Abstract
The magnetic separation tailings of saprolite laterite ore containing magnesium processed by roasting and magnetic separation are used as raw materials, and the product of magnesium oxide was obtained by the process of sulfuric acid leaching, removing iron, precipitating magnesium and roasting. The effects of sulfuric acid concentration, leaching temperature, leaching time, liquid–solid ratio, and stirring speed on the extraction degree of magnesium, nickel, and iron during magnetic separation tailings acid leaching were investigated. The optimal leaching conditions are as follows: the temperature of leaching was 80 °C, the time of leaching was 4 h, the sulfuric acid concentration was 15%, the liquid–solid ratio was 10:1 (mL g−1), and the stirring speed was 450 rpm. The iron in magnesium sulfate solution was removed by a neutralization hydrolysis method. The effect of different alkali addition on the precipitation of basic magnesium carbonate was investigated. The results showed that the addition of ammonium carbonate shortened the precipitation time and the precipitation efficiency of magnesium was relatively high, reaching 99.2% over 1.75 h. XRF analysis of basic magnesium carbonate (Mg2(OH)2CO3.H2O) showed that the precipitation efficiency of magnesium in the leaching solution reaches 98.6% after impurity removal.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnesium is an alkaline earth metal, and magnesium oxide is its alkali metal oxide. It is tasteless, nontoxic and odorless white solid at normal atmospheric temperature. Magnesium can be found in the form of chlorides, silicates, hydroxides, sulfates and carbonates. The most common magnesium minerals are dolomite (Ca Mg (CO3)2), magnesite (MgCO3), brucite (Mg (OH)2), carnallite (MgCl2·KCl·6H2O), bischofite (MgCl2·6H2O), olivine ((Mg, Fe)2SiO4), and ferromagnesian minerals that are within the serpentinite continuum ((Mg, Fe)3Si2O5(OH)4) [1,2,3]. These magnesium ore minerals are also the main raw material for smelting metal magnesium and making refractory materials. Magnesium oxide is soluble in acid and ammonium salt solutions and difficult to dissolve in water. Its solubility in water depends on the content of carbon dioxide in water. With the increase of carbon dioxide content, the solubility of magnesium oxide increases and it is accompanied by the production of Mg (OH)2. It exists in the air for a long time and is easy to absorb water and carbon dioxide in the air and gradually changes into basic magnesium carbonate.
At present, pyrometallurgy is mainly used to extract ferronickel from low-grade laterite ore of silicon magnesium type. In addition, additives such as sodium carbonate (Na2CO3), calcium carbonate (CaCO3), sodium chloride (NaCl), calcium chloride (CaCl2), and sodium sulfate (Na2SO4), as well as carbonaceous reducing agent, were used to selectively reduce nickel and iron from laterite ore to increase the migration and aggregation effect of ferronickel. Higher recovery extent of nickel and iron were obtained than that of applying only carbon as reductant. However, there are few studies on extracting magnesium from tailings of laterite nickel ore [4,5,6,7,8].
Pyrometallurgical processes extract iron and nickel from saprolite laterite ore. It means that the magnesium in the ore is difficult to be fully utilized, but the content of magnesium in the ore is generally high (30%–50%). The content of MgO in the low-grade laterite ore used in this paper is about three times of the total content of nickel and iron. Therefore, magnesium has high value in the saprolite laterite ore, and it is difficult to maximize the value of the ore if magnesium is abandoned [9]. China is a leading country in owning and producing magnesium in the world, producing approximately 900 kt per year as metal, and over 11,000 kt per year as a compound. Its magnesium output accounts for about 80% of the whole world [10]. Hydrometallurgy is mainly used to extract magnesium from ore [11,12,13,14,15,16]. At present, primary magnesium production from ores consisted of hydrometallurgical extraction followed by either thermal reduction or molten-salt electrolysis [17]. The most common method used for MgO production is the calcination of magnesite (MgCO3 → MgO + CO2) [18]. Some scholars also had studied the leaching efficiency of different acids (H2SO4, HCl, HNO3, HCOOH and CH3COOH, etc.) and ammonia salts on serpentine. They proved that sulfuric acid had better leaching results. The leaching efficiency reached between 75 and 94% [19,20,21,22,23,24]. The leaching temperature was generally controlled below 100 °C. The optimum leaching temperature of this research was about 80 °C. Teir et al. [25] studied that magnesium silicate was leached with hydrochloric acid and magnesium was precipitated with sodium hydroxide and carbon dioxide. Magnesium precipitated in the form of hydromagnesite (MgCO3·Mg(OH)2·4H2O), and achieved the purpose of carbon sequestration. And the acid concentration was between 1 and 3 M. They choosed to extract magnesium directly from serpentine. In this research, nickel and iron were extracted from serpentine minerals and magnesium was leached with sulfuric acid from tailings, which greatly reduced the influence of impurities on magnesium recovery. In this research, the leaching efficiency of magnesium is more than 99% which is higher than the average level. And the sulfuric acid concentration of this research is about 1.5 M which is lower enough.
Before pyrometallurgical or hydrometallurgical smelting, laterite ores often need to be beneficiated to enrich valuable metals according to different ore types. These beneficiation methods include sink-float (or dense media) separation, gravity separation, magnetic separation, electrostatic separation, (roasting) and flotation [26,27,28,29,30]. For silicon magnesium laterite ore with nickel content less than 1%, beneficiation seems to be of little significance. Comparing with previous studies, when treating laterite ore with nickel content less than 1%, pyrometallurgical smelting is used for pretreatment to eliminate the influence of nickel and iron on the subsequent extraction of magnesium with higher leaching degree and higher recovery of Ni, Fe and Mg in this research. A new process for comprehensive extraction of nickel, iron and magnesium from laterite nickel ore was proposed. The intermediate product high-grade ferronickel concentrate can be used for steelmaking and the final product magnesium oxide can be used for construction and industry, etc.
The present work is a fundamental research of magnesium extraction from tailings of silicon magnesium laterite ore, the objectives of this work are to obtain the technological process of sulfuric acid leaching, removing iron by neutralization hydrolysis, precipitation of magnesium and roasting the precipitate of magnesium and realize the comprehensive recovery and utilization of low-grade laterite ore. The effects of sulfuric acid concentration, leaching temperature, leaching time, liquid–solid ratio, and stirring speed on the extraction degree of Mg, Ni and Fe were investigated. It provided technical support for the efficient recovery and comprehensive utilization of a large number of silicon magnesium laterite nickel ores.
Materials and Methods
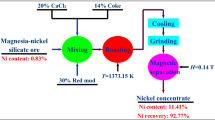
The composition of low-grade laterite ore from Yuanjiang, Yunnan, China was tested by chemical analysis. Under 7% of CaF2 for the adjuvant agent and 8% of anthracite for the reducing agent, the main metal nickel and iron in laterite ore (100 g) can be efficiently recovered after high temperature (1250 °C) reduction. The process is shown in Fig. 1. The reactions taking place at reduction stage are given in Eqs. (1)–(3), then the CaF2 has a complex chemical reaction. The relevant content will be studied later. The magnetic separation tailings that were finely ground (Passing 200 mesh sieve) and magnetically separated were used as raw materials for the acid leaching and magnesium precipitation process, and the chemical composition of the tailings was tested. The phase composition of raw ore and tailings was analyzed by XRD (Cu-Kα ray source, voltage of 35 kV, current of 20 mA, scanning speed of 10°/min, the diffraction angle (2θ) was scanned from 10° to 90°), the weight loss curve of raw ore was analyzed by TG-DSC (mass: 100 mg; heating rate: 10 °C/min), and the element distribution of tailings was further understood by EPMA (JXA-8230)
The magnetic separation tailings were used as raw materials for the acid leaching of magnesium. The waste liquid from magnetic separation was filtered by vacuum, and the filter residue was washed by deionized water. After that, it was placed into a drying oven for 48 h. Then, it was removed and finely ground in a vibration mill (XZM-100). The particle sizes of mineral powder were less than 0.074 mm passing through a 200-mesh sieve. When the reaction solution in the beaker was preheated to the same temperature as the water bath pan, the experiment began. Then 30 g of the prepared magnetic separation tailings were placed in the beaker. After that, the height of the Teflon stirring paddle and the stirring speed should be set up. After about 4 h, the acid leaching experiment was finished. And the main chemical reactions during acid leaching are Eqs. (4)–(8). The beaker was removed and the reaction solution was filtered by vacuum method to obtain magnesium sulfate solution and filter residue. A neutralization hydrolysis method was used to remove iron, and then H2O2 was added as a strong oxidant to the magnesium sulfate solution, the iron oxide which had not reduced or the metal iron that had not been completely separated by magnetic separation in the magnetic separation tailings, would be completely oxidized to Fe3+, so as to facilitate the subsequent removal of iron. The oxidation liquid stood for 10 min to ensure the oxidation reaction was complete. Ammonia was added to adjust the pH to 3–4 to form Fe (OH)3 floccules, which was separated by vacuum filtration to obtain refined magnesium sulfate solution. The reactions taking place at iron removal are given in Eqs. (9)–(11). During the process of magnesium precipitation, ammonia was added to the refined magnesium sulfate solution to adjust the pH value to 9.6–9.7, and then (NH4)2CO3 was added until the pH adjustment was complete. Then, the solution became a white suspension. After vacuum filtration, the liquid of the white suspension was drained. The rest of the solid and white material was placed to obtain white crystal in the drying oven at 105 °C for 6 h. After chemical composition analysis, it was shown to be Mg2(OH)2CO3•H2O. The reactions taking place at precipitation of hydromagnesite are given in Eqs. (12)–(13). Magnesium oxide was obtained by calcining basic magnesium carbonate. The reactions taking place at preparation of magnesium oxide are given in Eqs. (14). The experimental process is shown in Fig. 2.
Acid leaching
Iron removal
Precipitation of hydromagnesite:
Preparation of magnesium oxide:
Results and Discussion
Analysis of Low-Grade Laterite Ore and Magnetic Separation Tailings
The chemical analysis of low-grade laterite ore and magnetic separation tailings are shown in Tables 1 and 2. The nickel was reduced from 0.82% of the original ore to 0.3% of the tailings, and the iron content was reduced from 9.7 to 5.0% by magnetic separation. And, in the process of magnetic separation, most of CaF2 went into the magnetic separation tailings, and the other part went into the ferronickel concentrate. The CaF2 decomposes into Ca2+ and F − at high temperature. F− and O2− have the same ionic radius. In the reduction process, F − enters into the silicate structure which composed of Si–O tetrahedral structure. Reducing the bond energy of Si–O makes it easier to fracture at high temperature, so as to reduce the melting point of silicate. In the actual reduction process, the CaF2 reacts complicatedly with the serpentine (Mg3Si2O5(OH)4) of gangue phase to form tremolite (CaxMgy(Si4O11)2F2, X \(\approx \) 2; y \(\approx \) 5) which is low melting point (about 887 °C). Then the decrease of the melting point of the whole reduction system can increase the diffusion coefficient of atoms, accelerates the atomic movement rate between reactants, so as to promote the precipitation of metal particles along the boundary, and the rapid aggregation between particles [31, 32]. Then CaF2 of magnetic separation tailings entered to iron hydroxide precipitation and remaining solution after magnesium precipitation. CaF2 was mostly removed.
The XRD results of magnetic separation tailings and ferronickel alloy are shown in Figs. 3, 4. By analyzing the XRD and thermogravimetry DSC curves of raw laterite in Fig. 5, the ore components represent a weight loss of about 15%. It can be seen that the mass loss is mainly before 600 °C, and there are two endothermic reactions prior to 600 °C. The first endothermic reaction is free water that begins to be removed at about 103 °C. The second endothermic reaction is that the crystal water and structural water in the mineral are basically removed. The serpentine structure in the raw ore is dehydroxylated at 581.7 °C, and its structure is decomposed to produce free and highly active MgO and SiO2. The laterite ore had experienced the process of high temperature decomposition and recombination under the action of CaF2 and anthracite. The structure of the dissociated magnesium oxide and silica are changed to a glassy state at 600 °C, the serpentine structure in the original laterite ore decomposed and recombined in the reduction process and the dissociated magnesium oxide and silica recrystallize became Mg2SiO4 and enstatite MgSiO3 with increasing temperature [33]. The specific chemical reactions are as follows:
Some forsterites are associated with peridotite. The NiFe2O4 was formed by the reaction of NiO and Fe2O3 from the decomposition of serpentine. At the same time, because some iron oxide was not completely reduced, it remained in gangue in the form of Fe3O4. Iron was the main impurity element in the magnesium sulfate leaching solution.
The magnetic separation tailings were analyzed by electron probe microanalysis, and the characterization results are shown in Fig. 6. The representative points were selected for electron probe analysis. The detection positions are shown in Fig. 6a, b and c. The data of elemental mass percentage at different locations are shown in Table 3. There are points 3 and points 4 in Fig. 6a, point 2 in Fig. 6b and c. According to the energy spectrum data analysis, the color contrast of these four points is slightly deeper than that of the surrounding area, which is black and gray. The color regions with the same contrast at these points are all forsterite material, and the small areas are dense structures. Around the four darker spots, the energy spectrum data of five points are printed in the gray area, which are point 2 in Fig. 6a, point 1 in Fig. 6b, and points 1 and 3 in Fig. 6c. It is different from the darker region, and there are more calcium and aluminum elements. In the SEM (scanning electron microscope) images, the contrast of the micro region will be brighter because there are more elements from the back of the Periodic Table. Therefore, this region is forsterite. The Ca and Al contained in the micro zone replace Mg in forsterite with an isomorphic structure. Point 1 in Fig. 6a shows the paragenesis of forsterite and fayalite. Point 3 in Fig. 6b and point 4 in Fig. 6c are ferronickel particles, and there is a small amount of residual in the magnetic separation tailings. The reason is that the nickel and iron elements in the clay mineral are wrapped by a mass of serpentine and silica, and after high temperature reduction roasting, the elemental Ni and Fe become metal particles, which can be very small. At the same time, serpentine is decomposed and recrystallized as magnesium olivine and pyroxene. In the fine grinding process, the small particles are still coated with magnesium olivine and pyroxene. Then the magnetic field strength is small for the finer and coated metal particles, so a portion of the ferronickel particles enter the tailings. Combined with the comprehensive analysis of XRD of magnetic separation tailings, the main phase of magnetic separation tailings is forsterite, but a small amount of iron olivine coexists with it, and there are small amounts of Ca, Al, and a small amount of Fe–Ni alloy particles.
Leaching Experiment
The above research results had fully demonstrated that the extraction of ferronickel from minerals could improve the leaching efficiency of magnesium. For exploring the influence of sulfuric acid concentration, leaching temperature, leaching time, liquid–solid ratio, and stirring speed on the extraction degree of magnesium, nickel and iron, the following studies were performed.
The Effect of Sulfuric Acid Concentration
The above research results had fully demonstrated that high temperature reduction and fine grinding magnetic separation could reduce the impurity composition of the minerals containing magnesium and effectively improved the leaching efficiency of magnesium. To further explore the influence of sulfuric acid concentration, leaching temperature, leaching time, liquid–solid ratio, and stirring speed on the extraction degree of magnesium and iron, the following studies were performed.
According to the experimental results in Fig. 7, increased sulfuric acid concentration augments the magnesium extraction kinetics, but there are points of diminishing returns, above which the marginal increases in extraction rate is insignificant or extraction of impurities becomes problematic [10]. The content of ferronickel in the tailings is low, so the leaching of ferronickel is completed soon, while the content of Mg is large, and exist with the form of Mg2SiO4 and MgSiO3. With the increase of sulfuric acid concentration, the formation of H4SiO4, H2SiO3 and CaSO4 solids hinder the subsequent leaching reaction. The extraction degree of magnesium presents a continuous downward trend when the sulfuric acid concentration is greater than 15%. However, the effect of sulfuric acid concentration on the extraction degree of Ni and Fe is not obvious, which is maintained at about 98% and 70%, respectively. However, the leaching of Ni and Fe have been basically completed when the sulfuric acid concentration is 15%. It can be seen from Fig. 7 that when the sulfuric acid concentration is 15%, the magnesium extraction degree reached a maximum, so a sulfuric acid concentration of 15% is selected as the optimal sulfuric acid concentration.
The Effect of Leaching Temperature
The effect of different leaching temperatures on the extraction degree of magnesium, iron, and nickel from magnetic separation tailings under atmospheric pressure is shown in Fig. 8. The experiment was carried out in a water bath to maintain a steady temperature during the experiment.
According to the results in Fig. 8, with increased leaching temperature from 40 to 90 °C, the extraction degree of magnesium also increases from 62.6 to 99.9%. With the increasing temperature, the extraction degree of magnesium reached a maximum at 90 °C. Ribeiro et al. [21] studied the variation of Gibbs free energy of NiSO4, Fe2(SO4)3 and MgSO4. With the increase of temperature, their Gibbs free energy decreases. So, with the increase of temperature, it is conducive to form NiSO4, Fe2(SO4)3 and MgSO4. The chemical reaction between metal oxides and sulfuric acid is more thorough. The extraction degree of Ni, Fe, and Mg increase with leaching temperature and tend to plateau at 80–90 °C. The extraction degree of Ni increases from 81.3% at 40 °C to 96.4% at 80 °C. The extraction degree of Fe increases from 48.9 to 69.9% over the same temperature range. The extraction degree of Ni and Fe reach a maximum at 80 °C. When the temperature increases to 80 °C, the extraction degree of magnesium reach a higher level. Considering the economic benefits and extraction degree of Ni and Fe, the optimal leaching temperature is selected as 80 °C.
The Effect of Leaching Time
The effect of leaching time on extraction degree of magnesium, iron, and nickel from magnetic separation tailings under atmospheric pressure is shown in Fig. 9.
It can be seen from the leaching results in Fig. 9 that the extraction degree of magnesium reaches 87.1% after 1 h, which is a higher extraction degree, and the highest value of 99.9% is achieved after 6 h. Between 1 and 6 h, the extraction degree has increased 12.8%. The reason is that the forsterite structure is gradually separated with extended leaching time, and the MgO material is exposed. So, the contact area between MgO and sulfuric acid reactant increased, and the chemical reaction generated magnesium sulfate, which is a typical unreacted core model in metallurgical kinetics. With extended leaching time, the reaction between magnesium oxide and sulfuric acid reached equilibrium, and the extraction degree also tended to be stable. The extraction degree of Ni has reached 99% for 1 h, and there is no significant change with extended leaching time, which is due to the fact that nickel attached to the surface of forsterite reacts preferentially to Fe and reaches equilibrium [21]. The extraction degree of Fe is 61% after 1 h and increased to 69.9% after 5 h. It can be seen from Fig. 9 that the change in magnesium extraction degree is not obvious after 4 h. After comprehensive consideration, the optimal leaching time is chosen as 4 h.
The Effect of Liquid–Solid Ratio
The effect of the ratio of sulfuric acid and magnetic separation tailings (liquid–solid ratio in mL/g) on magnesium extraction degree under atmospheric pressure is shown in Fig. 10.
The leaching results in Fig. 10 shows that when the liquid–solid ratio is 2:1, the magnesium extraction degree is 38.0%, and a large amount of Mg remains in the leaching residue. When the liquid–solid ratio increases to 4:1, the magnesium extraction degree increases to 78.3%. As the liquid–solid ratio continued to increase to 8:1, the magnesium extraction degree changes greatly. When the liquid–solid ratio increases from 8:1 to 10:1, the extraction degree of Mg tends to be stable. The extraction degree of magnesium reaches a maximum value of 98.1% when the liquid–solid ratio reaches 10:1. With increasing liquid–solid ratio, the concentration of Ni2+, Fe3+ and Mg2+ is lower, which is conducive to the continuous leaching reaction. Therefore, the extraction degree of nickel, iron and magnesium increases with the liquid–solid ratio. However, excessive liquid–solid ratio will also increase the amount of leaching solution [34]. The extraction degree of Ni reaches a maximum of 99.6% when the liquid–solid ratio is 8:1. The extraction degree of Fe reaches a maximum of 75.1% when the liquid–solid ratio is 9:1. The optimum liquid–solid ratio is determined as 10:1.
The Effect of Stirring Speed
The effect of stirring speed on the extraction degrees of magnesium, iron, and nickel from magnetic separation tailings in acid leaching under atmospheric pressure is shown in Fig. 11.
Figure 11 shows that the leaching extent of Mg has reached a high value of 95.3% at a stirring speed of 150 rpm. With increasing of stirring speed, the leaching extent of Mg slightly increases, but the change is not obvious. When the stirring speed increases from 150 to 550 rpm, the overall leaching extent of magnesium increases by 4.6%. So, the thermodynamic conditions has a great influence on the reaction process, while the kinetic conditions have a small influence. The leaching extent of Ni and Fe increases by 5.9% and 5.5%, respectively, from 93.9% and 54.1% to 99.8% and 59.6%, respectively. Considering the leaching extent of magnesium and the actual experimental conditions, 450 rpm is selected as the optimal stirring speed.
Optimization Experiment of Leaching Conditions
According to the results of the single factor experiment, the optimal technological parameters of sulfuric acid leaching were obtained, and the magnetic separation tailings obtained by reduction roasting magnetic separation of laterite ore were determined. The optimal leaching process conditions of sulfuric acid leaching were as follows: leaching temperature 80 °C, leaching time 4 h, sulfuric acid concentration 15%, liquid–solid ratio 10:1 (mL/g), stirring speed: 450 rpm. To verify the reliability of these parameters and the stability of the leaching experiments, three groups of repeated experiments were carried out under optimal leaching conditions. The extraction degrees of Mg, Ni and Fe were calculated and their average values were taken. The experimental results are shown in Table 4.
By analyzing the extraction degree results of three groups of optimized repeated parallel experiments, it can be seen that the acid leaching experiment has good repeatability, and the extraction degree of Mg, Ni, and Fe and each process parameter index are relatively stable, which is consistent with the expected experimental results.
Impurity Removal from Acid Leaching Solution
Element Composition in Leaching Solution
The magnetic separation tailings obtained from laterite ore by reduction roasting-fine grinding magnetic separation are used as raw materials for sulfuric acid leaching of magnesium. Due to the first step of nickel and iron separation from laterite ore, the residual nickel and iron elements in magnetic separation tailings are greatly reduced, but there is still a small amount of residual. The chemical element analysis of magnesium sulfate leaching solution obtained by filtration separation after acid leaching was carried out. The elemental content in the leaching solution is shown in Table 5.
The contents of main elements in the acid leaching solution of magnetic separation tailings were detected and analyzed. Due to the low grade of nickel in the raw ore, the remaining nickel content in the magnetic separation tailings after magnetic separation was very low. Although basically 99% of the nickel completely reacted with sulfuric acid and entered the acid leaching solution, because of its small content, it did not affect the final magnesium products. From the elemental analysis of the leaching solution, it could be found that magnesium was the main metal element, and nickel and manganese were less, while iron was the main impurity element in the magnesium sulfate leaching solution. Therefore, the removal of iron was shown to be the main problem of the impurity removal process.
Iron Removal by Neutralization Hydrolysis Method
Some researchers also used calcium carbonate and sodium hydroxide to remove Fe, Al, Cr and other impurity elements [35]. Impurity elements such as calcium and sodium were added into the system. It was not conducive to magnesium precipitation. In this research, adjusting pH with ammonia and precipitating magnesium with ammonium carbonate was the main technical means of this research. Considering that the pH requirement of magnesium precipitation experiment was in alkaline environment, neutralization hydrolysis method was used to precipitate iron in alkaline environment without introducing new impurity ions and controlling economic cost. In the same way, a strong oxidant, H2O2, was added into the acid leaching solution of magnetic separation tailings to fully oxidize Fe2+ to Fe3+. The reactions taking place at iron removal are given in Eqs. (8)–(10). The beaker was preheated in a water bath, and the oxidized acid leaching solution was preheated to the preset temperature of the water bath at 90 °C. Ammonia was slowly added, and the pH value of the solution was adjusted. The chemical reaction between reactants was promoted in the process of adjusting pH, and then it was found that red flocculant substances would be produced and not redissolve. With the dripping of ammonia, red flocculent substances accumulated and gradually grew to fill the whole leaching solution. The pH value is between 3.3 and 4.0, and the solution is stirred continuously to cause iron ions precipitate as completely as possible. After the precipitation is completely separated by vacuum filtration, red filter cake and the solution of iron removal, which is colorless and transparent, are obtained, as shown in Fig. 12.
The precipitation of Fe (OH)3 obtained by neutralization hydrolysis method was analyzed by XRF, and the chemical elements of the solution after iron precipitation were analyzed. The main elemental contents are shown in Tables 6 and 7. In the solid precipitate, the main elements are oxygen, iron, sulfur, and small concentrations of fluorine, sodium, magnesium, and aluminum. The main precipitate is Fe (OH)3. The F, Na, and Al impurities coprecipitated together in the process of precipitation, filtration, and separation, and a small amount of MgSO4 is also shown to exist. The elemental F is primarily derived from the flux of CaF2 in the process of reduction roasting. From Table 7, it can be found that the content of Fe is as low as 5.4 mg/L, which is basically completely removed. It is beneficial to carry out the subsequent precipitation experiment of magnesium and obtain pure magnesium oxide products.
Precipitation of Hydromagnesite
After removing iron, NH3·H2O was added to adjust the pH to 9.6–9.7, and alkaline substances were slowly added into the solution several times to adjust the pH. It can be found that with the addition of NH3·H2O and alkaline precipitator, white turbid liquid is gradually formed in the progress. At the end of the reaction, the white turbid liquid was separated by vacuum filtration to obtain the filter cake of basic magnesium carbonate as shown in Fig. 13 and the colorless-transparent magnesium precipitation solution.
The effects of different alkaline substance on the precipitation of basic magnesium carbonate were investigated. The specific experimental results are shown in Table 8.
By adjusting the amount and ratio of alkali, it could be found that adding (NH4)2CO3 could shorten the time of magnesium precipitation and the precipitation efficiency of magnesium was relatively high. Therefore, (NH4)2CO3 was selected as the alkali precipitant. The precipitation of basic magnesium carbonate could be divided into two stages: double decomposition reaction and pyrolysis reaction. The specific chemical reaction equation was Eqs. (11)–(12).
The results are shown in Table 9 and Fig. 14. Cassia et al. used sulfuric acid as leaching agent to treat laterite ore, calcium carbonate to remove impurities, sodium carbonate as precipitant to deal with leaching solution. The highest precipitation extent of magnesium achieved was 96.2% [35]. In this research, by analysis of the white precipitate of hydromagnesite, it could be found that the main elements were Mg, O, C, and H. Combined with XRD phase analysis, the white precipitate obtained by precipitation was basic magnesium carbonate, and the total content of impurities was less than 2% of the total mass of precipitation. According to the calculation, the precipitation efficiency of magnesium in the leaching solution after impurity removal was 98.6%.
Preparation of Magnesium Oxide
The basic magnesium carbonate precipitate was dried in a drying oven at 20 0°C for 6 h to obtain white magnesium oxide. The basic magnesium carbonate precipitate was finely ground and placed in a quartz crucible. It was calcined at high temperature in T1700-L-B vertical jet furnace. The reactivity of MgO decreased as the calcining temperature and time increased [36, 37]. The reaction equation of high temperature decomposition of basic magnesium carbonate is Eqs. (13).
After the crucible was cooled in the furnace, the chemical composition of the decomposition product of basic magnesium carbonate was analyzed and the direct yield of magnesium was calculated. The grade of magnesium oxide obtained in this experiment reached the standard content of industrial grade MgO, and the XRD and chemical composition of the final product as shown in Fig. 15 and Table 10. The main impurities in the product are creedite.
Finally, the technological process of magnesium oxide products was obtained. The process studied might be suitable for comprehensive recovery valuable metal products such as Ni, Fe, Mg from laterite ore.
Conclusions
This research mainly put forward the technological process of comprehensive recovery of valuable metals (nickel, iron and magnesium) from a high silicon-magnesium laterite ore in Yunnan. The preparation process of magnesium oxide was emphatically studied, and the optimum process conditions of magnesium leaching and the chemical behavior of magnesium oxide leaching, purification and precipitation were obtained.
-
(1)
According to the research results, the decomposition of serpentine in silicon magnesium laterite was about 581.7 °C, and the phases of Mg2SiO4 and MgSiO3 began to appear. Then the melting point of the whole reduction system decreased by CaF2. It was very favorable for the migration and accumulation of Ni–Fe particles in laterite ore. The grade of ferronickel concentrate was 6.60% of Nickel and 62.1% of iron. It can be used for steelmaking.
-
(2)
There were points of diminishing returns, when the sulfuric acid concentration exceeded 20%, the extraction degree of Mg presented a continuous downward trend. There were basically no change of the extraction degree of Ni and Fe. When the leaching temperature reached 80 °C, the extraction degree of Mg, Fe and Ni start lowering, and the extraction degree of Mg reached a maximum of 99.9%. When the liquid–solid ratio was 9:1, the extraction degree of Fe reached a maximum of 75.1%. The extraction degree of Ni had reached 99%, while the leaching time was 1 h. And the repeated experiments got the extraction degree of Mg, Fe and Ni is 96.7%, 98.6% and 62.8% under optimal leaching conditions.
-
(3)
The pH value was between 3.3 and 4.0, and the solution was stirred continuously to cause iron ions nearly complete precipitation. Adding (NH4) 2CO3 made the precipitation efficiency of Mg reached a maximum of 99.2% within 1.75 h.
The valuable elements such as Ni and Fe were comprehensively recovered while extracting magnesium from laterite ore. In the process of magnesium extraction, high extraction degree and precipitation extent were also achieved. Combined with the previous research, the process technology of this research was a feasible method, which also provides a certain reference for the efficient and comprehensive utilization technology of laterite nickel ore resources.
References
Hosseini T, Selomulya C, Haque N, Zhang L (2014) Indirect carbonation of victorian brown coal fly ash for CO2 sequestration: multiple-cycle leaching-carbonation and magnesium leaching kinetic modeling. Energy Fuel 28(10):6481–6493. https://doi.org/10.1021/ef5014314
Harraz HZ (2017) Beneficiation and mineral processing of magnesium. Minerals. https://doi.org/10.13140/RG.2.2.18946.68806
Royani A, Sulistiyono E, Prasetiyo AB, Subagja R (2018) Extraction of magnesium from calcined dolomite ore using hydrochloric acid leaching. AIP Conf Proc. https://doi.org/10.1063/1.5038299
Shofi A, Rahmahwati A, Nurjaman F, Suharno B (2019) Effect of reduction temperature and sodium-based additives on nickel up-grading process of laterites ores. IOP Conf Ser. https://doi.org/10.1088/1757-899x/541/1/012002
Xiao J, Ding W, Peng Y, Chen T, Zou K, Wang Z (2020) Extraction of nickel from garnierite laterite ore using roasting and magnetic separation with calcium chloride and iron concentrate. Minerals. https://doi.org/10.3390/min10040352
Tian H, Pan J, Zhu D, Yang C, Guo Z, Xue Y (2020) Improved beneficiation of nickel and iron from a low-grade saprolite laterite by addition of limonitic laterite ore and CaCO3. J Market Res 9(2):2578–2589. https://doi.org/10.1016/j.jmrt.2019.12.088
Jiang M, Sun T, Liu Z, Kou J, Liu N, Zhang S (2013) Mechanism of sodium sulfate in promoting selective reduction of nickel laterite ore during reduction roasting process. Int J Miner Process 123:32–38. https://doi.org/10.1016/j.minpro.2013.04.005
Hang G, Xue Z, Wang J, Wu Y (2020) Mechanism of calcium sulphate on the aggregation and growth of ferronickel particles in the self-reduction of saprolitic nickel laterite ore. Metals. https://doi.org/10.3390/met10040423
Gu X, Qu T, Shi L, Wang Q, Yang B, Dai Y (2020) Extraction of magnesium from garnierite by carbothermal reduction in vacuum. Mater Res Express. https://doi.org/10.1088/2053-1591/ab6e35
González Y, Navarra A, Jeldres RI, Toro N (2021) Hydrometallurgical processing of magnesium minerals: a review. Hydrometallurgy. https://doi.org/10.1016/j.hydromet.2021.105573
Bray EL (2020) Magnesium Metal. In: Mineral Commodity Summaries. https://www.usgs.gov/centers/nmic/mineral-commodity-summaries
USGS (2020) Mineral Commodity Summaries U.S Department of the Interior. https://www.usgs.gov/centers/nmic/mineral-commodity-summaries
Myagkiy A, Truche L, Cathelineau M, Golfier F (2017) Revealing the conditions of Ni mineralization in the laterite profiles of New Caledonia insights from reactive geochemical transport modelling. Chem Geol 466:1–19. https://doi.org/10.1016/j.chemgeo.2017.06.018
Farrokhpay S, Filippov L, Fornasiero D (2018) Upgrading nickel in laterite ores by flotation. Miner Eng 121:100–106. https://doi.org/10.1016/j.revmed.2014.04.010
Farrokhpay S, Filippov L, Fornasiero D (2019) Pre-concentration of nickel in laterite ores using physical separation methods. Miner Eng 141:1–6. https://doi.org/10.1016/j.mineng.2019.105892
Myagkiy A, Truche L, Cathelineau M, Golfier F (2017) Revealing the conditions of Ni mineralization in the laterite profiles of New Caledonia: insights from reactive geochemical transport modelling. Chem Geol 466:1–19. https://doi.org/10.1016/j.chemgeo.2017.06.018
Agrılı H (2019) Magnesite and olivine deposits of Turkey. Solid Earth Sci. https://doi.org/10.1007/978-3-030-02950-0
Jose N, Ahmed H, Miguel B, Luis E, Jorge B (2020) Magnesia (MgO) production and characterization, and its influence on the performance of cementitious materials: a review. Materials (Basel) 13(21):2–5. https://doi.org/10.3390/ma13214752
Erlund R, Koivisto E, Fagerholm M, Zevenhoven R (2016) Extraction of magnesium from four Finnish magnesium silicate rocks for CO2 mineralisation—part 2: aqueous solution extraction. Hydrometallurgy 166:229–236. https://doi.org/10.1016/j.hydromet.2016.07.004
Ribeiro PPM, Santos IDD, Reiner N, Aline F, Bourdot DAJ (2019) Roasting and leaching behavior of nickel laterite ore. Metall Mater Trans 52(3):1739–1754. https://doi.org/10.1007/S11663-021-02141-6
Ribeiro PPM, Mendonça SLC, Reiner N, Santos IDD, Bourdot JA (2019) Nickel and cobalt losses from laterite ore after the sulfation-roasting-leaching processing. J Market Res 9(6):12404–12415. https://doi.org/10.1016/j.jmrt.2020.08.082
Santos ALA, Becheleni EMA, Viana PRM, Papini RM, Silvas FPC, Rocha SDF (2021) Kinetics of atmospheric leaching from a Brazilian nickel laterite ore allied to redox potential control. Mining Metall Explor 38(1):187–201. https://doi.org/10.1007/S42461-020-00310-W
Zhang X, Zeng Y, Li Z (2020) Enhanced hydromagnesite process for CO2 sequestration by desilication of serpentine ore in NaOH solution. Ind Eng Chem Res 59(25):11370–11380. https://doi.org/10.1021/acs.iecr.0c01600
Wang J, Li Z, Park AHA, Petit C (2015) Thermodynamic and kinetic studies of the MgCl2-NH4Cl-NH3-H2O system for the production of high purity MgO from calcined low-grade magnesite. AICHE J 61(6):1933–1946. https://doi.org/10.1002/aic.14789
Teir S, Eloneva S, Fogelholm CJ, Zevenhoven R (2009) Fixation of carbon dioxide by producing hydromagnesite from serpentinite. Appl Energy 86(2):214–218. https://doi.org/10.1016/j.apenergy.2008.03.013
Brest KK, Henock MM, Guellord N, Kimpiab M, Fabrice KK (2021) Statistical investigation of flotation parameters for copper recovery from sulfide flotation tailings. Results Eng. https://doi.org/10.1016/j.rineng.2021.100207
Farrokhpay S, Fornasiero D, Filippov L (2018) Upgrading nickel in laterite ores by flotation. Miner Eng 121:100–106. https://doi.org/10.1016/j.mineng.2018.02.021
Saeed F, Filippov L (2016) Challenges in processing nickel laterite ores by flotation. Int J Miner Process 151:59–67. https://doi.org/10.1016/j.minpro.2016.04.007
Kapiamba FK, Kimpiab M (2021) The effects of partially replacing amine collectors by a commercial frother in a reverse cationic hematite flotation. Heliyon 7:1–8. https://doi.org/10.1016/j.heliyon.2021.e06559
Keith Q, Jason NC, Skinner W, David JR, Jonas AM (2015) Preconcentration strategies in the processing of nickel laterite ores part 1: literature review. Miner Eng 79:261–268. https://doi.org/10.1016/j.mineng.2015.03.017
Mysen BO, David V (1985) