm{Na}\boldsymbol{\%}\right)\) is calculated by the following Eq. (5) (Todd and Mays 2005) where the evaluation of the sodium’s hazard and the SAR’s hazard also depends on the water’s electrical conductivity (Wilcox’s and Richard’s diagrams). The WQI is a powerful tool for the assessment of water quality with respect to drinking water or irrigation. WQI is based on the comparison of water quality parameters with the respective international standards. This index translates many of the data into simple terms (excellent, good, poor, etc.) (Horton 1965; Brown et al. 1970). The WQI is calculated by the following Eq. (6).
All the ions are expressed in meq L−1.
where Qi is the quality rating for the ith parameter, W is the unit weight of each parameter and n is the number of parameters.
Calculation of Q i value
Qi = [(Vi − V0)/(Si − v0)] Vi is the observed value of the ith parameter, V0 is the ideal value of the ith parameter in pure water, V0 is zero for all parameters except for pH 7.0, Si is the standard permissible value of the ith parameter. The calculation of the unit weight, Wi is inversely proportional to the standard permissible value Si for water quality parameters Wi = K/S I where K is the proportionality constant of the weights K = 1/\(\mathop \sum \nolimits_{i = 1}^{n} 1/S_{i}\). According to Wilcox’s and Richard’s methods, the assessment of sodium’s and SAR’s risk, respectively, requires the availability of the second parameter, which is the conductivity, as illustrated in Fig. 7. The classification of samples through the calculation of WQI is based on Table 2.
Results and discussion
Statistical analysis
The linear correlations EC vs. major elements
The linear correlation results of physical and chemical parameters with the EC (Table 3, Fig. 2) indicate that the pairs of (EC vs Ca2+), (EC vs. Mg2+), (EC vs Na+), (EC vs K+), (EC vs Cl−) and (EC vs SO42−) have a strong correlation, where the coefficient of correlation (R) oscillates between 0.76 and 0.89. This latter indicates that these elements largely contribute to water mineralization, which came from the leaching of evaporates. (Brinis et al. 2014). However, the linear correlation results of (EC vs HCO3−) and (EC vs NO3−) show a weak positive correlation with salinity (R = 0.30, and 0.15) which indicates that the carbonate rocks and nitrates have no importance in the increasing rate of salinity.
The linear correlations with major elements
The linear correlation results between the different chemical parameters (Ca2+, Mg2+, HCO−, SO42−, Na+, Cl− and Sr+2) (Table 3 and Fig. 3) show that the pairs of (Ca2+, HCO3−), (Mg2+, HCO3−) have a low correlation rate (R = 0.21, 0.28) because the dissolution of carbonate rocks does not have a significant importance. The pair of (Ca2+, Mg2+) has a strong correlation (R = 0.71) that can be explained by the reverse bases exchanges phenomena in which the sodium is fixed on the surface of clay minerals and the calcium and magnesium are released (Ben Alaya et al. 2014). (Na+, Cl−) and (K+, Cl−) pairs have a medium correlation (R = 0.65, 0.60) due to the leaching of the halite and sylvite, which have a medium importance. Finally, the pair of (Ca2+, SO42−) has a strong correlation (R = 0.95) which is explained by the high importance of the leaching of gypsum CaSO4·2H2O. The linear correlation results of physical and chemical parameters with the strontium (Sr+2) (Table 3), in which it is used as tracer element for indicating of water with evaporitic origin, indicate that the pairs of (Sr+2, Ca2+) and (Sr+2, SO42−) have a strong correlation (R = 0.82, 0.73). These results suggest a leaching of evaporitic rocks process which is mainly controlled by the leaching of gypsum CaSO4·2H2O that comes from the Triassic gypsum formations.
