Abstract
Purpose
Corneal diseases are the fourth reason for blindness around the world which can be treated with corneal transplantation as a gold standard approach. However, alternative strategies get more valuable to peruse due to the challenges of donor shortage and failing the corneal transplantation procedure. This study aims to find the trend of biological products introduced to the market for corneal regeneration.
Methods
Biological products introduced by different companies were evaluated in this review. The available and underevaluation products introduced to market are reported in this review. This search was done by keywords related to corneal/product, corneal/biological scaffold, corneal/biological product, corneal/allograft, corneal/xenograft, eye drops, biological eye drops, and amniotic membrane/cornea.
Results
Decellularized products of xenogeneic or allogeneic cornea and amniotic membrane matrixes were mostly employed as corneal scaffold. In addition, biological eye drops, gels, and (platelet-rich plasma) PRP are used in several reports as bioactive ingredients.
Conclusion
Herein, the most important issue about biological products that researchers are involved in is preserving the most active ingredients after decellularization or extraction process with minimum modification along with reasonable final cost.
Lay Summary
Although at first glance cornea appears as simple avascular collagenous tissue, corneal diseases are the fourth leading cause of blindness according to the WHO reports. Currently, corneal transplantation has been chosen as a gold standard treatment. In recent years, the rising growth of smart biomaterials can be considered as a turning point in modern medicine by using smart scaffolds, a hydrogel with self-healing properties that could be potentially loaded with a drug, autologous cells, or stem cells. Here, we review available and under evaluation products introduced to the market to some extent overcome the cornea transplantation side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
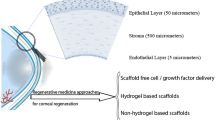
The transparent and avascular cornea is the anterior segment of the ocular with a thickness of about 575 μm. The cornea is formed in three layers (see Fig. 1); the outer layer is the non-keratinized epithelium that covers the middle layer of stromal connective tissue, and the innermost layer is a cuboidal endothelium. Bowman’s layer and Descemet’s membrane separate these layers [1, 2]. The epithelium layer of the cornea consists of four to six layers of cells that protect the inner parts of the cornea while being permeable toward oxygen and nutrients to be absorbed into indoor layers [3]. This layer could actively regenerate minor and superficial damages; however, deeper trauma with extension to the stroma leads to permanent opaque scars [4]. About 80–90% of corneal structure belongs to the stromal layer with 500 μm thickness with keratocytes entrapped within aligned collagen fibers [3]. Stromal layers of the cornea disable to regenerate extensive damage into these layers and lead to scar formation which disturbs the hydration of the cornea followed by loss of transparency [3]. One layer of endothelial cells covers the innermost layer of the cornea which pumps out excess fluid diffused by the anterior cornea to avoid the opacity of the cornea; this layer of cornea is unable to repair any damage [3, 4]. The functionality of each layer can be affected by different disorders that are mentioned in Table 1 [1, 5,6,7,8].
According to the World Health Organization (WHO), the fourth leading cause of blindness is due to corneal diseases [4, 9]. The most important function of the cornea is due to its radian and perfect transparency for light transmission into the eye which is related to the specific structure of collagenous fibers and the avascular character of the cornea that can be impressed by burn, infection, and trauma [10]. In comparison to other causes of blindness, younger populations are more affected by corneal blindness which is associated with increasing years of disability [11].
Corneal blindness is considered a reversible blindness disease with cornea transplantation [12]. In spite of progress toward corneal transplantation, the rate of undergoing surgery for treatable cases is about one out of seventy people which can be affected by different factors like social, economic, and political factors [11].
The first corneal transplantation was performed by Zirm in 1905 with full-thickness corneal transplantation (penetrating keratoplasty (PK)) which gradually progresses into partial corneal transplantation for selected diseased layers referred to as partial lamellar corneal surgery [12] which is associated with anterior lamellar keratoplasty (SALKFootnote 1, ALTKFootnote 2, and DALKFootnote 3) and posterior lamellar keratoplasty (DSAEKFootnote 4 and DMEKFootnote 5) [12, 13] with relatively similar outcomes to PK about the complications of pseudophakic bullous keratopathy (PBK), inflammation, and vascularization [13]. In spite of rather satisfying results for these procedures to overcome corneal blindness, there are some noticeable challenges to consider; the shortage of corneas to graft, graft failure, corneal infection, wound dehiscence, and the need for specialized centers to perform this procedure [13,14,15].
Applying keratoprosthesis is another strategy for corneal diseases that its primary studies began by Doane et al. in 1996 [16]. The artificial cornea was introduced to overcome the shortage of cornea donors and its related complications; however, these constructs are not able to integrate into native tissue and stimulate the biological function of corneal epithelial [2]. In spite of progresses in keratoprosthesis’s engineering, a half-life of 3 years was reported for Boston keratoprosthesis [13]; also, complications were reported such as retroprosthetic membrane formation, glaucoma, corneal melting, infectious keratitis, scleritis, suprachoroidal hemorrhages, retinal detachment, endophthalmitis, vitritis, and choroidal effusions and hypotony [17].
Due to the challenges that are ahead for current strategies to restore vision, biological scaffolds have emerged as the decellularized cornea, decellularized amniotic membrane, collagen, umbilical cord, and biological eye drops which are discussed in this review with a glimpse of products.
Materials and Methods
This study provided an overview of the commercially available biological products; however, the cell therapy approaches are excluded from this study as it has been discussed by previous studies [18,19,20]. This search was done by keywords related to corneal/product, corneal/biological scaffold, corneal/biological product, corneal/allograft, corneal/xenograft, eye drops, biological eye drops, and amniotic membrane/cornea. In this regard, any biological product for cornea which presented as a commercial product is reported in this review. Though, most of the reported products are not FDA-approved and have entered into clinical trials or product markets with desired preclinical results.
Result and Discussion
The quest was performed for commercially available biological products including scaffolds and small molecules for corneal regeneration, except cell-based therapies. Commercially available corneal scaffolds with biological origin are categorized into, allogeneic amniotic membrane, allograft cornea, xenograft cornea, collagen-based matrix, and umbilical cord matrix (Fig. 2) which are discussed in this review (Table 2). In addition, small molecules are presented as biological eye drops and are mostly derived from autologous or allogeneic serum, umbilical cord blood serum, platelet-rich plasma, amniotic extract, amniotic fluid extract, and sodium hyaluronate.
Amniotic Membrane
Reconstructing the ocular surface by human amniotic membrane (HAM) in symblepharon was the first usage of HAM in ophthalmology. Since that, the application of HAM has been increasing for corneal reconstruction in different situations like limbal stem cell deficiency, conjunctival reconstruction, glaucoma surgeries, ex vivo expansion of limbal stem cells, and sclera melt and perforation [21].
