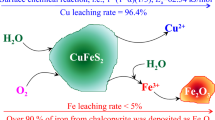
Abstract
In this paper, a novel process leaching complex chalcopyrite without acid under oxygen pressure is proposed, and the leaching behavior and kinetic characteristics of copper in the complex chalcopyrite is studied. The results show that in the process of oxygen pressure leaching of complex chalcopyrite the oxidation dissolution of FeS2 occurs first, and then the sulfuric acid required by the reaction is generated, which destroys the embedded structure of various mineral phases in chalcopyrite and makes it dissolve into the leaching solution. The copper in the mineral reacts with sulfuric acid to form copper sulfate which enter into the leaching solution, and the iron in the mineral is mainly transformed to hematite and then remained in the leaching slag. Under the optimal leaching conditions (initial sulfuric acid concentration 0 g/L, reaction temperature 200 ℃, partial pressure of oxygen 1.2 MPa, mineral particle size − 48 + 38 µm), the leaching efficiency of copper reached 99.86% after 120 min reaction. The analysis of the kinetic process of oxygen pressure leaching of complex chalcolite based on the "shrinkage core model" showed that the leaching process of chalcopyrite is mainly controlled by chemical reaction. The apparent activation energy of the reaction is 50.646 kJ/mol. In the process of chemical reaction control, the parameters of partial pressure of oxygen and initial radius of mineral particles are 4.040 and − 0.773, respectively. The kinetic equation can be expressed as \(1 - \left( {1 - X} \right)^{\frac{1}{3}} = 1.123 \times 10^{4} \times {\text{P}}_{{{\text{O}}_{2} }}^{4.040} r_{0}^{ - 0.773} e^{{\frac{ - 6092}{T}}} t\).
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chalcopyrite is the most widely distributed copper-bearing ore in nature [1,2,3,4,5], and it is also the main raw material for copper extracting. At present, 80–85% of the copper in the world is produced by pyrometallurgy, but along with the continuously exploitation of chalcopyrite ore, leading to the grade of the ore is more and more low. As the reason that the traditional method of pyrometallurgy mainly suitable for processing the chalcopyrite concentrate (copper content is generally greater than 18%), only poor economic and technical indicators can be arrived while it is used to deal with the complicated composition and low grade polymetallic complex chalcopyrite. In addition, a large amount of SO2 and other gases harmful to the environment and human body will be produced in the process of pyrometallurgy [6]. Compared with pyrometallurgical process, it is more economical and environmentally friendly to extract copper from complex chalcopyrite by hydrometallurgical method [7, 8]. However, due to the stable structure of chalcopyrite, the leaching rate and the recovery efficiency of valuable metal in the hydrometallurgical extraction process are not high. Therefore, it is urgent to improve the leaching efficiency of chalcopyrite, relieve the pressure of copper metallurgical resources, achieve economic and environmental win–win, and strengthen the research of low-grade complex chalcopyrite by hydrometallurgical leaching process.
At present, the hydrometallurgical leaching process of chalcopyrite can be divided into three categories: oxidative leaching [9,10,11], coordination leaching [12] and bioleaching [13, 14]. Bioleaching is a kind of metallurgical technology which only emerged in the latest 40 years. Its scientific principle is that bacteria are added in the leaching process to extract valuable metals from the ore. It is more suitable for the chalcopyrite with low grade, but its leaching cycle is long and bacteria are easy to die in the leaching process, so it is not suitable for industrial production. Coordination leaching is a method to form soluble complexes of copper and appropriate ligands [15] to realize chalcolite leaching. The most common ligands are chloride ions [16] and ammonium ions [17]. However, the corrosion in chloride medium conditions and defects such as ammonia volatilization to the environment during ammonia leaching have always been the difficulties in coordination leaching research. Compared with coordination leaching and bioleaching, oxidative leaching is more widely used and the technology is more mature. Oxidative leaching is usually divided into atmospheric pressure oxidative leaching [18, 19] and pressure oxidative leaching [11]. At present, most of the pressure oxidative leaching processes of chalcopyrite reported in the literature usually use sulfuric acid [6], oxalic acid [20] and other acidic solutions as leaching agents to leach copper in chalcopyrite, and few studies have reported that sulfuric acid produced by the dissolution of sulfur-containing minerals in chalcopyrite is used as the acid source of the leaching mineral.
In this paper, a typical complex chalcopyrite containing CuFeS2, FeS2, PbS and ZnS was taken as the research object, and distilled water was used as the leaching agent to study the leaching behavior and kinetic characteristics of copper under oxygen pressure. The effects of initial sulfuric acid concentration, reaction temperature, oxygen partial pressure and mineral particle size on copper leaching rate were studied, and the oxygen pressure leaching conditions were optimized to achieve high efficiency copper leaching from chalcopyrite. In addition, the role and mechanism of FeS2 in the leaching process were analyzed, and the phase composition and distribution before and after leaching were analyzed and discussed by X-ray diffraction (XRD), scanning electron microscopy (SEM) and other detection methods. Finally, the control steps of chalcopyrite dissolution reaction were analyzed and the kinetic equation of copper leaching was obtained.
Experimental
Experimental Materials
The raw material used in present study is secondary concentrate of chalcopyrite produced in a company in Yunnan China after flotation is performed. After ball milling and screening, its main components and content are shown in Table 1. It can be seen from the table that S, Fe, Cu, Pb and Zn account for the vast majority of the total mass of minerals, and the sum of these five elements accounts for 96.00% of the total mass. The XRD analysis results are shown in Fig. 1, and the SEM and EDS analysis are shown in Fig. 2.
As can be seen from Fig. 1 that chalcopyrite, bornite, pyrite, sphalerite and galena are the main mineral phases in the raw material. In addition, the chemical phase analysis of the raw materials showed that chalcopyrite and bornite accounted for 84.88% and 9.91% of the total copper containing minerals, respectively (mass fraction), indicating that copper in the raw material mainly existed in the form of chalcopyrite, followed by bornite. Iron was found in the form of pyrite (69.43%), chalcopyrite (29.33%) and bornite (0.68%). Zinc and lead exist as sphalerite and galena, respectively.
It can be seen from the energy spectrum diagram of point 1 in Fig. 2 that the main elements are zinc, sulfur and lead. Combined with the results of phase characterization analysis of the minerals in Figs. 1 and 2, the main components in this area are sphalerite and galena. Energy spectrum diagram can be found at point 2 that main element in this area is sulfur, copper and iron, and that in this region the main ingredients are chalcopyrite, bornite and a small amount of pyrite, and the SEM pattern shows that in this area the sphalerite and galena are associated and embedded together with an integrity and fine disseminated properties. The energy spectrum of point 3 mainly contains lead and sulfur, indicating that galena is the main species in this area. The most important elements in the energy spectrum of point 4 are iron and sulfur, indicating that pyrite is the main species in this area. In a word, multimetallic sulfides such as chalcopyrite, bornite, sphalerite, galena, pyrite, and etc. are embedded and disseminated with each other in the raw material with a fine particle size.
Experimental Equipment and Experimental Protocol
The experiment was carried out in an autoclave made of zirconium and driven by magnetic force (GSH-2/12.5, see Fig. 3) with a capacity of 2 L, working temperature < 250 ℃, working pressure < 10 MPa, and stirring speed of 1–750 rpm.
In each leaching experiment, 1000 mL leaching agent was first placed in the reaction kettle and heated to the specified temperature, then 100 g raw material was added, stirring was started (the stirring rate was fixed at 400 rpm), and oxygen at the specified pressure was fed in, and the partial pressure of oxygen and reaction temperature were kept constant during the reaction process for 120 min. Samples were taken every 12 min during the reaction to analyze the relationship between copper leaching rate and leaching reaction time. After the end of the reaction, turn off the heating and oxygen, and turn on the cooling water to cool the reaction system to room temperature, then turn off the stirring and open the vent valve to release the pressure in the kettle, followed by opening the pressurizer and taking out the slurry to filter on the vacuum filter device. The filter cake was washed three times with 15 mL distilled water, and the copper content of the filter liquid was measured and analyzed. The obtained filter cake (leaching residue) was put into an oven to dry at 110 ℃ for 2 h before analyzing and detecting.
Characterization and Analysis
Inductively coupled atomic emission spectrometer (ICP-AES) was used to determine the content of each element in the mineral, and the carbon and sulfur analyzer (ELTRA CS2000) manufactured from Germany was used to determine the sulfur content, and the iodine method was used to detect the copper content in the leaching solution. Equation (1) was used to calculate the copper leaching efficiency. X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) were used to characterize the phase composition and morphology of the minerals and leaching residue.
where V0 is the total volume of leaching solution, L; Vi is the volume of liquid samples taken out at intervals of every i times, L; Ci is the concentration of copper in the liquid sample taken out for the first time, g /L; W is the mass fraction of elements in the raw material, %.
Results and Discussion
Effects of Sulfuric Acid Concentration
Under the conditions of reaction temperature 200 ℃, partial pressure of oxygen 1.2 MPa and particle size − 48 + 38 µm, the influence of initial sulfuric acid concentration on chalcopyrite leaching process is shown in Fig. 4. As can be seen from Fig. 4 that the final leaching efficiency of copper is close to 100% regardless of the initial sulfuric acid concentration. This phenomenon is because there is a large amount of pyrite in the chalcopyrite, and pyrite is the source of sulfuric acid needed in the leaching process [21]. When the initial acidity is too low, especially when the initial acidity is 0 g/L, pyrite will dissolve before chalcopyrite and produce sulfuric acid needed for the dissolution of chalcopyrite. The reaction on the production of sulfuric acid from pyrite and the hydrolysis of ferric sulphate to produce ferric oxide (hematite) are shown in Eqs. (2) and (3).
As can be seen from Eq. (2) that Fe3+ will also be produced when pyrite oxidized and dissolved to produce sulfuric acid. With the continuous increase of Fe3+ concentration, the redox potential in the leaching system will also rise, and the increase of redox potential is widely considered to be the decisive factor to improve the dissolution rate of chalcopyrite.
In addition, it also can be found from the figure that, in the initial stage, the leaching rate of copper is faster with the increasing of initial concentration of sulfuric acid, this is because in early stages of the reaction, when the initial concentration of sulfuric acid is low, the leaching of copper from the mineral relies mainly on the sulfuric acid produced by the oxidation dissolution of pyrite which also needs a certain amount of time. However, when the initial sulfuric acid concentration which mainly depends on the artificial addition of sulfuric acid is at a high level, the initial acidity plays a dominant role in the leaching of copper. Since the initial acidity has little effect on the final leaching rate of copper, and too high acidity will erode the leaching equipment and cause damage to the experimental equipment. Therefore, the initial acidity of 0 g/L was appropriate for subsequent experiments.
Effects of Reaction Temperature
Under the conditions of initial sulfuric acid concentration 0 g/L, partial pressure of oxygen 1.2 MPa and particle size − 48 + 38 µm, the influence of reaction temperature on chalcopyrite leaching is shown in Fig. 5. As can be seen from Fig. 5 that the final leaching efficiency of copper also increases with the increase of temperature, and that the leaching efficiency of copper reaches 99.86% when the temperature is 200 ℃. This can be accounted by that the chemical reaction rate and diffusion rate of lixiviant in the leaching process will accelerate with the increase of reaction temperature. At the same time, it can be found that in the first period up to 24 min of the whole reaction duration, the increase of copper leaching efficiency is generally slow, but 24 min latter, the increase of copper leaching efficiency at almost all temperatures is faster. This is because in the whole leaching process, as the initial concentration of sulfuric acid is 0 g/L, pyrite is dissolved first to produce sulfuric acid and Fe3+ [18, 22], and then chalcopyrite reacts with the generated sulfuric acid and Fe3+ as well as combining with oxygen entered in the reaction system. In the first 24 min of the reaction process, the dissolution of pyrite is the main reaction, and the dissolution amount of chalcopyrite is not large. With the increasing amount of pyrite dissolution, the concentration of sulfuric acid and Fe3+ in the solution are also higher and higher, which leads to the acceleration of the dissolution of chalcopyrite. Since the copper leaching efficiency at 200 ℃ has reached more than 99.86%, in order to ensure the safety of the experiment and better leaching efficiency, and reduce energy consumption, 200 ℃ is the appropriate reaction temperature for subsequent experiments.
Effects of Oxygen Pressure
Under the conditions of initial sulfuric acid concentration 0 g/L, reaction temperature 200 ℃ and particle size − 48 + 38 µm, the effect of oxygen partial pressure on chalcopyrite leaching is shown in Fig. 6. It can be clearly seen from Fig. 6 that the partial pressure of oxygen has a great influence on the chalcopyrite leaching. Both the copper leaching efficiency during the leaching process and the final copper leaching efficiency increase with the increase of the partial pressure of oxygen. In addition, it can be seen that in the whole leaching process, the higher the partial pressure of oxygen, the faster the rate of copper leaching increases, because the increase of the partial pressure of oxygen will increase the dissolved oxygen in the leaching solution, and the increase of dissolved oxygen will make the mineral particles more fully contact with oxygen, and accelerate the chemical reaction. At the same time, with the increase of the partial pressure of oxygen, the pressure difference between the gas phase and the liquid phase also increases, and the increase of the pressure difference will accelerate the speed of oxygen entering the liquid phase from the gas phase, thus promoting the acceleration of the reaction rate. It can also be found from Fig. 6 that in the first 24 min of the whole reaction duration, the copper leaching efficiency under different oxygen partial pressure conditions is basically the same. However 24 min latter the copper leaching efficiency under 1.2 MPa condition increases with a significantly faster rate. This is because when the oxygen partial pressure is 1.2 MPa, the main reaction in the first 24 min of the whole reaction process is the dissolution of pyrite, and the dissolution of chalcopyrite starts to accelerate due to sufficient sulfuric acid and a large amount of Fe3+ production 24 min latter. Under the conditions of 0.8 MPa and 1.0 MPa, the time when turning point of accelerated chalcopyrite dissolution occurs is about 60 min. This is because the lower oxygen partial pressure will not only reduce the dissolution rate of chalcopyrite, but also reduce the dissolution rate of pyrite, thus leading to the decrease of sulfuric acid concentration in the system. In conclusion, 1.2 MPa is the appropriate oxygen partial pressure for subsequent experiments.
Effects of Particle Size
Under the conditions of initial sulfuric acid concentration 0 g/L, reaction temperature 200 °C and partial pressure of oxygen 1.2 MPa, the effect of mineral particle size on chalcopyrite leaching is shown in Fig. 7. The Fig. 7 shows that the leaching rate of copper increases with the reduce of mineral particle size, this is because the specific surface area of the mineral also increase with the decrease of the particle size, thereby increasing the contact area among mineral, leaching agent and dissolved oxygen, and accelerating the chemical reaction rate for getting more minerals dissolved. At the same time, the larger the mineral particle size is, the easier the formation of the passivation layer causes. This is the reason why the occurring time of turning point of copper leaching rate at − 48 + 38 µm is earlier than that at the other two particle size ranges. Therefore, it is better to choose − 48 + 38 µm mineral particle size in the experiment.
Optimal Leaching Conditions for Copper Leaching
According to the above experimental results, the optimal leaching conditions for copper leaching from chalcopyrite are as follows: initial sulfuric acid concentration 0 g/L, reaction temperature 200 ℃, oxygen partial pressure 1.2 MPa, mineral particle size − 48 + 38 µm. Under these conditions, the leaching efficiency of copper reached 99.86% after leaching for 2 h. The phases in the leaching residues were detected and analyzed by XRD and SEM, and the obtained results are shown in Figs. 8 and 9. It can be seen from the XRD spectra that lead iron vitriol, hematite, lead sulfide and elemental sulfur exist in the leaching residue, and that no chalcopyrite, bornite, pyrite, sphalerite and galena, have been completely reacted in the leaching process. Among them, the copper in the raw material reacts with sulfuric acid to produce the copper sulfide then entering into the leaching solution in the form of ions. Also it can be seen from the SEM figure that the structure of leaching residue is loose and is not as dense as raw material, showing that under the optimum leaching conditions the internal density structure of raw material is destructed which making the mineral phases like chalcopyrite, bornite and other substances exposed to be reacted fully with leaching agent in the leaching process. In addition, from the EDS spectrum diagram (Fig. 9) it can be found that elements such as oxygen, iron, sulfur, lead exist in the leaching residue, and neither copper nor zinc has been found. Which is proved the results characterized by XRD spectra (Fig. 8), at the same time, further illustration indicates that the chalcopyrite, bornite and zinc sulfide have reacted completely, and that the mineral phase of iron mainly exists in the form of hematite in the leaching residue. After certain treatment performed, the hematite can be used as high quality raw material for iron making.
Compared with the results reported by literatures involve the oxidative chalcopyrite leaching with hydrogen peroxide and organic additive in sulfuric acid solution which achieved about 55% copper leaching efficiency and 35% iron leaching efficiency [23], the oxygen pressure leaching in sulfuric acid which the copper and iron extraction rates were 98% respectively under the conditions of temperature 150 °C and oxygen partial pressure 0.7 MPa [24], the bioleaching process assisted by microbial fuel cells and catalyzed by silver-bearing ores which the copper extraction efficiency from chalcopyrite arrived 10.03%[25], and the ionic liquid leaching process with potassium dichromate which the maximum copper extraction yield of 90.2% from chalcopyrite which the copper leaching efficiency arrived 90.2%[26], the proposed process in present work for leaching valuable metals like copper and zinc from complex multi-metallic chalcopyrite are selective and effective.
Kinetics of Oxygen Pressure Leaching of Complex Chalcopyrite
Kinetics Model Choice
Due to the complex composition of chalcopyrite and other reactants used like lixiviant and oxygen, there will be multiphases reactions between gas–solid–liquid in the leaching process. Therefore, an appropriate kinetic model should be selected before the kinetic study. In the process of chalcopyrite leaching, sulfur elements and other substances will be generated and attached to the surface of the reactive mineral to form a passivation layer [27]. Therefore, the reaction mechanism diagram of chalcopyrite leached under oxygen pressure can be shown as Fig. 10.
It can be seen from Fig. 10 that there are three layers during the chalcopyrite leaching, which are mineral unreacted core, solid product layer and leaching agent diffusion layer. For the leaching process of chalcopyrite solid products layer consisting of elemental sulfur [10], alkaline ferric sulfate [28] and copper sulfide [29] and etc. form the parcel covering on the surface of unreacted mineral core, and the solid product layer is difficult to dissolve in the leaching process, causing the unreacted internal mineral is hard to contact with leaching agent and hampering the leaching reaction [30]. As can be seen from the figure, H2SO4 and O2 need pass through solid products layer and diffusion layer before participating leaching reaction, and the products like Cu+, Fe2+ and Fe3+ will enter into leaching solution, with the progress of leaching the unreacted core will become small.
According to the above analysis of chalcopyrite leaching process, we can describe the chalcopyrite leaching process with "shrinkage core model" [31], which mainly includes the following steps:
-
(1)
The liquid reactant (leachate) diffuses to the surface of the solid reactant through the leachate diffusion layer.
-
(2)
Diffusion of liquid reactants through the solid product layer to the unreacted layer.
-
(3)
The liquid reactant adsorbs on the surface of the unreacted layer and reacts with it chemically.
-
(4)
The liquid product diffuses to the solid surface through the solid product layer.
-
(5)
The liquid products are diffused into the leaching solution through the leaching agent diffusion layer around the mineral particles.
Generally speaking, if any of the above steps takes much more time than the other steps in the leaching process, then the total rate of the leaching reaction depends on the rate of this reaction step. Then the reaction rate equations controlled by chemical reaction control, internal diffusion control and their combined action are shown in Eqs. (4), (5) and (6), respectively [32,33,34].
where: X is the copper leaching efficiency, Kr, Kd and Km are the reaction rate constant controlled by chemical reaction, diffusion and mixture, respectively, and t is the reaction time (min).
Kinetics Analysis of Control Step of Pressure Leaching of Complex Chalcopyrite
According to the influence of various factors on chalcopyrite leaching process, chalcopyrite leaching process may be influenced by chemical reaction control and internal diffusion control of solid product layer. Therefore, the experimental data can be substituted into the kinetic equation, and the simulated R2 value can be used to determine whether the leaching process of chalcopyrite is mainly affected by chemical reaction control or internal diffusion control.
It can be seen from the above in “Effects of Reaction Temperature” Section that temperature is one of the largest factors influence on chalcopyrite leaching process. By changing reaction temperature it is easy to determine the controlling step whether the reaction process is controlled by chemical reaction or by internal diffusion [35], therefore, for making dynamic analysis of the influence of temperature the experimental data obtained in Fig. 5 were respectively substituted into Eqs. (4) and (5) for plotting which can be found as Fig. 11a, b. The fitting results are also shown in Fig. 11, and the parameters of the chemical reaction control model and internal diffusion control model are shown in Table 2. In addition, as the reason that poor fitting results were achieved when the experimental data obtained in Fig. 5 were substituted into Eq. (6), indicating the leaching behavior of copper does not conform to the mixture control steps. Therefore further work about the mixture control does not be mentioned in the present work.
Where R2 in Table 2 is the correlation coefficient of the fitted straight line. As can be seen from the R2 value given in Table 2 that the leaching process at each temperature is more in line with the control of chemical reaction, indicating that the oxygen pressure leaching process of copper from chalcopyrite is mainly controlled by chemical reaction. Therefore, the reaction rate constant K in the chemical reaction control model at different temperatures was substituted into the Arrhenius Equation (Eq. 7).
where A is the frequency factor; E is the apparent activation energy (kJ/mol); R is the gas equilibrium constant, 8.314 J/mol; T is the thermodynamic temperature (K).
Taking logarithm on both sides of Eq. 6 at the same time, the Eq. (8) can be got.
The plot of LnK against 1/T, and the its fitting result is shown in Fig. 12. Its linear correlation constant R2 is 0.9998, indicating that the fitting degree of the line is good. According to the slope of the fitting equation in Fig. 12, the apparent activation energy of this reaction is calculated to be 50.646 kJ/mol, which is greater than 41.8 kJ/mol, which further verifies that the oxygen pressure leaching process of chalcopyrite is controlled by chemical reaction. It is known that the leaching behavior is controlled by chemical reaction control while the apparent active energy is larger than 41.8 kJ/mol, and controlled by internal diffusion while the apparent active energy lays in the range of 4–18 kJ/mol [36,37,38,39].
Kinetics Equation of Main Control Step of Oxygen Pressure Leaching of Complex Chalcopyrite
In the process of studying the kinetics, in addition to determining its control steps by temperature, it is necessary to study the influence of other factors like partial oxygen pressure and particle size of raw material on the leaching process. Considering the influence of various factors on copper leaching rate, the relationship between the rate constant controlled by chemical reaction and various factors can be expressed as Eq. (9) [40,41,42,43].
where n1 and n2 are parameters of partial pressure of oxygen (MPa) and initial radius (µm), and Kʹ is rate constant related to temperature; E is the apparent activation energy (kJ/mol); R is the gas equilibrium constant, 8.314 J/mol; T is the absolute temperature (K).
For particles with particle size of "− dmax + dmin", the initial radius (r0) can be estimated by the following equation [44]:
The data in Figs. 6 and 7 were substituted into Eq. (3) and fitted to obtain the fitting equations of 1 − (1 − x)1/3 for different oxygen partial pressures, different mineral particle sizes, as shown in Fig. 13a, b, respectively. It can be seen from Fig. 13 that the copper leaching rate under different conditions presents good fitting effect, and the slope K of each fitted line is the rate constant under different oxygen partial pressure and different mineral particle size. LnK is used to plot lnPO2 and lnr0, and the results are shown in Fig. 14a, b, respectively. By fitting the slope of the line, the parameters of oxygen partial pressure and initial radius can be obtained as 4.040 and − 0.773, respectively. When the above data and apparent activation energy are substituted into Eq. (8), the value K’ is obtained as 1.123 × 104, and the macroscopic kinetic equation of copper oxygen pressure leaching is Eq. (11).
Conclusions
-
(1)
The treatment of the complex chalcopyrite by oxygen pressure leaching without sulfuric acid can well destroy the mosaic structure of the mineral phases in the raw material and make chalcopyrite easier to react. In the process of oxygen pressure leaching of the complex chalcopyrite without acid, pyrite will be preferably dissolved to produce the sulfuric acid needed for the dissolution of other minerals like chalcopyrite, sphalerite, galena, and etc. Copper and zinc in the mineral form the correspondence metal sulfate, which enters the leaching solution in the form of ions, while iron in the mineral is mainly left in the leaching slag in the form of hematite. The optimal leaching conditions of chalcopyrite are as follows: initial sulfuric acid concentration 0 g/L, reaction temperature 200℃, oxygen partial pressure 1.2 MPa, mineral particle size − 48 + 38 µm. Under these conditions, the copper leaching efficiency can reach 99.86%. The whole leaching process is green, pollution-free, low energy consumption, mild reaction conditions, small corrosion to equipment, and simple operation method, easy to realize industrial production.
-
(2)
The kinetics model for leaching copper from chalcopyrite is in line with the "shrinking core model". The leaching process is controlled by chemical reaction, and the apparent activation energy of the leaching reaction is 50.646 kJ/mol. In the chemical reaction control process, the parameters of oxygen partial pressure and the initial radius of mineral particles are 4.040 and − 0.773, respectively, and the kinetics equation can be expressed as \(1 - \left( {1 - X} \right)^{\frac{1}{3}} = 1.123 \times 10^{4} \times {\text{P}}_{{{\text{O}}_{2} }}^{4.040} r_{0}^{ - 0.773} e^{{\frac{ - 6092}{T}}} t\).
References
Zhao HB, Wang J, Qin WQ et al (2015) Electrochemical dissolution process of chalcopyrite in the presence of mesophilic microorganisms. Miner Eng 71:159–169
Yang BJ, Luo W, Wang XX et al (2020) The use of biochar for controlling acid mine drainage through the inhibition of chalcopyrite biodissolution. Sci Total Environ 737:139485
Yang BJ, Lin M, Fang JH et al (2020) Combined effects of jarosite and visible light on chalcopyrite dissolution mediated by Acidithiobacillus ferrooxidans. Sci Total Environ 698:134175
Zhao HB, Wang J, Gan XW et al (2015) Cooperative bioleaching of chalcopyrite and silver bearing tailing by mixed moderately thermophilic culture: an emphasis on the chalcopyrite dissolution with XPS and electrochemical analysis. Miner Eng 81:29–39
Wang J, Zhao HB, Qin WQ et al (2013) Investigation of interface reactions and electrochemical behaviors of chalcopyrite dissolution in different leaching mediums. Int J Electrochem Sci 12:12590–12599
Bai YL, Wang W, Xie F et al (2022) Effect of temperature, oxygen partial pressure and calcium lignosulphonate on chalcopyrite dissolution in sulfuric acid solution. Trans Nonferrous Met Soc China 32(5):1650–1663
Olubambi PA, Potgieter JH (2009) Investigations on the mechanisms of sulfuric acid leaching of chalcopyrite in the presence of hydrogen peroxide. Miner Process Extr Metall Rev 30(4):327–345
Chen ML, Zhang L, Gu GH et al (2008) Effects of microorganisms on surface properties of chalcopyrite and bioleaching. Trans Nonferrous Met Soc China 6:1421–1426
Lane DJ, Cook NJ, Grano SR et al (2016) Selective leaching of penalty elements from copper concentrates: a review. Miner Eng 98:110–121
Petrovic SJ, Bogdanovic GD, Antonijevic MM (2018) Leaching of chalcopyrite with hydrogen peroxide in hydrochloric acid solution. Trans Nonferrous Met Soc China 28(7):1444–1455
Han B, Altansukh B, Haga K et al (2017) Leaching and kinetic study on pressure oxidation of chalcopyrite in H2SO4 solution and the effect of pyrite on chalcopyrite leaching. J Sustain Metall 3(3):528–542
Yevenes LV, Miki H, Nicol M (2010) The dissolution of chalcopyrite in chloride solutions: Part 2: effect of various parameters on the rate. Hydrometallurgy 103(1–4):80–85
Panda S, Akcil A, Pradhan N et al (2015) Current scenario of chalcopyrite bioleaching: a review on the recent advances to Its heap-leach technology. Biores Technol 196:694–706
Fowler TA, Crundwell FK (1998) Leaching of zinc sulfide by thiobacillus ferrooxidans: experiments with a controlled redox potential indicate no direct bacterial mechanism. Appl Environ Microbiol 64(10):3570–3575
Moyo T, Petersen J (2016) Study of the dissolution of chalcopyrite in solutions of different ammonium salts. J South Afr Inst Min Metall 116(6):509–516
Zhong S, Li YB (2019) An improved understanding of chalcopyrite leaching kinetics and mechanisms in the presence of NaCl. J Market Res 8(4):3487–3494
Baba AA, Ghosh MK, Pradhan SR et al (2014) Characterization and kinetic study on ammonia leaching of complex copper ore. Trans Nonferrous Met Soc China 24(5):1587–1595
Okamoto H, Hiroyoshi N, Tsunekawa M (2009) Improved chalcopyrite leaching through optimization of redox potential. Can Metall Q 47(3):253–258
Ibanez T, Velasquez L (2013) The dissolution of chalcopyrite in chloride media. Rev Metal 49(2):131–144
Turan MD, Sari ZA, Nizamoglu H (2020) Pressure leaching of chalcopyrite with oxalic acid and hydrogen peroxide. J Taiwan Inst Chem Eng 118:112–120
McDonald RG, Muir DM (2007) Pressure oxidation leaching of chalcopyrite. Part I. Comparison of high and low temperature reaction kinetics and products. Hydrometallurgy 86(3–4):191–205
Olvera OG, Rebolledo M, Asselin E (2016) Atmospheric ferric sulfate leaching of chalcopyrite: thermodynamics, kinetics and electrochemistry. Hydrometallurgy 165(1):148–158
Ruiz-Sanchez A, Lapidus GT (2022) Decomposition of organic additives in the oxidative chalcopyrite leaching with hydrogen peroxide. Miner Eng 187:107783
Bai YL, Wang W, Xie F et al (2022) Effect of temperature, oxygen partial pressure and calcium lignosulphonate on chalcopyrite dissolution in sulfuric acid solution. Trans Nonferrous Met Soc China 32:1650–1663
Zhang X, Zhang S (2022) Enhanced copper extraction in the chalcopyrite bioleaching system assisted by microbial fuel cells and catalyzed by silver-bearing ores. J Environ Chem Eng 10:108827
Hu JX, Zi FT, Tian GC (2021) Extraction of copper from chalcopyrite with potassium dichromate in 1-ethyl-3-methylimidazolium hydrogen sulfate ionic liquid aqueous solution. Miner Eng 172:107179
Bai YL, Wang W, Zhao SR et al (2022) Effect of mechanical activation on leaching behavior and mechanism of chalcopyrite. Miner Process Extr Metall Rev 43(4):440–452
Nie ZY, Zhang WW, Liu HC et al (2019) Bioleaching of chalcopyrite with different crystal phases by Acidianus manzaensis. Trans Nonferrous Met Soc China 29(3):617–624
Wu SF, Yang CR, Qin WQ et al (2015) Sulfur composition on surface of chalcopyrite during its bioleaching at 50 °C. Trans Nonferrous Met Soc China 25(12):4110–4118
Elsherief AE (2022) The influence of cathodic reduction, Fe2+ and Cu2+ ions on the electrochemical dissolution of chalcopyrite in acidic solution. Miner Eng 15(4):215–223
Veglio F, Trifoni M, Pagnanelli F (2001) Shrinking core model with variable activation energy: a kinetic model of manganiferous ore leaching with sulphuric acid and lactose. Hydrometallurgy 60(2):167–179
Hiroyoshi N, Miki H, Hirajima T et al (2000) A model for ferrous-promoted chalcopyrite leaching. Hydrometallurgy 57(1):31–38
Mahajan V, Misra M, Zhong K et al (2008) Enhanced leaching of copper from chalcopyrite in hydrogen peroxide–glycol system. Miner Eng 20(7):670–674
Dickinson CF, Healb GR (1999) Solid–liquid diffusion controlled rate equations. Thermochim Acta 340(99):89–103
Shi GC, Liao YL, Su BW et al (2020) Kinetics of copper extraction from copper smelting slag by pressure oxidative leaching with sulfuric acid. Sep Purif Technol 241:116699
Wang HH, Li GQ, Zhao D et al (2017) Dephosphorization of high phosphorus oolitic hematite by acid leaching and the leaching kinetics. Hydrometallurgy 171:61–68
Li L, Bian Y, Zhang X et al (2018) Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching. Waste Manage 71:362–371
Mu WN, Lu XY, Cui FH et al (2018) Transformation and leaching kinetics of silicon from low-grade nickel laterite ore by pre-roasting and alkaline leaching process. Trans Nonferrous Met Soc China 28(1):169–176
Li X, Han PW, Wang YL et al (2019) Silver dissolution in a novel leaching system: reaction kinetics study. Int J Miner Metall Mater 26(02):28–37
Zhou HM, Zheng SL, Zhang Y et al (2006) A kinetic study of the leaching of a low-grade niobium-tantalum ore by concentrated KOH solution. Hydrometallurgy 80(3):170–178
Aydogan S, Aras A, Canbazoglu M (2005) Dissolution kinetics of sphalerite in acidic ferric chloride leaching. Chem Eng J 114(1–3):67–72
Li M, Zhang XW, Liu ZG et al (2013) Kinetics of leaching fluoride from mixed rare earth concentrate with hydrochloric acid and aluminum chloride. Hydrometallurgy 140:71–76
He GX, Zhao ZW, Wang XB et al (2014) Leaching kinetics of scheelite in hydrochloric acid solution containing hydrogen peroxide as complexing agent. Hydrometallurgy 144:140–147
Dimitrios F, Rao K, George PD (1997) A kinetic study on the acid pressure leaching of pyrrhotite. Hydrometallurgy 47(1):1–18
Acknowledgements
The authors express their sincere appreciation to the National Natural Science Foundation of China for financial support (Project No. 21978122 and 21566017).
Author information
Authors and Affiliations
Contributions
All authors have read and approved the content and agree to submit for consideration for publication in the journal.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Zhongwei Zhao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ji, G., Liao, Y., Xi, J. et al. Behavior and Kinetics of Copper During Oxygen Pressure Leaching of Complex Chalcopyrite Without Acid. J. Sustain. Metall. 9, 350–362 (2023). https://doi.org/10.1007/s40831-023-00658-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00658-5