Principal component analysis (PCA)
Twenty water samples were collected and 10 physicochemical parameters were determined. These water quality data were analyzed using principal component analysis (PCA). This statistical tool showed three significant factors. The first factor 1 explains 58.20% of the total variance (Fig. 4a), and has strong positive loadings on evaporitic elements: Ca2+, Mg2+, Na+, K+, Cl−, SO42−, and EC which were 0.863, 0.895, 0.951, 0.910, 0.747, 0.922 and 0.966, respectively (Table 4). High positive loadings indicated strong linear correlation between the factor and parameters. Thus, factor 1 can be termed as salinization factor. This axis reflects the role of the participation of some evaporite minerals: Halite and Triassic gypsum in the acquisition of saline load of groundwater in the region. Factor 2 explains 13.60% of the total variance of the dataset and has strong positive loading on pH and strong negative loading on HCO3− which were 0.873 and -0.707, respectively. This axis reflects the role of dissolution of some carbonate minerals of Constantine Neritic Nappe in the aquifer. Factor 3 explains 12.40% of the total variance of the dataset and has strong positive loading on NO3− which is 0.921. This axis explains the effect of anthropogenic pollution, which is usually identified in the urban and agriculture-intensive areas due to the domestic wastewater discharge and agricultural activities (Khelif and Boudoukha 2018).
Q-mode cluster analysis (Q-CA)
In our case study, Q-mode cluster analysis is used to categorize the waters samples into different groups based on their physico-chemical parameters. This analysis resulted in the grouping of water samples into two clusters relying on their physical and chemical characteristics (Fig. 4b):
-
Cluster 1: Formed by nine wells (P2, P3, P4, P5, P9, P10, P11, P17 and P20) with a mean value of EC conductivity equals to 1446.03 µS cm−1, indicating low salinity (min = 850.34 µS cm−1, max = 2436.95 µS cm−1, mean = 1446.03 µS cm−1). This group is dominated by calcium (min = 56.12 meq L−1, max = 212.43 meq L−1, mean = 130.93 meq L−1 and bicarbonate (min = 170.8 meq L−1, max = 372.1 meq L−1, mean = 262.3 meq L−1) (Table 5). The majority of the wells are localized between the carbonate massifs of Constantine neritic nappe.
-
Cluster 2: Formed by eleven wells (P1, P6, P7, P8, P12, P13, P14, P15, P16, P18 and P19) with a mean value of EC Conductivity equals to 2721.65 µS cm−1, indicating a very high salinity (min = 1275.51 µS cm−1, max = 4376.14 µS cm−1, mean = 2721.65 µS cm−1). This group is dominated by calcium (min = 76.16 meq L−1, max = 625.25 meq L−1, mean = 294.77 meq L−1 and sulfate (min = 175 meq L−1, max = 1140 meq L−1, mean = 490 meq L−1) (Table 5). The majority of wells are localized near the outcrops of the evaporitic triassic formations. Based on the results obtained from the previous Cluster analysis, we can say that the study area is influenced by two primordially processes:
-
The dissolution of the carbonate rocks process: corresponds to the Cluster 1, suggesting that the mineralization is mainly controlled by the dissolution of the carbonate massif of Jurassic and cretaceous carbonate of the Constantine neritic nappe. It generates a high quantity of calcium and bicarbonate where the rate of salinity is not important (mean EC = 1446.03 µS cm−1).
-
The leaching of the evaporitic rocks process: corresponds to the Cluster 2, suggesting that the mineralization is mainly controlled by the dissolution of the evaporitic rocks of Triassic gypsum, and generates a high quantity of calcium and sulfate. This group has an important impact on the increasing rate of salinity (mean EC conductivity = 2721. 65 µS cm−1) that reaches 4376.14 µS cm−1. It should be noted that the existence of the chloride and sodium with an important quantity in both Clusters suggests a secondary process of leaching the halite NaCl that synchronizes these processes.
Evaluation of Hydrogeochemical process and origin of salinity
Piper diagram and strontium-calcium ratio
The analyses results carried on the Piper’s diagram (Piper 1944) (Fig. 5) show that 19 wells (P1–P19) are positioned on the chloride and sulfate calcium and magnesium pole, while one sample (P20) is positioned on the bicarbonate calcium and magnesium pole. The positioning of the majority of samples (95%) in a chloride and sulfate calcium and magnesium pole confirms the common origin of the samples, which is influenced by the dissolution of evaporites (Brinis 2011), because of the enhanced leaching of Triassic formations (Ayadi et al. 2018). This process has generated several chemical elements, especially sulphates, chlorides, magnesium and calcium (Khedidja 2016; Khedidja and Boudoukha 2015). The water samples that represented the triangle relevant to the cations begin in the middle (mixed part) and tend toward the pole of calcium, whereas the water samples that represented the triangle of anions begin in the middle (mixed part) and tend toward the sulfated or chloride pole. To further confirm the results, we used the strontium because it is a very strong indicator to determine the origins of groundwater mineralization that is used in the hydrochemistry domain (Chabour et al. 2021; Lebid et al. 2016; Shout et al. 2020). Strontium (Sr2+) is a good tracer for the existence of evaporites. It makes it possible to distinguish between sulfates resulting from the oxidation of sulfide (low content) and those resulting from the dissolution of evaporites (high content) (Abdesselam et al. 2000). The ionic ratio (in molar concentration) Sr2+/Ca2+ is a very useful tool to indicate the groundwater origin. If it is equal or greater than 1 ‰, the groundwater has an evaporitic origin (Bakalowicz 1988). In this context, we have selected the water samples with high to very high mineralization that are considered as anomalies so as to make the dosage of strontium (Table 6). The obtained results show that the Sr2+/Ca2+ ratio is bigger than 1‰ in all cases. It actually exceeds 6 ‰ in all cases. These results confirm the primordially evaporitic origin of mio-plio-quaternary groundwater’s mineralization of Mchira-Teleghma that mainly depends on the dissolution of the evaporitic formations of triassic gypsum.
Chemical facies (water type) and hydrochemical process
The conductivity values in Table 7 illustrated from Fig. 6 show that the water samples have a Calcium Sulfate facies SO4–Cl–Ca–Na and SO4–Cl–Ca–Mg (typical facies of gypsum CaSO4·2H2O) and calcium chloride secondarily sulfate facies Cl–SO4–Ca–Mg and Cl–SO4–Ca–Na are characterized by a high to a very high mineralization with conductivity oscillates between 2177.7 µS cm−1 and 4376.14 µS cm−1 (P2, P12, P14, P15, P16, P17, P18, P19), settle on the northwest of the study area, around or near to the outcrops of Triassic clay–gypsum formations. The water samples have a calcium bicarbonate facies Ca–Mg–HCO3–Cl and Ca–Na–HCO3–Cl (typical facies of limestone CaCO3), magnesium bicarbonate facies Mg–Na–HCO3–Cl and Mg–Na–HCO3–SO4 (typical facies of the dolomite rocks CaMg(CO3)2) and Calcium Chloride secondarily bicarbonate facies Ca–Mg–Cl–HCO3 are characterized by a low to a medium mineralization with conductivity varying between 850.34 µS cm−1 and 1503.65 µS cm−1 (P3, P4, P5, P8, P9, P10, P11, P20), settle in the southeast part of the plain, between the carbonate massifs of Constantine neritic Nappe. The water samples have a Calcium Chloride facies, secondarily sodium Ca–Na–Cl–HCO3 are characterized by a medium mineralization with conductivity of 1482.91 µS cm−1 and 1596.98 µS cm−1 in P1 and P13 water samples, respectively, suggesting a secondary halite leaching process. The existence of calcium chloride facies is often an indicator of pollution, where the chloride (Cl) in the water is considered as tracer for groundwater Contamination. It comes from weathering of minerals, such as halite and other anthropogenic sources, such as domestic effluents, septic tanks, leachates from landfills, in addition to being the main chemical element in the composition of detergents and fertilizers (Marghade et al. 2011; Mihoubi 2009). P6 and P7 water samples that have a magnesium chloride facies Mg–Ca–Cl–SO4 with conductivity of 3142.11 µS cm−1 and 1690.31 µS cm−1, respectively, located in the center of the study area, suggest a mixture water state. The presence of sulphated facies in all samples that are characterized by high to very high mineralization validates that the evaporitic formations, especially of Triassic gypsum is the main factor for increasing the rate of salinity in the northwest, where the majority of these samples are included in Cluster 2.
Base exchange of Mchira-Teleghma groundwater
The CAI-I value of water samples varies from − 0.54 to 0.73 and the CAI-II values vary from − 0.17 to 0.83 (Table 7). These results show that 75% of the water samples have a positive chloro-alkaline which indicates the exchange between Na+ and K+ in groundwater with Ca2+ and Mg2+ in aquifer materials. The other 25% of the water samples show a negative chloro-alkaline which indicates the exchange of Na+ and K+ in aquifer materials with Ca2+ and Mg2+ in groundwater.
Saturation state of Mchira-Teleghma groundwater
According to Table 7 and Fig. 7a, b, the results showed that 55% and 65% of the water samples have an SI < 0, these samples are undersaturated, respectively, by calcite and dolomite (is the dominant), which suggests a dissolution process. 10% of water samples have an SI = 0, which suggests that these water samples are in equilibrium by calcite. 35% of water samples have an SI > 0, these water samples are supersaturated by the calcite and dolomite, which indicates the precipitation process. The totally of water samples are undersaturated (SI < 0) with gypsum, Anhydrite and halite suggesting that their soluble component Na+, Cl−, Ca2+ and SO4−2 concentrations are not limited by mineral equilibrium, which indicates the dissolution process (Belkhiri and Mouni 2013; Bouteraa et al. 2019).
Assessment of water quality for irrigation purposes
The following diagrams (Fig. 8) classify the water samples in relation to irrigation purposes. These latter indicate an excess of mineralization (conductivity corrected to 25 °C). According to Wilcox's method (Wilcox 1948), the percentage of sodium oscillates between 15.45 and 40.15%, where the water samples are classified into three categories: good to permissible quality category is the dominant group by 55% (P1, P3, P4, P5, P7, P8, P9, P10, P11, P13 and P20). This category indicates a low salinity hazard and risk of sodium where the majority of these samples are included in Cluster 1 in which its salinity is controlled by the dissolution of carbonate massif of neritic Constantine nappe, doubtful to unsuitable category dominates by 10% (P2 and P17) and unsuitable quality category dominates by 35% (P6, P12, P14, P15, P16, P18 and P19). This category indicates an important salinity hazard and low risk of sodium where these samples are totally included in cluster 2 in which its salinity is mainly controlled by the leaching of the evaporitic rocks process of Triassic gypsum. According to Richard’s method (Richards 1954), the SAR values oscillate between 0.87 and 2.56, where the water samples are classified into two classes: C3S1 class (admissible quality) by 55% (P1, P3, P4, P5, P7, P8, P9, P10, P11, P13 and P20). It characterized by a low alkali hazard SAR and low risk of mineralization; the majority of these samples are included in Cluster 1. C4S1 class (mediocre quality) dominates by 45% (P2, P6, P12, P14, P15, P16, P17, P18 and P19). It characterized by a low alkali hazard SAR and high risk of mineralization where the majority of these samples are included in Cluster2. These water classes are generally suitable for irrigation of certain crop species that tolerate salts in well-drained and leached soils (Gouaidia et al. 2012; Khedidja 2016). According to WQI’s method, the WQI values (Table 7) oscillate between 46.58 and 508.70 where 25% of wells (P2, P4, P5, P9 and P11) have a good water quality, these samples are totally included in Cluster 1. 20% of wells (P3, P10, P17 and P20) have a poor water quality, 15% of wells (P1, P7 and P13) have a very poor water quality and 40% of wells (P6, P8, P12, P14, P15, P16, P18 and P19) are unsuitable at all for irrigation. The wells that have a very poor water quality to unsuitable for irrigation purposes are totally included in the Cluster 2, in which its salinity is controlled mainly by the leaching of the evaporitic rocks process of Triassic gypsum.
Conclusion
Mchira-Teleghma MPQ groundwater suffers from a growth of salinity in the northwestern part (it can reach 4376.14 µS cm−1). The Sr2+/Ca2+ ratio and piper diagram suggest evaporite dominance in the hydrochemistry processes occurring in the studied aquifer, where the evaporitic elements are mainly responsible for anomalies of salinity because its strong correlation with EC conductivity. The assessment of these results by the statistical tool: principal component analysis (PCA) and Q-mode cluster analysis (Q-CA) confirms that the hydrochemistry of Mchira-Teleghma is controlled by two main processes: the dissolution of carbonate massif process of neritic Constantine nappe and the leaching of evaporite rocks process, synchronizes by secondary process of leaching the halite NaCl. The assessment of water quality by water type showed that the anomalies of salinity are mainly due to the leaching of Triassic gypsum process that outcrops in the northern part and western borders of the study area, this process gives sulfated facies that is characterized by high to very high mineralization. The studied groundwater suffers from anthropogenic pollution confirmed by a strong positive loading on NO3− which is 0.921, due mainly to the domestic wastewater discharge and agricultural activities. The assessment of base exchange process in water using the Chloro-Alkali Indices (CAI-I and CAI-II) showed that 75% of the water samples have a positive chloro-alkaline ( are the dominant) which indicates the exchange between Na+ and K+ in groundwater with Ca2+ and Mg2+ in aquifer materials, consequently an enrichment of the water table by calcium and magnesium. Saturation indices (SI) indicate that the water is undersaturated (55% calcite, 65% dolomite) with respect to most of the carbonate minerals, and supersaturated with a reduced percentage (35%), while evaporitic minerals are totally undersaturated with respect to evaporite minerals confirming water–rock interactions by dissolution and precipitation processes of carbonate minerals and the dissolution process only of evaporitic minerals. To assess the water quality of the studied groundwater for irrigation purposes, we used three methods: Wilcox, Richard and WQI methods. Wilcox’s method classified the water samples into three categories: good to permissible quality category: is the dominant group by 55%, doubtful to unsuitable category: dominates by 10% and unsuitable quality category dominates by 35%. Richard’s method classified the water samples into two classes: C3S1 class (admissible quality) by 55% and C4S1 class (mediocre quality) dominates by 45%. To perform the irrigation with these water classes, they have to be used with salt-tolerant species in well-drained and leached soils. The WQI values oscillate between 46.58 and 508.70. The WQI method classified the water samples into four categories: 25% of water samples have a good water quality, 20% of samples have a poor water quality, 15% of samples have a very poor water quality and 40% of water samples are unsuitable at all for irrigation. The water samples that have a good water quality characterized by low salinity. These water samples are totally included in Cluster 1 in which its salinity is controlled mainly by the dissolution of the carbonate formations rocks, while the water samples that have a very poor water quality to unsuitable for irrigation purposes characterized by a high salinity. These water samples are totally included in the Cluster 2, in which its salinity is controlled mainly by the leaching of the evaporitic rocks process of Triassic gypsum.
References
Abdesselam M, Mania J, Mudry J, Gélard JP, Chauve P, Lami H, Aigoun C (2000) Arguments hydrogéochimiques en faveur de Trias évaporitique non affleurant dans le massif du Djurdjura (dorsale kabyle, élément des Maghrébides). Rseau 13:155–166. https://doi.org/10.7202/705387ar
Agoubi B, Souid F, Kharroubi A, Abdallaoui A (2016) Assessment of hot groundwater in an arid area in Tunisia using geochemical and fuzzy logic approaches. Environ Earth Sci 75:1163. https://doi.org/10.1007/s12665-016-6296-8
Aubert G (1975) Les sols sodiques en Afrique du Nord. Annale de l’institut National. Agronomique. Alger
Ayadi Y, Mokadem N, Besser H, Redhaounia B, Khelifi F, Harabi S, Nasri T, Hamed Y (2018) Statistical and geochemical assessment of groundwater quality in Teboursouk area (Northwestern Tunisian Atlas). Environ Earth Sci 77:208. https://doi.org/10.1007/s12665-018-7523-2
Bakalowicz M (1988) La formation des travertins : aspects géochimiques. Essai de synthèse et discussion n°XVII, U.A.903. CNRS et ATP PIREN Aix-en-Provence
Barkat A, Bouaicha F, Bouteraa O, Mester T, Ata B, Balla D, Rahal Z, Szabó G (2021) Assessment of complex terminal groundwater aquifer for different use of Oued Souf Valley (Algeria) using multivariate statistical methods, geostatistical modeling, and Water Quality Index. Water 13:1609. https://doi.org/10.3390/w13111609
Belkhiri L, Mouni L (2013) Geochemical modeling of groundwater in the El Eulma area, Algeria. Desalin Water Treat 51:1468–1476. https://doi.org/10.1080/19443994.2012.699350
Belkhiri L, Boudoukha A, Mouni L, Baouz T (2011) Statistical categorization geochemical modeling of groundwater in Ain Azel plain (Algeria). J Afr Earth Sc 59:140–148. https://doi.org/10.1016/j.jafrearsci.2010.09.007
Ben Alaya M, Zemni T, Mamou A, Zargouni F (2014) Acquisition de salinité et qualité des eaux d’une nappe profonde en Tunisie: approche statistique et géochimique. Hydrol Sci J 59:395–419. https://doi.org/10.1080/02626667.2013.870663
Bouaicha F, Dib H, Bouteraa O, Manchar N, Boufaa K, Chabour N, Demdoum A (2019) Geochemical assessment, mixing behavior and environmental impact of thermal waters in the Guelma geothermal system, Algeria. Acta Geochim 38:683–702. https://doi.org/10.1007/s11631-019-00324-2
Bouteraa O, Mebarki A, Bouaicha F, Nouaceur Z, Laignel B (2019) Groundwater quality assessment using multivariate analysis, geostatistical modeling, and water quality index (WQI): a case of study in the Boumerzoug-El Khroub valley of Northeast Algeria. Acta Geochim 38:796–814. https://doi.org/10.1007/s11631-019-00329-x
Brinis N (2011) Caractérisation de la salinité d’un complexe aquifère en zone aride cas de l’aquifère d’el-Outaya région nord-ouest de Biskra. Algérie, faculté des sciences et de la technologie, département d’hydraulique et du génie civil, Doctorat en science, Université Mohamed Kheider–Biskra
Brinis N, Boudouka A, Djaiz F (2014) Etude de la salinité des eaux souterraines dans les zones arides Cas de l’aquifère d’El-Outaya Région Nord-ouest de Biskra-Algerie. Int J Environ Water 3(1):44–51
Brown RM, McClelland NI, Deininger RA, Tozer RG (1970) A Water Quality Index: do we dare? Water Sewage Works 117(10):339–343
Chabour N, Dib H, Bouaicha F, Bechkit MA, Messaoud Nacer N (2021) A conceptual framework of groundwater flowpath and recharge in Ziban aquifer: south of Algeria. Sustain Water Resour Manag 7:36p. https://doi.org/10.1007/s40899-020-00483-8
Foued B, Hénia D, Lazhar B, Nabil M, Nabil C (2017) Hydrogeochemistry and geothermometry of thermal springs from the Guelma region, Algeria. J Geol Soc India 90:226–232. https://doi.org/10.1007/s12594-017-0703-y
Gouaidia L, Guefaifia O, Boudoukha A, LaidHemila M, Martin C (2012) Évaluation de la salinité des eaux souterraines utilisées en irrigation et risques de dégradation des sols : exemple de la plaine de Meskiana (Nord-Est Algérien). Physio-Geo. https://doi.org/10.4000/physio-geo.2632
Haritash AK, Mathur K, Singh P, Singh SK (2017) Hydrochemical characterization and suitability assessment of groundwater in Baga-Calangute stretch of Goa, India. Environ Earth Sci 76:15. https://doi.org/10.1007/s12665-017-6679-5
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–305
Khedidja A, Boudoukha A (2015) Characterization of the salinity of the alluvial aquifer in the upper valley of Wadi Rhumel (eastern Algeria). Desalin Water Treat 56:629–637. https://doi.org/10.1080/19443994.2014.939856
Khedidja A (2016) Caractérisation des paramètres hydrodynamiques de l’aquifère de Tadjnant–Chelghoum Laid et impact de la pollution des eaux de surface sur les eaux souterraines, Thèse de doctorat en science, Faculté de technologie, Département d’hydraulique, université de batna2
Khelif S, Boudoukha A (2018) Multivariate statistical characterization of groundwater quality in Fesdis, East of Algeria. J Water Land Dev 37:65–74. https://doi.org/10.2478/jwld-2018-0026
Kouadra R, Demdoum A, Chabour N, Benchikh R (2019) The use of hydrogeochemical analyses and multivariate statistics for the characterization of thermal springs in the Constantine area, Northeastern Algeria. Acta Geochim 38:292–306. https://doi.org/10.1007/s11631-018-0298-z
Lebid H, Errih M, Boudjemline D (2016) Contribution of strontium to the study of groundwater salinity. Case of the alluvial plain of Sidi Bel Abbes (Northwestern Algeria). Environ Earth Sci 75:2000. https://doi.org/10.1007/s12665-016-5704-4
Marghade D, Malpe DB, Zade AB (2011) Geochemical characterization of groundwater from northeastern part of Nagpur urban, Central India. Environ Earth Sci 62:1419–1430. https://doi.org/10.1007/s12665-010-0627-y
Mebarki A (1994) Le barrage d’Hamman Grouz (haut Rhumel, Constantinois). Bilan et perspectives d’un aménagement hydraulique en zone semi-aride. Medit 80:15–22. https://doi.org/10.3406/medit.1994.2854
Mebarki A (2005) Hydrologie des bassins de l’Est algérien: Ressources en eau, aménagement et environnement, Thèse de doctorat d’Etat, Faculté des sciences de la terre, de la géographie et de l’aménagement du territoire, Département de l’aménagement du territoire, Université Constantine1
Mihoubi N (2009) Fonctionnement et de gestion hydrogéologique et hydrologique des ressources en eau du bassin du Hammam Grouz, Mémoire de magister, département des Sciences de la Terre, faculté des sciences de la terre, de la géographie et de l’aménagement du territoire, Université Mentouri Constantine
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans AGU 25:914. https://doi.org/10.1029/TR025i006p00914
Ravikumar P, Venkatesharaju K, Prakash KL, Somashekar RK (2011) Geochemistry of groundwater and groundwater prospects evaluation, Anekal Taluk, Bangalore Urban District, Karnataka, India. Environ Monit Assess 179:93–112. https://doi.org/10.1007/s10661-010-1721-z
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Agriculture handbook, vol 60. USDA, Washington D.C.
Rodier J (2009) L’analyse de l’eau 9 èmeédition. Dunod, Paris, France
Schoeller H (1959) Hydrologie des régions arides - progrès recents, Organisation des Nations Unies pour l’Education, la science et la culture (UNESCO): place de Fontenoy, Paris-70 Imprimeries Oberthur. p. 127
Shout H, Bouaicha F, Merrad Z (2020) Les ressources géothermiques du nord-est constantinois - étude comparative - cas de la région de Guelma et Telaghma (impact socio-économique et juridique). Sciences & technologie des hydrocarbures. Sonatrach-Devision Laboratoires (E&P/SONTRACH) N°02/Septembre 2020. Algérie
Todd DKM, Mays LW (2005) Groundwater hydrology, 3rd edn. Wiley, Hoboken
Vila J M (1977a) Cartegéologique de Sétif au 200000ème, avec sa notice explicative, Service de la carte géologique de l’Algérie
Vila J M (1977b) Cartes géologiques de Constantine au 200000ème, avec sa notice explicative, Service de la cartegéologique de l’Algérie
Wilcox LV (ed) (1948) The quality of water for agricultural use. Edit. U.S Department of Agriculture, Technical Bulletin, vol 962, Washington (USA)
Wilcox LV (1955) Classification and use of irrigation waters. U.S. Department of Agriculture, Circular 969, Washington, D.C.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohamadi, A., Demdoum, A., Bouaicha, F. et al. Evaluation of the quality of groundwater for its appropriateness for irrigation purposes using Water Quality Index (WQI), Mchira-Teleghma aquifer case study, northeastern Algeria. Sustain. Water Resour. Manag. 7, 97 (2021). https://doi.org/10.1007/s40899-021-00571-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-021-00571-3