The semi-transparent HAM with a thickness of 0.02–0.05 mm is the innermost layer of the placenta which is consisted of three layers; epithelium, basement membrane, and an avascular stroma. The epithelial cells of HAM are responsible for the homeostasis of amniotic fluid along with secretory activity. The permeability of HAM to water and soluble compounds is an important characteristic of this membrane. In addition, secretion of different growth factors, cytokines, and vasoactive peptides (EGFFootnote 6, bFGFFootnote 7, HGFFootnote 8, KGFFootnote 9, TGFFootnote 10 a, TGF b-1, b-2, and b-3 isoforms, IL-6, IL-8, amniotic IFN-C) makes it an interesting bioactive membrane for its anti-inflammatory, anti-angiogenic, and anti-microbial effects along with promoting epithelialization. Also, HAM is not an immunogenic structure that makes it a proper transparent graft to be transplanted without irritating the immune system to heal corneal wounds while retaining the physiologically moist environment. Following these facts, HAM presented as a suitable substrate for epithelial cells to growth, migration, and adhesion [21,22,23]. In addition to growth factors and cytokines, other special matrix components in HAM are high molecular weight hyaluronic acid (HA), heavy chain-HA complex, and Pentraxin 3 in a complex (HC-HA/PTX3) that are responsible for the therapeutic effects of HAM. Limbal epithelial stem cells can be expanded ex vivo by maintaining stem cell quiescence in the presence of the complex of HC-HA/PTX3, proving the potential of this complex to reconstruct the limbal stem cell niche [24, 25]. In addition, the anti-inflammatory and anti-scaring properties of HC-HA/PTX3 were proven for ophthalmology applications [26]. Taking the mentioned advantage of HAM, different commercially available HAMs are presented to be used for ocular surface diseases [27,28,29,30]. In addition, HAM is introduced as a suitable substrate for in vitro and ex vivo expansion of corneal epithelial cells or a cell vehicle for cultivated limbal stem cells to be transplanted [31,32,33]. AmnioGraft® is a cryopreserved amniotic membrane presented by BioTissue, Inc. to promote the healing process in corneal ulcers, dry eye, pterygium, chemical burns, excision of tumors, and Stevens–Johnson syndrome [34]. PROKERA® is a biologic cornea bandage that is another cryopreserved product by BioTissue, Inc., based on HAM to reduce inflammation and scar formation during the healing of the damaged cornea [35].
BioTissue, Inc. company uses the Cryo Tek technology besides the Steri Tek® preservation technique to bring FDA-approved products of decellularized HAM into the market [36]. However, other processing techniques were introduced by other companies to dehydrate HAM like the PURION Process presented by IOP Ophthalmics, Inc. which prepares the AmbioDisK as a dehydrated and acellular HAM graft [37]. The Tereo® process is another patented process to prepare a dried HAM which is presented by NuVision for products of Omnigen/OmniLenz [38]. Preservation of the biomedical properties of HAM with minimum damage to its structure is the most important issue to consider. It is reported by Cooke et al. [86] that the technology of CRYOTEK® (cryopreservation technology) can preserve the HC-HA/PTX3 as an important biofunctional component that retains the proper functionality of HAM and human umbilical cord (HUC), instead of the dehydration preservation method [34]. However, the dried form of HAM has advantages too; a dried HAM can be stored at room temperature for 2–5 years in free-standing status, but a cryopreserved HAM must be stored at −80°C while attached to a nitrocellulose paper [39]. Though, a recent study by Mao et al. [36] reported the superiority of decellularized dried HAM (Biovance®3L Ocular (Celularity, Florham Park, NJ)) in comparison to dried HAM (AMBIO2® (Katena, Parsippany, NJ)) and cryopreserved HAM (AmnioGraft® (BioTissue, Miami, FL)) in in vitro evaluation for human corneal epithelial cell (HCEC) activity. Different eye banks such as Veneto Eye Bank and Barcelona Tissue Bank provide HAM to graft damaged cornea, however not under a specific trade name.
Allogeneic and Xenogeneic Cornea
Corneal transplantation has been a promising technique for years to restore one’s vision, though the risk of failure of transplantation due to immunogenic responses is a concern. In a healthy condition, the risk of immunological rejection for transplanted cornea decreases due to the avascular nature of the cornea and the ocular immune privilege; however, a damaged environment is susceptible to rejecting the transplanted allogeneic cornea due to inflammation and infection [40]. Different decellularization methods have been proposed by various studies to decrease the rejection rate of the transplanted allogeneic or xenogeneic cornea, which is more important in the case of xenogeneic tissues. However, it is important to preserve the native structure and biological factors during any manipulation. Reported strategies for decellularized xenogeneic cornea can be divided into 3 categories: (1) physical methods (high hydrostatic pressure, N2 gas, freeze-thaw, supercritical CO2, electrophoresis), (2) chemical agents (sodium dodecyl sulfate (SDS), Triton X-100, sodium deoxycholate, hypertonic saline, peracetic acid, formic acid, glycerol with chemical cross-linking), (3) biological agents (phospholipase A2, trypsin, dispase, human serum, nucleases) [41, 42]. The decellularization methods for human cornea that were evaluated with different studies are hypertonic NaCl, sodium dodecylsulfate (SDS), Triton X-100 [43,44,45,46], trypsin-EDTA [45], sodium deoxycholate [40], liquid nitrogen [43], poly(ethylene glycol) [43], and nuclease [46].
In a comparison study by Huh et al. [45], the superiority of hypotonic trypsin-EDTA to SDS proved for complete decellularization and preserved recellularization of human corneal lenticule. In addition, it is reported by Shafiq et al. [43] that the NaCl decellularized cornea lenticule can support the growth of both epithelial and fibroblast cells, in comparison to SDS-treated cornea that can support the growth of fibroblasts [43, 46]. In a study by Mertsch, sodium deoxycholate monohydrate (NaDC) solution followed by DNase was used for decellularization of the corneal cell sheet derived from human corneal fibroblasts. This decellularized corneal sheet had the property to be implanted into the cornea as a substrate [47].
Different studies compared these methods toward investigation for a proper decellularization method that can conserve transparency and microstructure of decellularized cornea along with the complete removal of cells (Fig. 3). These methods are mostly based on using chemical agents that might damage the extracellular matrix (ECM) and their residuals are toxic. The technology of supercritical carbon dioxide (scCO2) extraction is presented as a great alternative method that can remove cells without toxicity and damage to ECM [48].
Currently, some allogeneic or xenogeneic decellularized corneas are qualified to be commercially available. In ACRO Biomedical Inc., the scCO2 technology is used to decellularize porcine cornea to prepare ABCcolla® collagen ophthalmic matrix. This corneal scaffold is prepared in different thicknesses (20μm, 50μm, 80 μm) to be useable for different corneal transplantation techniques. Also, this company provided decellularized corneal powder for bioprinting applications for 3D structured cornea [49].
ACORNEA® (AcuHerb Marketing International Corporation (AMIC)) is another decellularized porcine cornea that is presented by AMIC ACUHERB Inc.; this product is recommended for cornea ulceration without perforation and replacement of the impaired tissue of Bowman’s membrane. Trends toward using allograft cornea instead of xenograft led to the emergence of OptiGraft® cornea by Lions Eye Institute. OptiGraft® (Lions Eye Institute for Transplant and Research’s (LEITR)) is a sterile gamma irradiated cornea with a decreased rate of immunological rejection and a long shelf life of up to 2 years. The CorneaGen company introduced another cryogenically treated allogeneic cornea with gamma irradiation sterilization (VisionGraft) as a substitute for corneal surgery [50]. Mostly, eye banks and institutes introduced acellular allogeneic cornea for corneal transplantation and related surgeries which are not represented as commercially available products.
Collagen
The most abundant structural protein in the cornea is collagen type I; however, other types of collagen are present in fewer amounts. About 70% of the weight in the dry cornea is collagen, because of this fact, collagen is one of the most important choices to be used in corneal tissue engineering. Different studies evaluated different kinds of collagen-based scaffolds. However, there are some challenges ahead for collagenous scaffolds for the cornea as poor mechanical properties and the absence of native fibril structure of corneal stromal, considering the fact that the specific organization of fibrils has a crucial rule in transparency [51].
Previous studies evaluated the efficiency and safety of the human recombinant collagen type III (FibroGen, Inc., San Francisco, USA) for cornea stromal reconstruction [52, 53]. These recombinant collagenous scaffolds are not susceptible to rejection by the immune system and also are safe for disease transmission which is a concern in allografts and xenografts [52, 54, 55]. However, this product is not widely used for cornea stromal reconstruction.
Other sources of collagen which have been used for corneal scaffolds are the porcine cornea and fish scale which have commercially available products. The Ologen® biocornea [56] is a corneal patch based on collagen type I derived from a fish scale that is presented to patch temporarily the perforated cornea for hours to days till a proper donor cornea can be transplanted [57]. Ologen® collagen matrix and implants are derived from the porcine cornea and are presented for different ophthalmic surgeries [58,59,60,61,62]. Xenia® is another collagen-based matrix derived from the porcine cornea that is introduced for keratoconus; this product can be transplanted into the cornea instead of customary cornea transplantation. The Xenia can be prepared for individual patient, as a custom-made device with a rationally simple procedure to implant [63].
Umbilical Cord
The therapeutic effects of human umbilical cord (HUC) blood for cancer and other hematopoietic disorders were proven by different studies and this biologic product is approved by the US Food and Drug Administration (FDA) for lymphomas, leukemia, sickle cell disease, and Wiskott–Aldrich syndrome. However, the biological application of HUC tissue was evaluated later. Similar to HAM, the HUC contains growth factors and cytokines that can promote the proliferation and differentiation of cells along with tissue regeneration and growth [64]. It was the first time in the 1970s that Irving and Herbert used the segments of HUC for skin grafts, science that other studies emerged to introduce other applications of this biocompatible structure for vascular lesion repair, gastroschisis, spina bifida defects, and wound repair [64]. In the case of ophthalmologic surgeries, the efficiency of the HAM-HUC patch was evaluated in reducing the glaucoma shunt tube exposure and showed the properties of low immunogenicity, and high tensile strength with good integration of host-tissue [65].
The AmnioGuard® (BioTissue, Inc.) is an umbilical cord–derived graft that is presented for different ocular surgeries. The high tensile strength and thickness of the AminoGuard® make it easy to handle and suture-able for the reconstruction of the cornea, conjunctiva, socket, sclera, and eyelid [66]. The CRYOTEK® cryopreservation technology is used to prepare the AminoGuard® with preserved properties to reduce inflammation and scar formation along with promoting regenerative healing. Another HUC product is a topical gel, eye drop form of AMUC that is presented by BioTissue/Tissue Tech Inc. with the trade name of Regenesol™, this product should be administrated twice a day for patients with dry eye and after phototherapeutic keratectomy (PTK), EPI-off cross-linking, and photorefractive keratectomy (PRK) [23, 67].
Biological Eye Drops
The crucial rule of growth factors in corneal epithelial regeneration is proven in different studies [68]. Biological eye drops that are rich in growth factors and cytokines from autologous serum [69], allogeneic serum [70], amniotic membrane extract [71], amnion fluid [23], umbilical cord [72], and finger-prick autologous blood [73] can promote corneal regeneration.
The similarity of components in blood serum to natural tears led to the production of serum eye drops for corneal regeneration. The presence of EGF, TGF-β, fibronectin, and vitamin A in serum eye drops promotes corneal regeneration [69]. Rehabilitation of the corneal epithelium occurred via applying the autologous serum eye drop (ASED) to the limbal stem cell deficiency patients [74,75,76]. In addition to promoting the healing process of corneal epithelial after ocular surgeries [77] and epithelium-off cross-linking [78], the efficiency of ASED is proven with various studies for different ocular surface diseases like graft-versus-host disease (GVHD), dry eye, keratoconjunctivitis, Sjögren’s disease [75].
Human platelet derivatives such as platelet-rich plasma (PRP) eye drops, platelet gels, and human platelet lysate are rich in growth factors (PDGFFootnote 11, TGF, EGF, bFGF, IGF-I, HGF, NGF, VEGFFootnote 12) to regenerate the damaged limbal niche [79]. Other bioactive factors for corneal niche reconstruction are based on umbilical cord serum and HAM derivatives. In the case of umbilical cord serum eye drops, substance P, EGF, NGF, and TGF-β are responsible for regenerative effects on the corneal epithelium [72, 80]. Amniotic membrane extracts eye drops are prepared by homogenizing the AM and centrifugation for gathering the supernatant that is full of growth factors [89].
OptiSerum by Next Biosciences is an eye drop derived from umbilical cord blood serum that is rich in growth factors, proteins, and neurotrophic factors to promote corneal healing. The preparation method for OptiSerum is centrifuging the clotted cord blood to separate cellular fractions of serum fraction.
There are different strategies to prepare the HAM eye drops. One of these methods is given step by step as follows: (1) washing the AM (normal saline containing penicillin and streptomycin), (2) using a scalpel blade for chopping into small pieces, (3) submerging in liquid nitrogen, (4) homogenization and centrifugation, (5) collection of the supernatant, (6) centrifugation, (7) sterilizing using a 0.25 mm filter. In other methods, the cryopreserved or dehydrated HAM can be pulverized, micronized, or morselized to prepare the proper HAM extraction as an eye drop. The importance of different processing methods is due to the different amounts of remaining bioactive components in the final products [23]. Another desired biofunction component in eye drops is hyaluronic acid (sodium hyaluronate) which can promote the healing process of corneal wounds by improving cell migration of corneal epithelial cells [81, 82]. Tisseel and Tissucol are two tissue adhesive agents introduced for corneal ulcers to improve healing [83].
Discussion and Conclusion
The scarcity of donor cornea for corneal transplantation as a global issue causes the development of alternative approaches to donor cornea. Regarding the important role of special features in collagen fibers with hexagonal space lattice ultrastructure for presenting the unique optical and mechanical properties of the stromal cornea, most studies rely on allograft or xenograft corneal substitutes. However, these biological scaffolds must be modified toward a proper construct for corneal transplantation to carry the properties of the natural cornea. The main purpose of product processing is to reduce potential risks along with increasing the biofunctionality, biocompatibility, and biomechanical characteristics. Different decellularization methods are applied to the allogeneic cornea to reduce immune rejection, while the proper method must protect the natural ECM components and architecture of the cornea. The importance of decellularization is more concerned in the case of xenogeneic corneal grafts [48, 49, 84]. In addition, cross-linking was introduced to improve the mechanical and functional behavior of a decellularized cornea [85]. In the case of HAM, a proper decellularization method along with a preservation method is crucial to protect the functionality of biofunctional components of HAM [86]. Collagen scaffolds are other introduced alternatives for cornea transplantation. Although fish and porcine collagen are used for commercially available corneal implants, recombinant human collagen can be considered to be a less immunogenic source of biomaterials for corneal implants [52, 53, 56, 61, 87]. Though, the trend of recent preclinical studies can bring insight into the development of natural hydrogels as a corneal substitute, especially hydrogels composed of corneal ECM [88, 89]. Developing bioadhesive hydrogels which can be photo-cross-link to fulfill a defect of cornea is another research near to clinic, known as the sutureless approach, in preclinical research reported as GelCore with satisfying results in rabbit corneal defect [90]. Another promising preclinical study for corneal reconstruction is based on a 3D fiber hydrogel construct. Synthetic polymers of poly (ε-caprolactone)-poly (ethylene glycol) microfibrous are used to mimic the topological structure of the cornea and improve the mechanical properties of gelatin methacrylate (GelMA) hydrogel [91]. Besides a proper scaffold, the rehabilitation of damaged cornea can be achieved via eye drops and gels with regenerative biomolecules. However, most of these products are encountered high production costs and accessibility to good tissue practice (GTP) or good manufacturing practice (GMP) facilities, which are the main limitations of these products; in this regard, a novel intervention by the use of finger-prick autologous blood (FAB) as an accessible alternative for corneal surface diseases is introduced recently [73, 92, 93]. The safety and effectiveness of FAB were proved for severe dry eye disease [73, 92], and the same results were reported by other studies for persistent epithelial defect [93, 94], though, this approach is not categorized as a commercially available product. In spite of introducing several approaches for corneal substitutes, mimicking the specific ultrastructure of corneal stroma or conserving this structure during processing steps is still a crucial concern.
Future Perspective
According to the rising growth of promising results reported in stem cell therapy, smart biomaterials, and artificial intelligence, the integration of these emerging technologies with conventional treatments in corneal disease seems to be inevitable. Although at first glance the cornea appears as simple avascular collagenous tissue, the improvement of these limited biomaterials introduced to the market has evoked great expectations due to the lack of long-time functionality or failure transplantation. Stem cell therapies have become a very promising and advanced scientific research topic. A wide variety of possibilities makes this cutting-edge a turning point in modern medicine, such as using smart scaffolds with self-healing properties loaded with autologous cells or stem cells. The role of synthetic biomaterials especially hydrogels with enhanced functional and compatible properties for the improvement of the biological products market also seems to be neglected. Thereupon, a possible snapshot of future commercially available products to overcome the cornea transplantation side effects could be a graft containing a combination of biomimetic synthetic and natural polymers that at least act as transparent mechanical and structural support and cell, drug, and protein carriers.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Notes
Superficial anterior lamellar keratoplasty
Automated lamellar therapeutic keratoplasty
Deep anterior lamellar keratoplasty
Descemet’s stripping automated endothelial keratoplasty
Descemet’s membrane endothelial keratoplasty
Epithelial growth factor
Basic fibroblast growth factor
Hepatocyte growth factor
Keratinocyte growth factor
Tumor growth factors
Platelet-derived growth factor
Vascular endothelial growth factor
References
Oie Y, Nishida K. Regenerative medicine for the cornea. BioMed Res Int. 2013;2013 https://doi.org/10.1155/2013/428247.
Nosrati H, Abpeikar Z, Mahmoudian ZG, Zafari M, Majidi J, Alizadeh A, et al. Corneal epithelium tissue engineering: recent advances in regeneration and replacement of corneal surface. Regen Med. 2020;15(8):2029–44. https://doi.org/10.2217/rme-2019-0055.
Palchesko RN, Carrasquilla SD, Feinberg AW. Natural biomaterials for corneal tissue engineering, repair, and regeneration. Adv Healthc Mater. 2018;7(16):1701434. https://doi.org/10.1002/adhm.201701434.
Guérin L-P, Le-Bel G, Desjardins P, Couture C, Gillard E, Boisselier É, et al. The human tissue-engineered cornea (hTEC): recent progress. Int J Mol Sci. 2021;22(3):1291. https://doi.org/10.3390/ijms22031291.
Lagali N. Corneal stromal regeneration: current status and future therapeutic potential. Curr Eye Res. 2020;45(3):278–90. https://doi.org/10.1080/02713683.2019.1663874.
Vaidyanathan U, Hopping GC, Liu HY, Somani AN, Ronquillo YC, Hoopes PC, et al. Persistent corneal epithelial defects: a review article. Med Hypothesis Discov Innov Ophthalmol. 2019;8(3):163.
Bandeira F, Goh T-W, Setiawan M, Yam GH-F, Mehta JS. Cellular therapy of corneal epithelial defect by adipose mesenchymal stem cell-derived epithelial progenitors. Stem Cell Res Ther. 2020;11(1):1–13. https://doi.org/10.1186/s13287-019-1533-1.
Feizi S. Corneal endothelial cell dysfunction: etiologies and management. Ther Adv Ophthalmol. 2018;10:2515841418815802. https://doi.org/10.1177/2515841418815802.
Colby K, Dana R. Foundations of corneal disease: past, present and future. Springer; 2019. https://doi.org/10.1007/978-3-030-25335-6.
Shang Q, Chu Y, Li Y, Han Y, Yu D, Liu R, et al. Adipose-derived mesenchymal stromal cells promote corneal wound healing by accelerating the clearance of neutrophils in cornea. Cell Death Dis. 2020;11(8):1–15. https://doi.org/10.1038/s41419-020-02914-y.
Mathews PM, Lindsley K, Aldave AJ, Akpek EK. Etiology of global corneal blindness and current practices of corneal transplantation: a focused review. Cornea. 2018;37(9):1198–203. https://doi.org/10.1097/ICO.0000000000001666.
Singh R, Gupta N, Vanathi M, Tandon R. Corneal transplantation in the modern era. Indian J Med Res. 2019;150(1):7. https://doi.org/10.4103/ijmr.IJMR_141_19.
Antunes-Foschini R, Adriano L, Murashima AAB, Barbosa AP, Nominato LF, Dias LC, et al. Limitations and advances in new treatments and future perspectives of corneal blindness. Arq brasil oft. 2021;84:282–96. https://doi.org/10.5935/0004-2749.20210042.
Greenrod EB, Jones MN, Kaye S, Larkin DF, NHS B, Group TOTA. Center and surgeon effect on outcomes of endothelial keratoplasty versus penetrating keratoplasty in the United Kingdom. Am J Ophthalmol. 2014;158(5):957–66. e1. https://doi.org/10.1016/j.ajo.2014.07.037.
Ang M, Soh Y, Htoon HM, Mehta JS, Tan D. Five-year graft survival comparing Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Ophthalmology. 2016;123(8):1646–52. https://doi.org/10.1016/j.ophtha.2016.04.049.
Doane MG, Dohlman CH, Bearse G. Fabrication of a keratoprosthesis. Cornea. 1996;15(2):179–84. https://doi.org/10.1097/00003226-199603000-00011.
Lee WB, Shtein RM, Kaufman SC, Deng SX, Rosenblatt MI. Boston keratoprosthesis: outcomes and complications: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122(7):1504–11. https://doi.org/10.1016/j.ophtha.2015.03.025.
Kao WW, Thomas VJC. Cell therapy of corneal diseases. Cornea. 2016;35(Suppl 1):S9. https://doi.org/10.1097/ICO.0000000000001010.
Chakrabarty K, Shetty R, Ghosh A. Corneal cell therapy: with iPSCs, it is no more a far-sight. Stem Cell Res Ther. 2018;9(1):1–15. https://doi.org/10.1186/s13287-018-1036-5.
Fuest M, Yam GH-F, Peh GS-L, Mehta JS. Advances in corneal cell therapy. Regen Med. 2016;11(6):601–15. https://doi.org/10.2217/rme-2016-0054.
Jirsova K, Jones GL. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting—a review. Cell Tissue Bank. 2017;18(2):193–204. https://doi.org/10.1007/s10561-017-9618-5.
Schuerch K, Baeriswyl A, Frueh BE, Tappeiner C. Efficacy of amniotic membrane transplantation for the treatment of corneal ulcers. Cornea. 2020;39(4):479–83. https://doi.org/10.1097/ICO.0000000000002179.
Murri MS, Moshirfar M, Birdsong OC, Ronquillo YC, Ding Y, Hoopes PC. Amniotic membrane extract and eye drops: a review of literature and clinical application. Clin Ophthalmol. 2018;12:1105. https://doi.org/10.2147/OPTH.S165553.
Tseng SC. HC-HA/PTX3 purified from amniotic membrane as novel regenerative matrix: insight into relationship between inflammation and regeneration. Invest Ophthalmol Vis Sci. 2016;57(5):ORSFh1–8. https://doi.org/10.1167/iovs.15-17637.
Tseng SCG, Chen S-Y, Mead OG, Tighe S. Niche regulation of limbal epithelial stem cells: HC-HA/PTX3 as surrogate matrix niche. Exp Eye Res. 2020;199:108181. https://doi.org/10.1016/j.exer.2020.108181.
Ogawa Y, He H, Mukai S, Imada T, Nakamura S, Su CW, et al. Heavy chain-hyaluronan/pentraxin 3 from amniotic membrane suppresses inflammation and scarring in murine lacrimal gland and conjunctiva of chronic graft-versus-host disease. Sci Rep. 2017;7:42195. https://doi.org/10.1038/srep42195.
Khokhar S, Natung T, Sony P, Sharma N, Agarwal N, Vajpayee RB. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea. 2005;24(6):654–60. https://doi.org/10.1097/01.ico.0000153102.19776.80.
Plummer CE. The use of amniotic membrane transplantation for ocular surface reconstruction: a review and series of 58 equine clinical cases (2002–2008). Vet Ophthalmol. 2009;12:17–24. https://doi.org/10.1111/j.1463-5224.2009.00741.x.
Hick S, Demers PE, Brunette I, La C, Mabon M, Duchesne B. Amniotic membrane transplantation and fibrin glue in the management of corneal ulcers and perforations: a review of 33 cases. Cornea. 2005;24(4):369–77. https://doi.org/10.1097/01.ico.0000151547.08113.d1.
Ke L, Shen D, Wang H, Qiao C, Zeng Q. Lamellar keratoplasty combined with amniotic membrane transplantation for the treatment of corneal perforations: a clinical and in vivo confocal microscopy study. BioMed Res Int. 2020;2020 https://doi.org/10.1155/2020/7403842.
Koizumi N, Inatomi T, Suzuki T, Sotozono C, Kinoshita S. Cultivated corneal epithelial transplantation for ocular surface reconstruction in acute phase of Stevens-Johnson syndrome. Arch Ophthalmol. 2001;119(2):298–300.
Fatima A, Sangwan V, Iftekhar G, Reddy P, Matalia H, Balasubramanian D, et al. Technique of cultivating limbal derived corneal epithelium on human amniotic membrane for clinical transplantation. J Postgrad Med. 2006;52(4):257.
Zhao Y, Ma L. Systematic review and meta-analysis on transplantation of ex vivo cultivated limbal epithelial stem cell on amniotic membrane in limbal stem cell deficiency. Cornea. 2015;34(5):592–600. https://doi.org/10.1097/ICO.0000000000000398.
Shanbhag SS, Hall L, Chodosh J, Saeed HN. Long-term outcomes of amniotic membrane treatment in acute Stevens-Johnson syndrome/toxic epidermal necrolysis. Ocul Surf. 2020;18(3):517–22. https://doi.org/10.1016/j.jtos.2020.03.004.
Suri K, Kosker M, Raber IM, Hammersmith KM, Nagra PK, Ayres BD, et al. Sutureless amniotic membrane ProKera for ocular surface disorders: short-term results. Eye Contact Lens. 2013;39(5):341–7. https://doi.org/10.1097/ICL.0b013e3182a2f8fa.
Mao Y, Protzman NM, John N, Kuehn A, Long D, Sivalenka R, et al. An in vitro comparison of human corneal epithelial cell activity and inflammatory response on differently designed ocular amniotic membranes and a clinical case study. J Biomed Mater Res Part B Appl Biomater. 2023;111(3):684–700. https://doi.org/10.1002/jbm.b.35186.
Paul D. Luong, Edward S. Bennett, Stephanie L. Woo, Raymond J. Brill. The role of amniotic membrane transplantation. A look at the clinical efficacy of using AMs for ocular surface disorders and their utility in primary eye care. https://www.clspectrum.com/issues/2016/march-2016/the-role-of-amniotic-membrane-transplantation (2016).
Xanthopoulou PT, Elanwar M, Alzyadi M, Lavaris A, Kopsachilis N, Elanwar MFM, et al. Α novel sutureless pterygium excision surgery using human-derived dehydrated amniotic membrane. Cureus. 2022;14(4). https://doi.org/10.7759/cureus.23839.
Malhotra C, Jain AK. Human amniotic membrane transplantation: different modalities of its use in ophthalmology. World J Transplant. 2014;4(2):111. https://doi.org/10.5500/wjt.v4.i2.111.
Polisetti N, Schmid A, Schlötzer-Schrehardt U, Maier P, Lang SJ, Steinberg T, et al. A decellularized human corneal scaffold for anterior corneal surface reconstruction. Sci Rep. 2021;11(1):1–15. https://doi.org/10.1038/s41598-021-82678-3.
Fernández-Pérez J, Ahearne M. Decellularization and recellularization of cornea: progress towards a donor alternative. Methods. 2020;171:86–96. https://doi.org/10.1016/j.ymeth.2019.05.009.
Isidan A, Liu S, Li P, Lashmet M, Smith LJ, Hara H, et al. Decellularization methods for developing porcine corneal xenografts and future perspectives. Xenotransplantation. 2019;26(6):e12564. https://doi.org/10.1111/xen.12564.
Shafiq MA, Gemeinhart RA, Yue BY, Djalilian AR. Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma. Tissue Eng Part C Methods. 2012;18(5):340–8. https://doi.org/10.1089/ten.tec.2011.0072.
Wilson SL, Sidney LE, Dunphy SE, Dua HS, Hopkinson A. Corneal decellularization: a method of recycling unsuitable donor tissue for clinical translation? Curr Eye Res. 2016;41(6):769–82. https://doi.org/10.3109/02713683.2015.1062114.
Huh M-I, Lee K-P, Kim J, Yi S, Park B-U, Kim HK. Generation of femtosecond laser-cut decellularized corneal lenticule using hypotonic trypsin-EDTA solution for corneal tissue engineering. J Ophthalmol. 2018;2018. https://doi.org/10.1155/2018/2590536
Yam GH-F, Yusoff NZBM, Goh T-W, Setiawan M, Lee X-W, Liu Y-C, et al. Decellularization of human stromal refractive lenticules for corneal tissue engineering. Sci Rep. 2016;6(1):1–11. https://doi.org/10.1038/srep26339.
Mertsch S, Hasenzahl M, Reichl S, Geerling G, Schrader S. Decellularized human corneal stromal cell sheet as a novel matrix for ocular surface reconstruction. Tissue Eng Regen Med. 2020;14(9):1318–32. https://doi.org/10.1002/term.3103.
Chou P-R, Lin Y-N, Wu S-H, Lin S-D, Srinivasan P, Hsieh D-J, et al. Supercritical carbon dioxide-decellularized porcine acellular dermal matrix combined with autologous adipose-derived stem cells: its role in accelerated diabetic wound healing. Int J Medical Sci. 2020;17(3):354–67. https://doi.org/10.7150/ijms.41155.
Huang YH, Tseng FW, Chang WH, Peng IC, Hsieh DJ, Wu SW, et al. Preparation of acellular scaffold for corneal tissue engineering by supercritical carbon dioxide extraction technology. Acta Biomater. 2017;58:238–43. https://doi.org/10.1016/j.actbio.2017.05.060.
Pan Q, Jampel H, Ramulu P, Schwartz G, Cotter F, Cute D, et al. Clinical outcomes of gamma-irradiated sterile cornea in aqueous drainage device surgery: a multicenter retrospective study. Eye. 2017;31(3):430–6. https://doi.org/10.1038/eye.2016.230.
Ahearne M, Fernández-Pérez J, Masterton S, Madden PW, Bhattacharjee P. Designing scaffolds for corneal regeneration. Adv Funct Mater. 2020;30(44):1908996. https://doi.org/10.1002/adfm.201908996.
Fagerholm P, Lagali NS, Merrett K, Jackson WB, Munger R, Liu Y, et al. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med. 2010;2(46):46ra61. https://doi.org/10.1126/scitranslmed.3001022.
Fagerholm P, Lagali NS, Ong JA, Merrett K, Jackson WB, Polarek JW, et al. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials. 2014;35(8):2420–7. https://doi.org/10.1016/j.biomaterials.2013.11.079.
Buznyk O, Pasyechnikova N, Islam MM, Iakymenko S, Fagerholm P, Griffith M. Bioengineered corneas grafted as alternatives to human donor corneas in three high-risk patients. Clin Transl Sci. 2015;8(5):558–62. https://doi.org/10.1111/cts.12293.
Ong JA, Auvinet E, Forget KJ, Lagali N, Fagerholm P, Griffith M, et al. 3D corneal shape after implantation of a biosynthetic corneal stromal substitute. Invest Ophthalmol Vis Sci. 2016;57(6):2355–65. https://doi.org/10.1167/iovs.15-18271.
Bachmann B, Matthaei M, Enders P, Altay L, Schuitmaker J, Stijnen T, et al. First-in-human clinical trial of a fish scale based biomaterial for the emergency management of corneal perforations. Invest Ophthalmol Vis Sci. 2022;63(7):4340–A0277.
Schuitmaker H. The fish scale based Biocornea: an off-the shelf device for managing acute corneal perforations. Acta Ophthalmol. 2019:97. https://doi.org/10.1111/j.1755-3768.2019.8028.
Radhakrishnan O, Pahuja K, Patel K, Chandna S. OLOGEN® implant in the management of glaucoma in an unusual case of Axenfeld–Rieger Syndrome. Oman J Ophthalmol. 2014;7(2):90. https://doi.org/10.4103/0974-620X.137170.
Yuan F, Li L, Chen X, Yan X, Wang L. Biodegradable 3D-Porous Collagen Matrix (Ologen) Compared with mitomycin C for treatment of primary open-angle glaucoma: results at 5 years. J Ophthalmol. 2015;2015:637537. https://doi.org/10.1155/2015/637537.
He M, Wang W, Zhang X, Huang W. Ologen implant versus mitomycin C for trabeculectomy: a systematic review and meta-analysis. PLoS One. 2014;9(1):e85782. https://doi.org/10.1371/journal.pone.0085782.
Cho C-H, Lee S-B. Biodegradable collagen matrix (Ologen™) implant and conjunctival autograft for scleral necrosis after pterygium excision: two case reports. BMC Ophthalmol. 2015;15(1):1–6. https://doi.org/10.1186/s12886-015-0130-z.
Tanito M, Okada A, Mori Y, Sano I, Ikeda Y, Fujihara E. Subconjunctival implantation of Ologen collagen matrix to treat ocular hypotony after filtration glaucoma surgery. Eye. 2017;31(10):1475–9. https://doi.org/10.1038/eye.2017.98.
El-Massry A, Ibrahim O, Abdalla M, Osman I, Mahmoud S. Safety and indicative effectiveness of porcine corneal lenticular implants in patients with advanced keratoconus and post LASIK ectasia: a retrospective clinical study. Clin Ophthalmol. 2021;15:3165. https://doi.org/10.2147/OPTH.S325666.
Velarde F, Castañeda V, Morales E, Ortega M, Ocaña E, Álvarez-Barreto J, et al. Use of human umbilical cord and its byproducts in tissue regeneration. Front Bioeng Biotechnol. 2020;8:117. https://doi.org/10.3389/fbioe.2020.00117.
Sheha H, Tello C, Al-Aswad LA, Sayed MS, Lee RK. Outcomes of the shunt tube exposure prevention study: a randomized clinical trial. Ophthalmol Glaucoma. 2019;2(6):392–401. https://doi.org/10.1016/j.ogla.2019.08.003.
Bunin LS. Reconstruction with umbilical amnion following ocular evisceration: a case study. Am J Ophthalmol. 2022;26:101462. https://doi.org/10.1016/j.ajoc.2022.101462.
Cheng AM, Chua L, Casas V, Tseng SC. Morselized amniotic membrane tissue for refractory corneal epithelial defects in cicatricial ocular surface diseases. Transl Vis. Sci Technol. 2016;5(3):9. https://doi.org/10.1167/tvst.5.3.9.
Fu-Shin XY, Yin J, Xu K, Huang J. Growth factors and corneal epithelial wound healing. Brain Res Bull. 2010;81(2-3):229–35. https://doi.org/10.1016/j.brainresbull.2009.08.024.
Azari AA, Rapuano CJ. Autologous serum eye drops for the treatment of ocular surface disease. Eye & Contact Lens. 2015;41(3) https://doi.org/10.1016/j.ophtha.2019.08.018.
Chiang C, Chen W, Lin J, Tsai Y. Allogeneic serum eye drops for the treatment of persistent corneal epithelial defect. Eye. 2009;23(2):290–3. https://doi.org/10.1038/sj.eye.6703079.
Samarkanova D, Cox S, Hernandez D, Rodriguez L, Pérez ML, Madrigal A, et al. Cord blood and amniotic membrane extract eye drop preparations display immune-suppressive and regenerative properties. Sci Rep. 2021;11(1):1–12. https://doi.org/10.1038/s41598-021-93150-7.
Giannaccare G, Carnevali A, Senni C, Logozzo L, Scorcia V. Umbilical cord blood and serum for the treatment of ocular diseases: a comprehensive review. Ophthalmol Ther. 2020;9(2):235–48. https://doi.org/10.1007/s40123-020-00239-9.
Hassan A, Balal S, Cook E, Dehbi HM, Pardhan S, Bourne R, et al. Finger-prick autologous blood (FAB) eye drops for dry eye disease: single masked multi-centre randomised controlled trial. Clin Ophthalmol. 2022;16:3973–9. https://doi.org/10.2147/opth.S384586.
Harritshøj LH, Nielsen C, Ullum H, Hansen MB, Julian HO. Ready-made allogeneic ABO-specific serum eye drops: production from regular male blood donors, clinical routine, safety and efficacy. Acta Ophthalmol. 2014;92(8):783–6. https://doi.org/10.1111/aos.12386.
Semeraro F, Forbice E, Braga O, Bova A, Di Salvatore A, Azzolini C. Evaluation of the efficacy of 50% autologous serum eye drops in different ocular surface pathologies. BioMed Res Int. 2014;2014. https://doi.org/10.1155/2014/826970
Jeng BH, Dupps WJ Jr. Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea. 2009;28(10):1104–8. https://doi.org/10.1097/ICO.0b013e3181a2a7f6.
Lekhanont K, Jongkhajornpong P, Choubtum L, Chuckpaiwong V. Topical 100% serum eye drops for treating corneal epithelial defect after ocular surgery. BioMed Res Int. 2013;2013 https://doi.org/10.1155/2013/521315.
Kirgiz A, Akdemir MO, Yilmaz A, Kaldirim H, Atalay K, Nacaroglu SA. The use of autologous serum eye drops after epithelium-off corneal collagen crosslinking. Optom Vis Sci. 2020;97(4):300–4. https://doi.org/10.1097/OPX.0000000000001500.
You J, Hodge C, Hoque M, Petsoglou C, Sutton G. Human platelets and derived products in treating ocular surface diseases–a systematic review. Clin Ophthalmol. 2020;14:3195. https://doi.org/10.2147/OPTH.S265701.
Yoon KC. Use of umbilical cord serum in ophthalmology. Chonnam Med J. 2014;50(3):82–5. https://doi.org/10.4068/cmj.2014.50.3.82.
Gomes J, Amankwah R, Powell-Richards A, Dua H. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br J Ophthalmol. 2004;88(6):821–5. https://doi.org/10.1136/bjo.2003.027573.
Ling K, Bastion M-LC. Use of commercially available sodium hyaluronate 0.18% eye drops for corneal epithelial healing in diabetic patients. Int Ophthalmol. 2019;39(10):2195–203. https://doi.org/10.1007/s10792-018-1057-1.
Kim MS, Kim JH. Effects of tissue adhesive (Tisseel) on corneal wound healing in lamellar keratoplasty in rabbits. Korean J Ophthalmol. 1989;3(1):14–21. https://doi.org/10.3341/kjo.1989.3.1.14.
Wang Y, Xu L, Zhao J, Liang J, Zhang Z, Li Q, et al. Reconstructing auto tissue engineering lamellar cornea with aspartic acid modified acellular porcine corneal stroma and preconditioned limbal stem cell for corneal regeneration. Biomaterials. 2022;289:121745. https://doi.org/10.1016/j.biomaterials.2022.121745.
Wilson A, Jones J, Marshall J. Biomechanical evaluation of decellularized and crosslinked corneal implants manufactured from porcine corneas as a treatment option for advanced keratoconus. Front Bioeng. Biotechnol. 2022:491. https://doi.org/10.3389/fbioe.2022.862969.
Cooke M, Tan E, Mandrycky C, He H, O’Connell J, Tseng S. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J Wound Care. 2014;23(10):465–76. https://doi.org/10.12968/jowc.2014.23.10.465.
Van Essen T, Van Zijl L, Possemiers T, Mulder A, Zwart S, Chou C-H, et al. Biocompatibility of a fish scale-derived artificial cornea: cytotoxicity, cellular adhesion and phenotype, and in vivo immunogenicity. Biomaterials. 2016;81:36–45. https://doi.org/10.1016/j.biomaterials.2015.11.015.
Ahearne M, Fernández-Pérez J. Fabrication of corneal extracellular matrix-derived hydrogels. Methods Mol Biol. 2020;2145:159–68. https://doi.org/10.1007/978-1-0716-0599-8_11.
Zhou Q, Guaiquil VH, Wong M, Escobar A, Ivakhnitskaia E, Yazdanpanah G, et al. Hydrogels derived from acellular porcine corneal stroma enhance corneal wound healing. Acta Biomater. 2021;134:177–89. https://doi.org/10.1016/j.actbio.2021.08.011.
Shirzaei Sani E, Kheirkhah A, Rana D, Sun Z, Foulsham W, Sheikhi A, et al. Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Sci Adv. 2019;5(3):eaav1281. https://doi.org/10.1126/sciadv.aav1281.
Kong B, Chen Y, Liu R, Liu X, Liu C, Shao Z, et al. Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat Commun. 2020;11(1):1–12. https://doi.org/10.1038/s41467-020-14887-9.
Erikitola O-o, Williams O, Fern A, Lyall D. Fingerprick autologous blood in the treatment of severe dry eyes and ocular surface disease. Cornea. 2021;40(9):1104–9. https://doi.org/10.1097/ICO.0000000000002624.
Balal S, Nitiahpapand R, Hassan A, Than J, Patel A, Kumar B, et al. Finger-prick autologous blood in the treatment of persistent corneal epithelial defects. Cornea. 2020;39(5):594–7. https://doi.org/10.1097/ICO.0000000000002230.
Pujari R, Deshmukh R, Sheth C, Rajan MS. Maternal finger-prick allogenic blood for persistent corneal epithelial defects. BMJ-BRIT Med J. 2021;14(4):e241138. https://doi.org/10.1136/bcr-2020-241138.
Zhou TE, Robert M-C. Comparing ProKera with amniotic membrane transplantation: indications, outcomes, and costs. Cornea. 2022;41(7):840–4. https://doi.org/10.1097/ICO.0000000000002852.
Choi CM, Jeon HS. Clinical outcomes of in-office sutureless amniotic membrane transplantation in persistent epithelial defect. Korean J Ophthalmol. 2022;36(2):87–96. https://doi.org/10.3341/kjo.2021.0095.
Supokawate J. Comparison of cryopreserved amniotic membrane grafts versus dry amniotic membrane grafts combined with intraoperative mitomycin C for primary pterygium excision using fibrin glue technique. Eye South East Asia. 2018;13(2):14–9.
Mimouni M, Trinh T, Sorkin N, Cohen E, Santaella G, Rootman DS, et al. Sutureless dehydrated amniotic membrane for persistent epithelial defects. Eur J Ophthalmol. 2022;32(2):875–9. https://doi.org/10.1177/11206721211011354.
Hofmann N, Salz A-K, Kleinhoff K, Möhle N, Börgel M, Diedenhofen N, et al. AmnioClip-Plus as sutureless alternative to amniotic membrane transplantation to improve healing of ocular surface disorders. Transplantology. 2021;2(4):425–32. https://doi.org/10.3390/transplantology2040040.
Kotomin I, Valtink M, Hofmann K, Frenzel A, Morawietz H, Werner C, et al. Sutureless fixation of amniotic membrane for therapy of ocular surface disorders. PLoS One. 2015;10(5):e0125035. https://doi.org/10.1371/journal.pone.0125035.
Dua HS, Ting DSJ, AlSaadi A, Said DG. Management of limbal stem cell deficiency by amnion-assisted conjunctival epithelial redirection using vacuum-dried amniotic membrane and fibrin glue. Br J Ophthalmol. 2021; https://doi.org/10.1136/bjophthalmol-2020-318496.
Garcin T, Gain P, Thuret G. Treatment of complex macular holes using a large disc of trypan blue stained-lyophilized amniotic membrane: a 10 cases series with 1-year follow-up. Acta Ophthalmol. 2021:99. https://doi.org/10.1111/j.1755-3768.20200031.
Delbarre M, Boucenna W, Froussart-Maille F. Sutureless lyophilized amniotic membrane grafting for corneal epithelial defects. Eye & Contact Lens. 2022;48(10):430–2. https://doi.org/10.1097/ICL.0000000000000913.
Al-Swailem SA, AlHilali S, Maktabi AM. Acute sterile keratolysis after deep anterior lamellar keratoplasty in a patient with keratoconus: a case report. Cornea. 2022;10:1097. https://doi.org/10.1097/ICO.0000000000003271.
Khodaparast M, Shahraki K, Jabbarvand M, Shahraki K, Rafat M, Moravvej Z. Sutureless femtosecond laser-assisted anterior lamellar keratoplasty using a bioengineered cornea as a viable alternative to human donor transplantation for superficial corneal opacities. Cornea. 2020;39(9):1184–9. https://doi.org/10.1097/ICO.0000000000002394.
Rafat M, Jabbarvand M, Sharma N, Xeroudaki M, Tabe S, Omrani R, et al. Bioengineered corneal tissue for minimally invasive vision restoration in advanced keratoconus in two clinical cohorts. Nat Biotechnol. 2022; https://doi.org/10.1038/s41587-022-01408-w.
Jankov MR, Elmassry A. Lenticular intrastromal keratoplasty for keratoconus. Keratoconus: Springer; 2022. p. 989–98.
Fea AM, Aragno V, Testa V, Machetta F, Parisi S, D’Antico S, et al. The effect of autologous platelet lysate eye drops: an in vivo confocal microscopy study. Biomed Res Int. 2016;2016:8406832. https://doi.org/10.1155/2016/8406832.
López-García JS, García-Lozano I, Rivas L, Martínez-Garchitorena J. Use of autologous serum in ophthalmic practice. Arch Soc Esp Oftalmol. 2007;82(1):9–20. https://doi.org/10.4321/s0365-66912007000100004.
Meni-Babakidi N, Viramontes-Gamboa G, Ibarra-Bracamontes LA, Luna-Reyes I, editors. Evaluation of a microinjection system for severe dry eye treatment. J Phys Conf Ser. IOP Publishing; 2021. https://doi.org/10.1088/1742-6596/1723/1/012016.
Alzamil H, Pearce EI. Effectiveness of 3% trehalose and 0.15% sodium hyaluronate eye drop in an adverse dry environment. J Invest Dermatol. 2019;60(9):304.
Scalcione C, Ortiz-Vaquerizas D, Said D, Dua H. Fibrin glue as agent for sealing corneal and conjunctival wound leaks. Eye. 2018;32(2):463–6. https://doi.org/10.1038/eye.2017.227.
Funding
This study was supported by the Shahid Beheshti University of Medical Sciences (Grant No. 28988).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boroumand, S., Hamedi, E., Sigaroodi, F. et al. Biological Materials Introduced to the Market for Blurred Cornea Regeneration. Regen. Eng. Transl. Med. 10, 172–188 (2024). https://doi.org/10.1007/s40883-023-00319-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-023-00319-9