Abstract
The aim of this paper is to study the demographic impact of malaria on the host population and investigate the possibility to eliminate it with a strategy based on prevention (e.g., the use of bed nets). In this paper, a deterministic model for the transmission of malaria (with variable human and mosquito population sizes) has been studied. The model equilibria have been found. Their local and global stability have been analyzed. An exact formula for the critical case fatality required to stop the growth of the host has been found. The conditions to eliminate malaria with a strategy based on the use of bed nets have been found.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Malaria is a life-threatening vector-borne disease caused by parasites which are transmitted to people through the bites of infected mosquitoes. Recently, it has been estimated that there were about 219 million cases of malaria in 2010 (with an uncertainty range of 154–289 million) and an estimated 660,000 deaths (with an uncertainty range of 490,000–836,000) [31].
The mathematical modeling of malaria has a long history since the discovery of malaria mode of transmission. This history goes back to the work of the pioneers (Ross [26] and Macdonald [17]) in this field. Ross [26] set the first model that describes the transmission dynamics of malaria. Then, Macdonald [17] considered major extensions for Ross’s model, where he improved the model to a two-dimensional model with one variable representing humans and one variable representing mosquitoes. An important extension of the model was proposed by Dietz et al. [12] who added the inclusion of immunity. Thereafter, other extensive studies for malaria models have been carried out (see [3, 19, 21, 22, 29] and the references therein). Other studies concerning the effect of climatic changes and weather on malaria dynamics are shown in [13, 23, 24]. Some good reviews on the mathematical modeling and analysis of malaria are in [2, 4, 10, 15, 18, 20]. However, almost all these studies concentrate on the epidemiology of malaria infection, while this study gathers the epidemiology and demography of malaria infection on the host population.
We consider an SIS model for malaria, where the human as well as the mosquito populations are assumed to have variable (in time) size. The human population is assumed to be exponentially growing with a growth rate \(\rho \). Since the infectious period of malaria is relatively short (in days), we assumed that individuals dying as a result of malaria infection die after passing the infectious period, an assumption that we believe to be more realistic than assuming differential mortality approach as considered in the literature ( see for example [8, 22, 32]). Therefore, we consider the proportional fatality approach [28]. We further investigate the impact of malaria on the demography of the host.
The paper is organized as follows. In Sect. 2, the model is formulated and the corresponding model in sub-population proportions is introduced. In Sect. 3, the reduced model is considered and thoroughly analyzed, where the endemic equilibria have been found. Both the local and global stability of equilibria are evaluated in Sect. 4. The effect of case fatality on the demography of the host is shown in Sect. 5. In Sect. 6, conditions to eliminate malaria with a strategy based on prevention (e.g., the use of bed nets) are found. Finally, summary and conclusion on the results are given in Sect. 7.
2 Formulation of the Model
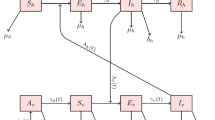
The model supposes a homogeneous mixing of the human and vector (mosquito) populations so that each mosquito bite has equal chance of transmitting the virus to susceptible humans in the population. The total host (human) population at time t, whose size is denoted by \(N_h(t)\), is subdivided into two sub-populations: susceptible humans \(S_h(t)\) and infectious humans \(I_h(t)\), so that \(N_h(t)=S_h(t)+I_h(t)\). Similarly, the total vector (mosquitoes) population at time t, whose size is denoted by \(N_v(t)\), is split into two sub-populations: susceptible mosquitoes \(S_v(t)\) and infectious mosquitoes \(I_v(t)\), so that \(N_v(t)=S_v(t)+I_v(t)\).
The susceptible human population is generated via recruitment of humans by birth at rate \(\beta _h\). This population is decreased due to natural deaths at rate \(\mu _h\) or due to an infection by effective contacts with infectious vectors at rate \(\lambda _h(t)\) (the force of infection), given by \(\lambda _h=C_{vh}\frac{I_v}{N_v}\). Thus, the rate of change in the susceptible human population is given by,
Infected humans either die with natural death rate \(\mu _h\), die due to the infection with rate \(c\gamma _h\), or recover with temporal immunity to be susceptible again with rate \((1-c)\gamma _h\). Thus,
Physically, the parameter c represents the proportion of removed individuals (from the \(I_h\) state) who die as a result of malaria in the human population.
The susceptible vector population is generated by births at rate \(\beta _v\). This susceptible population is reduced by infection following effective contact rate \(\lambda _v(t)=C_{hv}\frac{I_h}{N_h}\) and natural deaths at rate \(\mu _v\). Infected vectors die with rate \(\mu _v\). Thus,
Since mosquitoes bite both susceptible and infected humans, it is assumed that the average number of mosquito bites received by humans depends on the total sizes of the population of mosquitoes and humans in the community. It is assumed further that each susceptible mosquito bites an infected human at an average biting rate \(b_s\), and the human hosts are always sufficient in abundance so that it is reasonable to assume that the biting rate \(b_s\) is constant. Let \(C_{hv}=P_{hv}b_s\), be the rate at which mosquitoes acquire infection from infected humans, where \(P_{hv}\) is the transmission probability from an infected human to a susceptible mosquito and \(b_s\) is the number of human bites one mosquito has per unit time [8].
Similarly, let \(C_{vh}=P_{vh}b_i\), be the rate at which humans acquire infection from infected mosquitoes, where \(P_{vh}\) is the transmission probability from an infected mosquito to a susceptible human and \(b_i\) is the number of mosquito bites one human receives per unit time [8]. A full description for the model variable states and parameters is given in Tables 1 and 2, respectively.
The mathematical representation of the model is
All parameters are strictly positive with the exception of the parameter c, representing the proportional fatality, which is assumed to be non-negative. The mosquito birth rate \(\beta _v\) is assumed to be bigger than the mosquito death rate \(\mu _v\) to ensure that there is a stable positive mosquito population.
2.1 Reformulation of the Model
Let us now reformulate model (1) to express it in terms of proportions rather than numbers. Assume that \(s_h=\frac{S_h}{N_h}\) , \(i_h=\frac{I_h}{N_h}\) , \(s_v=\frac{I_v}{N_v}\) , \(i_v=\frac{I_v}{N_v}\) where \(s_h+i_h=1\) and \(s_v+i_v=1\). Thus, the model (1) reads, see Appendix 1:
For this system the closed set \(\mathcal {D} = \{(s_h, i_h, s_v, i_v)\in \mathbb {R}^4: 0\le s_h, i_h, s_v, i_v\le 1, s_h+i_h = 1, s_v+i_v = 1\}\) is positively invariant, and the model (2) is mathematically and epidemiologically well posed, in the sense that solutions starting in \(\mathcal {D}\) remain in it all the time.
3 Reduced Model
Since \(s_h+i_h = 1\) and \(s_v+i_v = 1\), then model (2) can be reduced to the following model with two equations
where \(\lambda _h = C_{vh} i_v\) and \(\lambda _v = C_{hv} i_h\).
3.1 Existence and Stability of the Infection-Free Equilibrium and the Basic Reproduction number
To find the equilibria we put the derivatives in the left-hand side in model (3) equal zero. The infection-free equilibrium (IFE) is obtained by putting \(i_h = i_v = 0\) and solving the resulting system with respect to \(i_h\) and \(i_v\). Therefore, the IFE is \(E_{0}=(i_h^0, i_v^0)^t=(0, 0)^t\), where t denotes vector transpose.
To find the basic reproduction number we use the next generation operator method [30] to find the matrices F (for the new infection terms) and V (for the transition terms) as, see Appendix 2
It follows then that the basic reproduction number is given by
where \(\rho \) is the spectral radius (dominant eigenvalue in magnitude) of the matrix \(\mathbf {F}\mathbf {V}^{-1}\).
To establish the local stability of the infection-free equilibrium we consider its corresponding jacobian matrix
It is clear that the trace of \(J_{E_{0}}\) is less than zero, while its determinant is positive if and only if the basic reproduction number \(R_0 < 1\). Therefore, we show the following proposition:
Proposition 1
The infection-free equilibrium \(E_0\) is locally asymptotically stable (LAS) if and only if \(R_0 < 1\).
The proof of this proposition is done using the next generation matrix method [30] and is deferred to Appendix 3.
3.2 Endemic Equilibria
3.2.1 The Characteristic Epidemiologic Equation
The endemic equilibria are obtained by solving the following non-linear algebraic system of two equations in the variables \(i_v\) and \(i_h\)
We further assume that \(i_h \ne i_v \ne 0\). Therefore, we get
On reducing \(i_h\) between the last two equations, we get an equation in the proportion of endemic infected vector \( i_v\) as
where
Equation (9) is the characteristic epidemiologic equation whose solutions have one-to-one correspondence with the endemic equilibria of the system (3). A feasible solution of this equation satisfies the inequality \(0< i_v < 1\). Moreover, the left-hand side of (9) is a polynomial of the second degree and henceforth it can have up to two feasible (non-negative) roots, depending on the values of the model parameters. However, to study the possibility for the existence of positive equilibria, we analyze the bifurcation near the infection-free equilibrium.
3.2.2 Bifurcation Points
The scalar equation (9) could be seen as a bifurcation equation. We keep the parameters \(C_{vh}, \beta _v, \beta _h, \gamma _h\), and c fixed and express the equation in terms of \(C_{hv}\) and \(i_v\). Since the infection-free equilibrium exists for all parameter values, we put \(i_v = 0\) in Eq. (9) to get \(C_{hv} = 0\) or \(C_{hv} = C_{hv}^0\) where
Hence, there are two bifurcation points, (0, 0) and \((C_{hv}^0, 0)\). We shall compute the direction of bifurcation at each of them. It is shown in Appendix 4 that the bifurcation is forward at the bifurcation point (0, 0).
3.2.3 Center Manifold Analysis Near the Infection-Free Equilibrium
Since the infection-free equilibrium exists for all parameter values, we put \(i_v = 0\) in Eq. (9) to get \(C_{hv} = 0\) or \(C_{hv} = C_{hv}^0\) where,
Hence, \((C_{hv}^0, 0)\) is a bifurcation point at which we compute the direction of bifurcation. We use the center manifold theory [6, 7, 30] to analyze the model near the infection-free equilibrium. To this end, we consider the jacobian matrix \(J_{E_{0}}\) evaluated at the infection-free equilibrium with \(C_{hv} = C_{hv}^0\) (denoted by \(J^*\)) which becomes
It is easy to check that the matrix \(J^*\) has a simple zero eigenvalue with another one having negative real part. Therefore, the center manifold theory [6, 7, 30] can be used to analyze the model. The method is based on evaluating a left eigenvector of \(J^*\) (corresponding to the zero eigenvalue), given by \(V=(\beta _v,C_{vh})\) and a right eigenvector of it which is given by \(W=(C_{vh},(\beta _h+\gamma _h))^T\). Then we compute the two quantities a and b which are given by
Therefore, according to theorem 4.1 of [7], the direction of bifurcation at the bifurcation point \((C_{hv}^0, 0)\) is forward and we show the following proposition.
Proposition 2
The model we consider has two bifurcation points (0, 0) and \((C_{hv}^0,0)\) at which the bifurcation direction is forward (i.e., at these points the bifurcation is supercritical).
3.2.4 Existence of Endemic Equilibria
It can be checked that
and
Hence, there are two cases:
-
Case 1 \(R_0 < 1\). In this case \(G(0) < 0\) which implies that there is a unique positive solution for (9). This solution is given by
$$\begin{aligned} i_v^{+} = \frac{-B + \sqrt{D}}{2A}, \end{aligned}$$(16)where
$$\begin{aligned} D= & {} B^2 - 4AC\nonumber \\= & {} \beta _v^2 \left\{ [(\beta _h+\gamma _h-C_{vh}) + c \gamma _h\beta _v]^2 + 4 C_{vh}(\beta _h + (1-c)\gamma _h)C_{hv}^2 \right\} >0.\qquad \quad \end{aligned}$$(17)Thus, for model (3), there is a unique endemic equilibrium \(E^{+} =(i_h^{+}, i_v^{+})^{t}\) where,
$$\begin{aligned} i_h ^{+}= \frac{\beta _v i_v^{+}}{C_{hv}(1-i_v^{+})}. \end{aligned}$$(18) -
Case 2 \(R_0 > 1\). In this case, \(G(0) >0, G(1) >0\), and \(D>0\). Also, it is easy to check that \(G^{\prime }(0) < 0\). Therefore, equation (9) has two positive solutions \(i_v^{+}\) (given by (16)) and \(i_v^{-}\), given by
$$\begin{aligned} i_v^{-} = \frac{-B - \sqrt{D}}{2A}. \end{aligned}$$(19)Hence, model (3) has two equilibria \(E^{+} =(i_h^{+}, i_v^{+})^{t}\) and \(E^{-} =(i_h^{-}, i_v^{-})^{t}\), where \(i_h^{-}\) has a similar form like (18).
We summarize our result in the following proposition.
Proposition 3
In addition to the infection-free equilibrium \(E_0\), the reduced model (3) has an endemic equilibrium if \(R_0 < 1\) and two endemic equilibria if \(R_0 > 1\).
4 Stability Analysis
4.1 Local Stability Analysis of the Endemic Equilibria
To study the stability of the endemic equilibria of model (3) we make a small perturbation \((x(t), y(t))^{t}\) around a general equilibrium \((i_h, i_v)^{t}\) of that model. It is easy to get the linearized system of model (3) in the matrix form as
We denote the coefficient matrix by J. Hence the characteristic equation of J is
where, using (6)
and
It is shown in Appendix 5 that \(tr(J) <0\). Therefore, we get the following proposition.
Proposition 4
The endemic equilibrium \({E^{+}} = (i_h^{+}, i_v^{+})^{t}\) is unstable whenever it exists, while the other one \(E^{-} =(i_h^{-}, i_v^{-})^{t}\) is locally asymptotically stable whenever it exists (i.e., for \(R_0 > 1\)).
4.2 Global Stability
To study the global stability, we consider the reduced model (3) and define the set \(\Omega := \{(i_h,i_v): 0 < i_h, i_v < 1 \}\). On putting \(i_h = 0\) in (3.1) we get \(di_h/dt = C_{vh} i_v > 0\) for all \(i_v > 0\) and putting \(i_v = 0\) in (3.2) we get \(di_v/dt = C_{hv} i_h > 0\) for all \(i_h > 0\). Therefore, the first quadrant and consequently the set \(\Omega \) are positively invariant regions for the model, for any solution of (3) starting in the interior of \(\Omega \). Hence the \(\omega -limit\) set of trajectory must be contained in \(\Omega \). Now, assume that
and consider the Dulac’s function
where both \(i_h\) and \(i_v\) are positive. Hence
Therefore, using the Dulac’s criterion [25], the reduced model (3) has no limit cycle in the first quadrant. We show the following proposition.
Proposition 5
The reduced model (3) has no periodic solutions.
Hence, based on the Poincare–Bendixon theorem and propositions 4 and 5, the local stability of the equilibria \(E_0\) and \(E^{-}\) implies their global stability. Thus, we show the following proposition.
Proposition 6
For model (3), the following hold:
-
1.
If \(R_0 < 1\), then an unstable endemic equilibrium \(E^{+}\) exists in addition to the infection-free equilibrium which is globally stable, see Fig. 2.
-
2.
If \(R_0>1\), then two endemic equilibria (\(E^{-}\) and \(E^{+}\)) exist in addition to the infection-free equilibrium. Both the infection-free equilibrium \(E_0\) and the endemic equilibrium with higher infection level \(E^{+}\) are unstable, while the endemic equilibrium with lower infection level \(E^{-}\) is globally asymptotically stable, see Fig. 3.
Bifurcation diagram showing the endemic equilibrium values for the proportion of infectious mosquito population for the parameters in Table 3. The figure shows that there are two bifurcation points, at \(R_0 = 0\) and at \(R_0=1\), at which the bifurcation is supercritical. In addition to the infection-free equilibrium \(E_0\), an unstable endemic equilibrium \(E^{+}\) (represented by the dashed curve, \(i_v^{+}\)) exists for all values of \(R_0\). For \(R_0 < 1\), \(E_0\) is globally stable, while for \(R_0 > 1\), \(E_0\) looses its stability and another endemic equilibrium \(E^{-}\) (represented by the solid curve, \(i_v^{-}\)) exists and is globally stable
A bifurcation diagram, with parameter values in Table 3, has been created, Fig. 1. The figure shows that there are two bifurcation points; one at \(R_0 = 0\) and the other at \(R_0 = 1\). It further shows that at both points, the bifurcation is supercritical. The equilibrium solution represented by the curve bifurcating at \(R_0 = 0\) is always unstable while that represented by the curve bifurcating at \(R_0 = 1\) is stable.
Figure 2 shows the proportion of the infectious mosquito population for many randomly generated initial conditions, for parameter values in Table 3 and with \(C_{hv} = 0.06\) per day which corresponds to a \(R_0 = 0.25 < 1\). It shows that an unstable endemic equilibrium (\(i_v = i_v^+ = 0.9752\)) co-exists with the infection-free equilibrium (\(i_v = 0\)) that attracts all solutions (i.e., it is globally stable).
Time-dependent solutions in the region \(R_0 <1\). An unstable endemic equilibrium, \(E^{+}\), co-exists with the infection-free equilibrium which is the global attractor. Simulations are made with parameter values in Table 3 and for \(C_{hv} = 0.06\) per day which means that \(R_0 = 0.25\)
Time-dependent solutions in the region \(R_0 > 1\). Two positive equilibria exist. The infection-free equilibrium looses its stability, while the equilibrium infection level corresponding to the equilibrium solution \(E^{-}\) is the global attractor. The other equilibrium solution \(E^{+}\) is unstable. Simulations are made with parameter values in Table 3 and for \(C_{hv} = 0.48\) per day which means that \(R_0 = 2\)
Simulations for the same parameter values except for \(C_{hv}\) which has been increased to \(C_{hv} = 0.48\) per day (i.e., \(R_0 = 2\)) are shown in Fig. 3. The figure shows that solutions are attracted by an endemic positive equilibrium (\(i_v = i_v^- = 0.6604\)) that is globally stable and lie in between an unstable endemic equilibrium (\(i_v = i_v^+ = 0.9996\)) and the infection-free equilibrium (\(i_v = i_v^0 = 0\)) which looses its stability at \(R_0 = 1\).
5 Effects on Demography
To study the effects of malaria infection on the human population demography, we assume that \(\rho \) is the growth/decay rate of the human population. Since, system (1) is homogeneous, then the stationary solution of the human model [the first two equations in model (1)] is not a stationary (fixed) point, but an exponential “persistent” solution, i.e., a solution of the form \((S_h, I_h)e^{\rho t}\). Thus, on applying the theory of finite-dimensional homogeneous systems ([5, 14, 27]), we get
which implies
Now, we rewrite (8) to get
On omitting \(i_v\) and \(i_h\) among Eqs. (26), (27), and (28) we get an equation in the exponent of growth \(\rho \)
i.e.,
It should be noted that the relations (27) and (28) imply
or equivalently,
where \(\rho _0 = \beta _h-\mu _h\) is the malthusian growth rate (i.e., the growth rate in the absence of infection) for the human population. Thus, there is a one-to-one correspondence between solutions of (9) and those of (30). On the other hand, the case fatality ([11, 16, 28]) is
Now, we investigate whether the exponent of growth \(\rho \) can be driven to zero and what the case fatality is required to do so. To this end, we put \(\rho = 0\) and \(c=c^\star \) in (29) to get
where \(R_D = \beta _h/\mu _h\) is the demographic reproduction number for the human population. Thus, the critical case fatality \(f^\star \) [28] required to drive the human population growth rate to zero is
where
-
\(D_{Ih} = 1/(\mu _h+\gamma _h)\) is the average time spent in the \(I_h\) state for a human,
-
\(L_0 = 1/\mu _h\) is the expected time of life at birth in the absence of infection for a human,
-
\(P_{Ih} =D_{Ih}/L_0\) is the proportion of life spent in the \(I_h\) state for a human.
It is clear that the existence of the critical case fatality \(f^\star \) depends on the existence of a feasible value of the proportion c, i.e., the critical proportion \(c^\star \) must satisfy \(c^\star \in [0, 1]\). We summarize our results in the following proposition.
Proposition 7
If the model parameters satisfy that \(c^\star \in [0, 1]\), then the critical case fatality \(f^\star \) required to stop the growth of the human population is given by (35) and any slightly higher case fatality could drive the population to extinction.
6 Elimination of the Infection
Since the infection-free equilibrium is the global attractor if \(R_0 < 1\), then eliminating the infection could be implemented if \(R_0\) is reduced to slightly below one. One way to do so is to use nets as a barrier to prevent mosquitoes biting. In the infection transmission process, the bite is important when it occurs between an infected human and a susceptible mosquito or vice versa (i.e., when it occurs between an infected mosquito and a susceptible human). Now, using nets could be applied in three different scenarios: by susceptible humans only, by infected humans only, or by both (susceptible and infected humans). We shall study the three different scenarios.
-
Scenario \(\# 1\) In this case, we assume that a proportion p of susceptible human individuals would use nets to protect themselves from the biting of mosquitoes. Thus, a proportion \((1-p)\) of susceptible human individuals are subject to get bitten by infected mosquitoes. Hence, the total number of susceptible humans available for contacts with infected mosquitoes is reduced to \((1-p) S_h\). In other words, the effect of applying this strategy on model (1) appears in the term representing new infections in humans, where the term \(\lambda _h S_h\) in the first two equations of model (1) modifies to \((1-p)\lambda _h S_h = (1-p)C_{vh} i_v S_h\). The mathematical analysis of the model does not get affected, except we replace every \(C_{vh}\) with \((1-p)C_{vh}\). Thus, the basic reproduction number for the modified model is simply \(\sqrt{(1-p)}R_0\) and eliminating the infection could be achieved if a proportion p of the susceptible humans use nets such that
$$\begin{aligned} p>1-\frac{1}{{R_0}^2}. \end{aligned}$$(36) -
Scenario \(\# 2\) In this case, we assume that a proportion q of infected human individuals would use nets to prevent susceptible mosquitoes from infecting itself while taking its meals from the infected humans. This means that a proportion q of infected humans are excluded from contacts with mosquitoes and, therefore, the probability that a susceptible mosquito gets its meal from an infected human individual will be reduced from \(I_h/N_h\) to \((1-q)I_h/N_h\), which in turn means that the term \(\lambda _v S_v\) representing new infections in the vector population in model (1) is modified to \((1-q)\lambda _v S_v\). The effect of this modification can be considered if we replace every \(C_{hv}\) with \((1-q)C_{hv}\) in the analysis of the original model. This implies that the basic reproduction number for the modified model becomes \(\sqrt{(1-q)}R_0\). Hence, to eliminate the infection, a proportion q of the infected humans could use nets such that
$$\begin{aligned} q>1-\frac{1}{{R_0}^2}. \end{aligned}$$(37) -
Scenario \(\# 3\) In this case we assume that the above two scenarios are combined together, i.e., a proportion p of susceptible humans and a proportion q of infected humans would use the nets. As, in the above two scenarios we replace \(C_{vh}\) with \((1-p)C_{vh}\) and \(C_{hv}\) with \((1-q)C_{hv}\). Thus, the basic reproduction number for the new model reads \(\sqrt{(1-p)(1-q)} R_0\). Hence, eliminating the infection requires
$$\begin{aligned} \sqrt{(1-p)(1-q)}<\frac{1}{R_0}. \end{aligned}$$(38)We notice that if \(p = q :=\hat{p}\), then a proportion
$$\begin{aligned} \hat{p} > 1-\frac{1}{R_0} \end{aligned}$$(39)of the total human population is required to use nets to ensure an effective elimination of the infection. We end this section by the following proposition.
Proposition 8
Malaria infection could be eliminated from the population if
-
1.
a proportion \(p > 1 - 1/{R_0}^2\) of the susceptible human population uses nets to protect themselves from the biting of infected mosquitoes, or
-
2.
a proportion \(q > 1 - 1/{R_0}^2\) of the infected human population uses nets to prevent susceptible mosquitoes from getting infected while taking its meals from the infected humans, or
-
3.
a proportion \(\hat{p} > 1-{1}/{R_0}\) of the total human population uses nets to prevent the incidence of new infections.
7 Summary and Conclusion
As malaria is one of the most serious health problems facing the world today, several studies have been made in an attempt to understand its dynamics and predict what may happen and then suggest strategies to eliminate it [3, 8, 10, 12, 15, 21, 22].
In this paper, a deterministic model (SIS for humans and SI for mosquitoes) has been studied. Both the human and mosquito populations are of varying size. Though it’s simple, the model shows interesting dynamics. Its analysis shows that in addition to the infection-free equilibrium (denoted by \(E_0\)) two endemic equilibria exist. One of them (denoted by \(E^{+}\) and has high level of endemic infection) exists forever, while the other (denoted by \(E^{-}\) and has low level of endemic infection) exists only if \(R_0 > 1\). The analysis shows further that \(E_0\) is globally stable if \(R_0 < 1\) and is unstable if \(R_0>1\), while \(E^{+}\) is unstable but \(E^{-}\) is globally stable whenever they exist. Thus any control strategy aiming to eliminate malaria should consider the inequality \(R_0 < 1\). In other words, reducing \(R_0\) to slightly below one is not only a necessity but a sufficient condition to eliminate malaria.
Since malaria is preventable (i.e., preventing the recruitment of new infections), our model has been modified to study the possibility to eliminate malaria using nets. The analysis shows that there are three scenarios to eliminate malaria. The first is that a proportion \(p > 1- 1/{R_0}^2\) of the susceptible human population uses nets to protect themselves from the bites of infected mosquitoes. This scenario is applicable if the total number of infected humans is much bigger than that of the susceptible human population. The second is that a proportion \(q > 1- 1/{R_0}^2\) of the infected humans should use nets to prevent the transmission of infection to susceptible mosquitoes while taking its meals. This is applicable if the number of infected humans is much less than that of susceptible human population. Finally, if the proportion of infected humans is close to 50 % then a proportion \(\hat{p} > 1- 1/{R_0}\) of the total human population should use bed nets as barriers to prevent the transmission of malaria to new cases.
On the other hand, the impact of malaria fatality on the demography of the host has been investigated. It has been shown that malaria case fatality could stop the growth of the host if the model parameters are such that the critical proportional fatality \(c^\star \in [0, 1]\).
References
Abu-Raddad, L.J., Patnaik, P., Kublin, J.G.: Dual infection with HIV and Malaria fuels the spread of both diseases in sub-Saharan Africa. Science 314(5805), 1603–1606 (2006)
Anderson, R.M., May, R.M.: Infectious Diseases of Humans: Dynamics and Control. Oxford University Press, Oxford (1991)
Aron, J.L.: Mathematical modeling of immunity to malaria. Math. Biosci. 90, 385–396 (1988)
Aron, J.L., May, R.M.: The population dynamics of malaria. In: Anderson, R.M. (ed.) The Population Dynamics of Infectious Disease: Theory and Applications, pp. 139–179. Chapman and Hall, London (1982)
Busenberg, S., Hadeler, K.P.: Demography and epidemics. Math. Biosci. 101, 63–74 (1990)
Carr, J.: Applications of Center Manifold Theory. Springer, New York (1981)
Castillo-Chavez, C., Song, B.: Dynamical models of tuberculosis and their applications. Math. Biosci. Eng. 1, 361–404 (2004)
Chitnis, N., Cushing, J.M., Hyman, J.M.: Bifurcation analysis of a mathematical model for malaria transmission. SIAM J. Appl. Math. 67, 24–45 (2006)
Chitnis, N., Hyman, J.M., Cushing, J.M.: Determining important parameters in the spread of malaria through the sensitivity analysis of a mathematical model. Bull. Math. Biol. 70, 1272–1296 (2008)
Dietz, K.: Mathematical models for transmission and control of malaria. In: Wernsdorfer, W., McGregor, Y. (eds.) Principles and Practice of Malariology, pp. 1091–1133. Churchill Livingston, Edinburgh (1988)
Dietz, K., Heesterbeek, J.A.P.: Daniel Bernoulli’s epidemiological model revisited. Math. Biosci. 180, 1–21 (2002)
Dietz, K., Molineaux, L., Thomas, A.: A malaria model tested in the African savannah. Bull. World Health Organ. 50, 347–357 (1974)
Gething, P.W., Smith, D.L., Patil, A.P., Tatem, A.J., Snow, R.W., Hay, S.I.: Climate change and the global malaria recession. Nature 465, 342–346 (2010)
Hadeler, K.P.: Periodic solution of homogeneous equations. J. Differ. Equ. 95, 183–202 (1992)
Koella, J.C.: On the use of mathematical models of malaria transmission. Acta Trop. 49, 1–25 (1991)
Ma, J., van den Driessche, P.: Case fatality proportion. Bull. Math. Biol. 70, 118–133 (2008)
Macdonald, G.: The Epidemiology and Control of Malaria. Oxford University Press, London (1957)
Mandal, S., Sarkar, R.R., Sinha, S.: Mathematical models of malaria. Malar. J. 10, 202 (2011). doi:10.1186/1475-2875-10-202
Mideo, N., Day, T., Read, A.F.: Modelling malaria pathogenesis. Cell Microbiol. 10, 1947–1955 (2008)
Nedelman, J.: Introductory review: some new thoughts about some old malaria models. Bull. Math. Biosci. 73, 159–182 (1985)
Ngwa, G.A.: On the population dynamics of the malaria vector. Bull. Math. Biol. 68, 2161–2189 (2006)
Ngwa, G.A., Shu, W.S.: A mathematical model for endemic malaria with variable human and mosquito populations. Math. Comput. Model. 32, 747–763 (2000)
Paijmans, K.P., Read, A.F.: Understanding the link between malaria risk and climate. Proc. Nat. Acad. Sci. USA 106, 13844–13849 (2009)
Parham, P.E., Michael, E.: Modeling the effects of weather and climate changes on malaria transmission. Environ. Health Perspect. 118, 620–626 (2010)
Perko, L.: Differential Equations and Dynamical Systems. Springer, New York (1991)
Ross, R.: The Prevention of Malaria, 2nd edn. Murray, London (1911)
Safan, M.: Spread of Infectious Diseases: Impact on Demography, and the Eradication Effort in Models with Backward Bifurcation, PhD Thesis, Faculty of Mathematics and Phyiscs, Eberhard-Karls University of Tuebingen (2006)
Safan, M., Dietz, K., Hadeler, K. P.: Demographic effect of infection lethality in an SEIR model for an exponentially growing population (In preparation)
Smith, T., Maire, N., Ross, A., Penny, M., Chitnis, N., Schapira, A., Studer, A., Genton, B., Lengeler, C., Tediosi, F., De Savigny, D., Tanner, M.: Towards a comprehensive simulation model of malaria epidemiology and control. Parasitology 135, 1507–1516 (2008)
van den Driessche, P., Watmough, J.: Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 180, 29–48 (2002)
WHO: World Malaria Report: Fact Sheet. http://www.who.int/malaria/publications/world_malaria_report_2012/wmr2012_factsheet (2012)
Zaman, G.: Qualitative behavior of giving up smoking models. Bull. Malays. Math. Sci. Soc. 34, 403–415 (2011)
Acknowledgments
The authors would like to thank the editor as well as the anonymous referees very much for their invaluable and comprehensive comments which helped in improving the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ataharul M. Islam.
Appendices
Appendix 1
Since \(N_h = S_h + I_h\) and \(N_v = S_v + I_v\), then
Hence,
Since \(s_h = \frac{S_h}{N_h}\), then
Similarly, \(i_h = \frac{I_h}{N_h}\) implies that
Also,
Appendix 2
To obtain the martinis F and V we rearrange the model (2) so that the equations representing the infections come first. Therefore,
The above system can be written as \(\dot{x_i} = f_i(x) = \mathcal {F}_i(x) - \mathcal {V}_i(x)\), where \(i = 1, 2, 3, 4\); \(x_1 = i_h, x_2 = i_v, x_3 = s_h, x_4 = s_v\); \(\mathcal {F}_i(x)\) is the rate of appearance of new infections in compartment i, and \(\mathcal {V}_i(x) = \mathcal {V}_i^{-}(x) - \mathcal {V}_i^{+}(x)\) with \(\mathcal {V}_i^{+}(x)\) representing the rate of transfer of individuals into compartment i by all other means and \(\mathcal {V}_i^{-}(x)\) representing the rate of transfer of individuals out of compartment. It has the infection-free equilibrium \(x_0 = (i_h^0, i_v^0, s_h^0, s_v^0)^{\prime } = (0, 0, 1, 1)^{\prime }\). Thus,
Hence,
Appendix 3
1.1 Proof of proposition 1
Let
Following the approach shown in [30], the stability of the infection-free equilibrium is determined by the sign of the quantity \(s(j_1)\) representing the maximum real part of all the eigenvalues of the matrix \(J_1\) (i.e., it is the spectral abscissa of \(J_1\)). Thus the proof summarizes in showing that
-
1.
\(s(J_1) < 0 \Longleftrightarrow R_0 = \rho (F V^{-1}) < 1,\)
-
2.
\(s(J_1) = 0 \Longleftrightarrow R_0 = \rho (F V^{-1}) = 1,\)
-
3.
\(s(J_1) > 0 \Longleftrightarrow R_0 = \rho (F V^{-1}) > 1\).
It is easy to evaluate that
Now, if \(R_0 < 1\), then the value of the expression under the square root is less than \([\beta _h+\gamma _h+\beta _v]^2\) which implies that \(s(J_1) < 0\) and vice versa. Also, if \(R_0 > 1\) then the expression under the square root will have a value that is bigger than \([\beta _h+\gamma _h+\beta _v]^2\) which induces that \(s(J_1) > 0\). It is easy to check that a value of \(R_0 = 1\) makes \(s(J_1) = 0\). Thus, the infection-free equilibrium is locally stable if and only if \(s(J_1) < 0 \Longleftrightarrow R_0 = \rho (F V^{-1}) < 1,\) while it is unstable if and only if \(s(J_1) > 0 \Longleftrightarrow R_0 = \rho (F V^{-1}) > 1\).
Appendix 4
1.1 Direction of bifurcation at (0, 0)
Using the implicit function theorem, the direction of bifurcation at a point depends on the sign of the expression
at this point. It is easy to check that
Hence
Thus, the bifurcation is forward at the point (0, 0).
Appendix 5
We have
However, from the second equation of (6) we have
Also, from the first equation of (6) we have
This implies that
Hence,
where
Now, we use (9) in (41) to reduce the degree by one. Therefore, we get
where
It is clear that H is linear in \(i_v\). Moreover, \(H(0) = {H_1}/{A} > 0\) and \(H^{\prime }(0) = - {H_2}/{A} < 0\). On the other hand, since \(i_v^{-}\) tends to 1 as \(C_{hv}\) tends to \(\infty \), then \(H(1) = {\beta _v}^2\), this could be obtained also if we put \(i_v = 1\) in (40). Thus \(H(i_v^{-}) > 0\). Hence, \(tr(J)<0\) for \(i_v = i_v^{-}\).
Rights and permissions
About this article
Cite this article
Safan, M., Ghazi, A. Demographic Impact and Controllability of Malaria in an SIS Model with Proportional Fatality. Bull. Malays. Math. Sci. Soc. 39, 65–86 (2016). https://doi.org/10.1007/s40840-015-0181-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40840-015-0181-6