Abstract
This paper studied chalcopyrite’s leaching behavior and kinetics under pressure oxidation conditions in water. The results show that it is possible to efficiently dissolve copper from chalcopyrite only by water, i.e., without any oxidant, and the copper leaching rate reaches 96.4% under the optimal conditions of a temperature of 180 °C, a total pressure of 1.5 MPa, a stirring speed of 900 rpm, and a leaching time of 90 min. XRD and SEM analysis shows that chalcopyrite might not dissolve as Cu2+ into the liquid phase directly but first transformed into sulfide such as CuS and FeS2, which is easier to leach. Then, the newly formed FeS2 act as a sulfuric acid source to promote the subsequent copper leaching. The kinetics is analyzed using the shrinking core model, and the results show that the surface chemical reaction mainly controls the chalcopyrite leaching process. The apparent activation energy of the chalcopyrite leaching is 62.34 kJ/mol, and the reaction order concerning temperature is approximately 18.0.
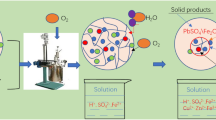
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper is an essential metal and is widely used in the fields such as electrical and electronics, transportation, petrochemical, and some new high-tech areas [1, 2]. The copper resources in nature can be divided into primary sulfide ore and oxide ore [3], and chalcopyrite (CuFeS2) is the raw material for copper extracting, which accounts for about 70 wt% of the earth’s total copper resources [3,4,5,6]. Currently, more than 80% of copper is produced from chalcopyrite via the pyrometallurgical process [5, 7, 8]. However, there are some critical issues, such as the emission of SO2, the copper grade getting lower and lower, copper ore being more complex, etc., are challenging traditional pyrometallurgical process [5, 9,10,11]. Therefore, hydrometallurgical methods (e.g., leaching), which are more environmentally friendly and efficient technology, have drawn attention as an alternative recently, and it is reported that around 25% of copper was produced by that route. The hydrometallurgical chalcopyrite leaching methods can be mainly divided into three categories, i.e., bioleaching [10, 12,13,14], coordination leaching [9, 15,16,17], and oxidative leaching [3, 4, 18,19,20,21,22,23]. There has been growing interest in using pressure oxidative leaching for treating chalcopyrite. However, it must be pointed out that chalcopyrite is a refractor mineral in hydro environments due to the formation of a passivation layer even at oxidative pressure conditions, a strong oxidant such as sulfuric acid [3, 22], hydrogen peroxide [21, 24], etc. is required for assisting and achieving efficient copper dissolution from chalcopyrite. Subsequently, these oxidants, i.e., acids, can cause environmental problems and strongly corrode the used equipment [25]. In addition, acid consumption increases when treating raw materials which contain vast amounts of gunge minerals [25]. Acid leaching also dissolves impurities (Fe etc.), the presence of which creates complications for the subsequent processing stages. However, non or fewer reports have focused on the pressure water leaching method, i.e., without acid addition.
This research systematically studies the leaching behavior and kinetics of copper from chalcopyrite in water under pressure oxidative conditions. The effect temperature (120 ~ 180 °C), total pressure (0 ~ 2.0 MPa), and stirring speed (300 ~ 900 rpm) on the leaching behaviors of chalcopyrite are investigated. The phase changes during chalcopyrite leaching are studied by using X-ray diffraction (XRD) and scanning electron microscopy (SEM). Finally, the kinetics of chalcopyrite oxidative leaching is analyzed by fitting the surface chemical reaction and product layer diffusion control model, and the activation energy is calculated according to the Arrhenius formula.
Materials and Methods
Materials
The chalcopyrite (CuFeS2) used in this study was obtained from a mine in Yunnan, China. The chalcopyrite was first dry-crushed to below 10 mm, and then the crushed mineral was “hand-picked” to reject any pieces containing visible non-sulfide mineral impurities. Then, the minerals were dry-screened to extract three (3) size fractions, i.e., − 47, + 47–74, and + 74–106 μm. The mineralogical and chemical studies on each ore sample were carried out by X-ray diffraction (XRD, D8 ADVANCE, Bruker, Germany) and X-ray fluorescence spectrometry (XRF, Bruker S8 TIGER, Germany), and scanning electron microscope (SEM, ZEISS Co., Ltd., Sigma 500, Germany), respectively. The XRD pattern (see Fig. 1) and the chemical analysis (see Table 1) confirmed that the chalcopyrite samples have excellent purity.
Methods
All pressure-oxidative leaching experiments were conducted using a stainless steel autoclave (Shanghai LABE Instrument Co., Ltd, AB-500) with a Teflon vessel (500 mL). A 4 g of ore sample was placed into a Teflon vessel with a total volume of 500 mL, and added with 150 mL of water. The vessel containing a slurry sample was then put into the Autoclave, followed by agitation using a magnetic impeller at speeds ranging from 300 to 900 rpm. The charged slurry/feed into an Autoclave was heated to the required temperature (120–180 °C), and pure oxygen gas was injected into the vessel to maintain the total target pressure (sum of vapor and oxygen partial pressure: 0.8–2.0 MPa), and then the reaction proceeded further for a period ranging from 0 to 90 min. It must be pointed out that the parameters are determined based on previous studies [26,27,28]. After the completion of pressure-oxidative leaching, the feed into the vessel was cooled down by a circulation water system and filtered through a 0.45-μm membrane to obtain pregnant leach solution (PLS) and solid residue, which are subjected to the chemical and mineralogical analyses using inductively coupled plasma-optical emission spectroscopy (ICP-OES, Jena Analytical Instruments Co., Ltd., PQ LC-Plasma Quant MS, Germany), XRD, and SEM, respectively. The leaching rate of each metal (RM) was calculated by the following Eq. (1).
where Rm is the metal leaching rate, %, CL and CF are the concentrations of metal in PLS (mg/L) and feed (mg/kg), respectively. VL is the volume of PLS (L), and MF is the dry mass of the feed (kg).
Results and Discussions
Effect of Particle Size
The effect of particle size on chalcopyrite oxidative leaching in a water system was conducted under different size fractions of − 47, − 74 + 47, and − 106 + 74 μm, while the temperature of 180 °C, a total pressure of 1.5 MPa, stirring speed of 500 rpm, and leaching time of 0, 30, 60, and 90 min.
It can be seen from the result in Fig. 2 that the copper leaching rate increased by extending the leaching time and decreasing the particle size. The recovery of copper after 30, 60, and 90 min leaching was 10.10%, 18.32%, and 30.48%; 28.52%, 40.40%, and 67.74%; and 44.7%, 60.1%, and 83.7%, while the size fraction was − 106 + 74, − 74 + 47, and − 47 μm, respectively. It is clear to see that the finer the particles, the higher the copper extraction from the original sample. The reason for that might be that the surface area of the mineral dramatically increases with reducing the particle size in unit weight, i.e., the finner mineral particle has a more significant surface area interacting with the lixiviant. Therefore, the more dissolved oxygen and water contact with the chalcopyrite surface results in a significant copper leaching rate and enhances the chalcopyrite leaching kinetics. Additionally, as mentioned by previous researchers [29], for smaller particles, a larger surface area is available for interaction with the liquid, and the boundary layer is thinner, so the leaching rate would be faster. As can be seen from Table 2 in Sect. “Chalcopyrite leaching kinetics in a pressure-water system”, the rate constant fitting by the surface reaction model is 0.005 with a particle size of − 47 μm, which is 20 times higher than that with a coarse particle, indicating that reducing the particle size could significantly enhancing the leaching kinetics of chalcopyrite. On the other hand, as reported by Ji et al., the larger the mineral particle size, the easier the passivation layer formation causes [4]. Therefore, the chalcopyrite with a particle size of − 47 μm is selected as the sample for subsequent tests. On the other hand, the leaching rate of iron (not shown in this paper) was not significant in all of the tests, which was less than 5%.
Effect of Leaching Temperature
The influence of temperature on chalcopyrite oxidative leaching in a water system was conducted under the different conditions of 120, 140, 160, and 180 °C, while the particle size was -47 μm, total pressure was 1.5 MPa, stirring speed was 500 rpm, and leaching time was in a range of 0–90 min.
It can be seen from Fig. 3 that leaching temperatures have a significant effect on chalcopyrite leaching. The copper leaching rate was only 2.93%, 6.59%, and 9.66% at 120 °C for 30 min, 60 min, and 90 min, respectively. With increasing the temperature to 140, 160, and 180 °C, the leaching rate of copper changed to 9.85%, 22.80%, and 25.50%; 28.90%, 43.90%, and 47.10%; and 30.4%, 67.7%, and 83.70%, while the reaction time was 30, 60, and 90 min, respectively. However, it changed to around 84% with increasing the temperature to 180 °C. As seen, the dissolution of copper from chalcopyrite is a temperature-dependent process because the increased leaching temperature could accelerate the chemical reaction rate and diffusion rate of the reagent. Additionally, it can be seen from Fig. 3 that the increase of copper leaching efficiency at 120–160 °C is generally slow; however, the rise of the leaching efficiency of copper at 180 °C is always higher than that at a relatively lower temperature. On the other hand, as the results are given in Table 2 of Sect. “Chalcopyrite leaching kinetics in a pressure-water system”, the apparent rate constants are 0.004, 0.0011, 0.0025, and 0.0050 fitting with the chemical reaction model when the reaction temperature is set at 120, 140, 160, and 180 °C, respectively. It can be seen that the rate constant of 180 °C is 10 times higher than that of 120 °C, indicating the dissolution of chalcopyrite is a temperature-dependent reaction. The activation energy analyzed by the specific temperature was 62.34 kJ/mol, which is more than 40 kJ/mol [29–31], illustrating the surface reaction model controlled the reaction.
As reported by Han et al., the chalcopyrite could not wholly dissolve, or significantly more minor copper is released into the solution at a relevant lower temperature, caused by the formation of passive film such as S [27]. However, the passive film of S could be destroyed by higher oxidative conditions (higher temperature) and enhance the formation of sulfuric acid (see Eq. (2)) which could promote the dissolution of chalcopyrite. The generated sulfuric acid supports chalcopyrite leaching together with oxygen, according to Eq. (3), while the ferric sulfate was produced. Meanwhile, the ferric sulfate converts to ferrous sulfate by oxidation via Eq. (4), and the ferric sulfate is reformed via Eq. (5), in which a cycling system between a portion of ferric and ferrous ions was formed at a higher oxidation condition provided by higher temperature.
It can be expected that the leaching of chalcopyrite will be complete by increasing the temperature to a higher level, such as 200 °C; however, in consideration of the energy consumption and Autoclave, the 180 °C was selected as the optimal temperature for the following experiments.
XRD patterns of the residue obtained after 90 min-leaching at 140–180 °C are shown in Fig. 4. As seen in Fig. 4, the primary phase of the sample is still chalcopyrite when the leaching temperature is set at 120 °C and 140 °C, while one new peak of hematite is found in the residue. This finding is consistent with the leaching result that the maximum copper leaching rate is less than 20% at the temperature of 120 °C and 140 °C. More and more hematite peaks are generated in the leaching residue with increasing temperature, resulting from the hydrolyzation of ferric sulfate at these temperatures (around 180 °C) to forming hematite via Eq. (6). It is reported that the ferric sulfate could hydrolyze to hematite, hydronium jarosite, or basic ferric sulfate depending on the free acidity in the system [28]; however, only hematite is found in this research because of the specified conditions. Additionally, it is interesting to point out that several covellite peaks are found in the residue obtained from 180 °C -leaching (as shown in Figs. 4 and 5). A possible explanation for this might be that the leaching of chalcopyrite is not directly dissolving copper into solution, or not all of the chalcopyrite leaching via Eqs. (3) and (4), but some of the secondary copper sulfide minerals formed during the leaching or must pass a phase transition stage before releasing the copper ion (see Eqs. (6–11)) [2,3,4, 7, 30].
Effect of Total Pressure
The effect of total pressure on chalcopyrite oxidative leaching in a water system was conducted in a range of 0.8–2.0 MPa, while the particle size of − 47 μm, the temperature of 180 °C, stirring speed of 500 rpm, and leaching time of 0, 30, 60, and 90 min.
It can be seen from the result in Fig. 6 that the total pressure has a significant effect on the chalcopyrite leaching. The copper does not leach anymore when the total pressure is 0.8 MPa, i.e., vapor pressure only. However, the copper leaching rate increased with increasing the total pressure as well as extending the reaction time, and a maximum leaching rate of 93% was obtained at the condition of 2.0 MPa in water for 90 min. Additionally, the copper leaching rate at 1.0, 1.5, and 2.0 MPa for 30, 60, and 90 min dissolution is 4.5%, 17.6%, and 38.8%; 30.4%, 67.7%, and 83.0%; and 47.8%, 82.7%, and 93.2%, respectively. It can be seen that the efficiency of copper leaching depends significantly on the total pressure. It must be pointed out that the total pressure is the sum of the vapor and oxygen partial pressure; therefore, substantially, the dissolution of chalcopyrite is an oxygen partial pressure-dependent process. With the increasing oxygen partial pressure, the dissolved oxygen level, which will directly work with the minerals, might be improved. Therefore, the passive film of S could be quickly destroyed by the dissolved oxygen and formed as sulfuric acid (Eq. (2)), which will further promote the dissolution of chalcopyrite by the combined action with dissolved oxygen via Eq. (3), resulting in a high-efficiency chalcopyrite leaching. However, by increasing the total pressure from 1.5 to 2.0 MPa, the increased efficiency of copper leaching changed slightly compared to that of 0.8–1.0 MPa and 1.0–1.5 MPa, which is about 40% and 44%, respectively. It can be expected that if the oxygen partial pressure is further increased, the influence on the leaching will no longer be apparent. This result agrees with the observation made by some researchers who concluded that due to surface saturation by oxygen, leaching is not affected by oxygen partial pressure after a certain pressure [26].
The XRD patterns of the residue obtained at different total pressure after 90 min-leaching are shown in Fig. 7. It can be seen that the majority of the residue sepsis is still chalcopyrite, which is almost the same as the original sample without leaching. Different from the case of 0.8 MPa, the peaks of pyrite and covellite are found in the residue obtained at 1.0 MPa after 90 min-leaching. However, the peaks of pyrite and covellite disappeared, and the majority phase in the residue changed to hematite when the total pressure was set to 1.5 and 2.0 MPa (Fig. 8).
Effect of Stirring Speed
The influence of stirring speed on chalcopyrite oxidative leaching in a water system was conducted under the different conditions of 300, 500, 700, and 900 rpm, while the particle size was − 47 μm, total pressure was 1.5 MPa, and the leaching time was in a range of 0–90 min. The results are presented in Fig. 9.
The leaching rate of copper increased with adjusting the stirring speed from 300 to 700 rpm, and the leaching rate reached 96% at 700 rpm for 90 min. However, a similar result was obtained when increasing the stirring speed to 900 rpm compared to 700 rpm. The XRD patterns of the residue obtained at different stirring speeds after 90 min-leaching are shown in Fig. 10. It can be seen that compared with the original sample, covellite peaks were generated in the residue obtained after 90 min-leaching at 300 rpm. However, the majority phase of the residue changed to hematite, and the covellite peak disappeared, increasing the stirring speed to a higher level, such as 500, 700, and 900 rpm.
Chalcopyrite Leaching Kinetics in a Pressure-Water System
Experimental data were analyzed using a shrinking core model expressed in Eq. (12) to determine the mechanism of copper leaching from chalcopyrite under the pressure oxidative water system.
The apparent rate constants and correlation coefficients were calculated using Eq. (13) and Eq. (14), representing the surface chemical reaction and product layer diffusion control models.
where kr and kd are the rate constant of the reaction (min−1), t is the reaction time (min), and α is the leaching rate.
The activation energy of the chalcopyrite leaching can be calculated according to the Arrhenius plot given in Eq. (15).
where k is the rate constant of reaction (min−1), A is the frequency factor, R is the isobaric heat capacity of an ideal gas (8.314 kJ/mol), \({E}_{a}\) is the apparent activation energy (kJ/mol), and T is the thermodynamic temperature (K).
The results obtained from regression and correlation analysis using the different kinetic models are summarized in Table 2. The results show that the linear coefficients between particle size, temperature, total pressure, and stirring speed is greater than 0.97, 0.96, 0.97, and 0.95, respectively, when fitting the data using the surface chemical reaction model. In the case of fitting using the product layer diffusion-controlled model, the linear correlation coefficients between particle size, temperature, total pressure, and stirring speed is greater than 0.94, 0.94, 0.78, and 0.95, respectively. However, results suggest that there is little difference in the linear correlation coefficient between the product layer diffusion control model and the surface chemical reaction control model. In addition, to study the influence of temperature on the reaction rate of chalcopyrite, the Arrhenius equation calculated the apparent activation energy of chalcopyrite according to kr and kd. Figure 11 shows the Arrhenius plots o ln k versus 1000/T for the chalcopyrite leaching by fitting the chemical reaction and product layer diffusion model. The activation energies of the product layer diffusion and surface chemical reaction control models are 121.34 and 62.34 kJ/mol, respectively. However, the activation energy of chalcopyrite under the product layer diffusion control model is always less than 20 kJ/mol, which indicates that the diffusion model may not control chalcopyrite leaching. Additionally, activation energy (62.34 kJ/mol) calculated by fitting the chemical reaction modal confirms that the chemical reaction model can represent the chalcopyrite leaching process. This result is consistent with the data obtained by Ji et al. [4].
To determine the reaction order, a linear correlation plot between the logarithms of the slope of a line and the logarithms of temperature is created (see Fig. 12). The empirical reaction order concerning different temperatures by fitting the chemical reaction model is approximately 18.0, which indicates that the temperature is the most important factor for chalcopyrite pressure oxidative leaching in water.
Conclusion
This research conducted a leaching and kinetics study of copper dissolution from chalcopyrite under pressure oxidative conditions in a water system. The results can be summarized as follows.
(1) An efficient copper dissolution of about 96% was achieved under pressure oxidative conditions only in an H2O media, i.e., without any addition of oxidant such as sulfuric acid.
(2) The dissolution mechanism of chalcopyrite from the chalcopyrite has been confirmed to be a multistage reaction, i.e., chalcopyrite first transformed into sulfides such as CuS and FeS2. Then the newly formed FeS2 acts as a sulfuric acid source to promote the subsequent copper leaching. The passivation layer in the chalcopyrite leaching process is mainly Fe2O3, CuS, and FeS2; however, both of these do not or less affect copper leaching at all.
(3) The leaching kinetics of chalcopyrite under a pressure water system can be expressed by the surface chemical control model, i.e., 1 − (1 − α)(1/3), and its apparent activation energy is 62.34 kJ/mol.
(4) Over 90% of iron from chalcopyrite was deposited as Fe2O3, and only less than 5% was extracted into the pregnant leach solutions, which indicated that copper could selectively separate from iron by pressure oxidative water leaching.
References
Schipper BW, Lin HC, Meloni MA, Wansleeben K, Heijungs R, van der Voet E (2018) Estimating global copper demand until 2100 with regression and stock dynamics. Resour Conserv Recy 132:28–36. https://doi.org/10.1016/j.resconrec.2018.01.004
Ji GX, Liao YL, Wu Y, Xi JJ, Liu QF (2022) A Review on the research of hydrometallurgical leaching of low-grade complex chalcopyrite. J Sustain Metall 8(3):964–977. https://doi.org/10.1007/s40831-022-00561-5
Xi JJ, Liao YL, Ji GX, Liu QF, Wu Y (2022) Mineralogical characteristics and oxygen pressure acid leaching of low-grade polymetallic complex chalcopyrite. J Sustain Metall 8(4):1628–1638. https://doi.org/10.1007/s40831-022-00594-w
Ji GX, Liao YL, Xi JJ, Liu QF, Wu Y, Ma HF et al (2023) Behavior and kinetics of copper during oxygen pressure leaching of complex chalcopyrite without acid. J Sustain Metall 9(1):350–362. https://doi.org/10.1007/s40831-023-00658-5
Chen GB, Sun JR, Yang HY, Ma PC, Gao SX (2021) Rapid atmospheric leaching of chalcopyrite using a novel reagent of trichloroisocyanuric acid. Minerals. https://doi.org/10.3390/min11091012
Toro N, Pérez K, Saldaña M, Jeldres RI, Jeldres M, Cánovas M (2020) Dissolution of pure chalcopyrite with manganese nodules and waste water. J Mark Res 9(1):798–805. https://doi.org/10.1016/j.jmrt.2019.11.020
Yang CR, Jiao F, Qin WQ (2018) Leaching of chalcopyrite: an emphasis on effect of copper and iron ions. J Cent South Univ 25(10):2380–2386. https://doi.org/10.1007/s11771-018-3922-5
Abdelraheem MTO, Agacayak T (2023) Investigation of the effect of some polar organic solvents on the leaching and dissolution kinetics of chalcopyrite in hydrogen peroxide and sulfuric acid solution. B Chem Soc Ethiop 37(3):779–788. https://doi.org/10.4314/bcse.v37i3.19
Torres D, Ayala L, Jeldres RI, Cerecedo-Saenz E, Salinas-Rodriguez E, Robles P et al (2020) Leaching chalcopyrite with high MnO2 and chloride concentrations. Metals. https://doi.org/10.3390/met10010107
Ma PC, Yang HY, Luan ZC, Sun QF, Ali A, Tong LL (2021) Leaching of chalcopyrite under bacteria-mineral contact/noncontact leaching model. Minerals. https://doi.org/10.3390/min11030230
Atesoglu G, Atilgan I (2022) Effect of roasting temperature on the leaching of chalcopyrite concentrate in sulphuric acid. Min Metall Explor 39(5):2199–2208. https://doi.org/10.1007/s42461-022-00669-y
Cheloni LMDS, Martins FL, Pinto LM, Rodrigues MLM, Leao VA (2023) Chemical and biological leaching of chalcopyrite- elemental sulfur reaction products. Miner Process Extr Metall Rev. https://doi.org/10.1080/08827508.2023.2181347
Noguchi H, Okibe N (2020) The role of bioleaching microorganisms in saline water leaching of chalcopyrite concentrate. Hydrometallurgy. https://doi.org/10.1016/j.hydromet.2020.105397
Esmailbagi MR, Schaffie M, Kamyabi A, Ranjbar M (2018) Microbial assisted galvanic leaching of chalcopyrite concentrate in continuously stirred bioreactors. Hydrometallurgy 180:139–143. https://doi.org/10.1016/j.hydromet.2018.07.020
Zhang RY, Mao YY, Liu C, Ni W (2022) Synergistic catalytic effect of chloride ion and ammonium ion on the leaching of chalcopyrite in sulfuric acid solution. Miner Eng. https://doi.org/10.1016/j.mineng.2022.107686
Chen H, He JF, Zhu LT, Liu B, Zhou K, Xu J et al (2022) Eco-friendly oxidation leaching from chalcopyrite powder and kinetics assisted by sodium chloride in organic acid media. Adv Powder Technol. https://doi.org/10.1016/j.apt.2022.103547
Ma YL, Yang Y, Fan R, Gao XY, Zheng L, Chen M (2021) Chalcopyrite leaching in ammonium chloride solutions under ambient conditions: Insight into the dissolution mechanism by XANES Raman, spectroscopy and electrochemical studies. Miner Eng. https://doi.org/10.1016/j.mineng.2021.107063
Marzoughi O, Li L, Pickles C, Ghahreman A (2022) Thermal treatment of lanxess lewatit (R) AF 5 resin used in the atmospheric chalcopyrite leaching process: regeneration and sulfur recovery. Chemosphere. https://doi.org/10.1016/j.chemosphere.2022.133890
Toledo AGR, Tayar SP, Arena FA, Benedetti AV, Bevilaqua D (2022) New insights into oxidative-reductive leaching of chalcopyrite concentrate using a central composite factorial design. Miner Eng. https://doi.org/10.1016/j.mineng.2022.107467
Ruiz-Sanchez A, Lapidus GT (2022) Electrochemical and leaching studies to better understand the role of ethylene glycol in the oxidative acid dissolution of chalcopyrite. Electrochim Acta. https://doi.org/10.1016/j.electacta.2022.140343
Turan MD, Sari ZA, Nizamoglu H (2021) Pressure leaching of chalcopyrite with oxalic acid and hydrogen peroxide. J Taiwan Inst Chem Eng 118:112–120. https://doi.org/10.1016/j.jtice.2020.10.021
Mojtahedi B, Rasouli S, Yoozbashizadeh H (2020) Pressure leaching of chalcopyrite concentrate with oxygen and kinetic study on the process in sulfuric acid solution. Trans Indian Inst Met 73(4):975–987. https://doi.org/10.1007/s12666-020-01882-3
Bai YL, Wang W, Xie F, Lu DK, Jiang KX, Dreisinger D (2022) In-situ electrochemical study of chalcopyrite pressure oxidation leaching from 110 degrees C to 150 degrees C under saturated vapor pressure. Arab J Chem. https://doi.org/10.1016/j.arabjc.2022.104139
Moraga GA, Jamett NE, Hernandez PC, Graber TA, Taboada ME (2021) Chalcopyrite leaching with hydrogen peroxide and iodine species in acidic chloride media at room temperature: technical and economic evaluation. Metals. https://doi.org/10.3390/met11101567
Nurtazina N, Uvarov N, Azhigulova R, Tyapkin P (2022) Chalcopyrite leaching by amino acid solutions in the presence of hydrogen peroxide. Physicochem Probl Miner Process. https://doi.org/10.37190/ppmp/157067
Han B, Altansukh B, Haga K, Takasaki Y, Shibayama A (2017) Copper recovery from silicate-containing low-grade copper ore using flotation followed by high-pressure oxidative leaching. Resour Process 64(1):3–14. https://doi.org/10.4144/rpsj.64.3
Han B, Altansukh B, Haga K, Takasaki Y, Shibayama A (2017) Leaching and kinetic study on pressure oxidation of chalcopyrite in H2SO4 solution and the effect of pyrite on chalcopyrite leaching. J Sustain Metall 3(3):528–542. https://doi.org/10.1007/s40831-017-0135-3
Han BS, Altansukh B, Haga K, Stevanovic Z, Jonovic R, Avramovic L et al (2018) Development of copper recovery process from flotation tailings by a combined method of high-pressure leaching solvent extraction. J Hazard Mater 352:192–203. https://doi.org/10.1016/j.jhazmat.2018.03.014
Faraji F, Alizadeh A, Rashchi F, Mostoufi N (2022) Kinetics of leaching: a review. Rev Chem Eng 38(2):113–148. https://doi.org/10.1515/revce-2019-0073
Quezada V, Roca A, Benavente O, Cruells M, Melo E (2021) The effects of sulphuric acid and sodium chloride agglomeration and curing on chalcopyrite leaching. Metals. https://doi.org/10.3390/met11060873
Acknowledgements
This work was supported by the Outstanding Young Talents Project of University of Science and Technology Liaoning (Grant Number 2021YQ05).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
The contributing editor for this article was Zhongwei Zhao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miao, J., Leng, H. & Han, B. Leaching and Kinetic Study of Chalcopyrite Without Acid in an O2–H2O System. J. Sustain. Metall. 9, 1279–1288 (2023). https://doi.org/10.1007/s40831-023-00730-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00730-0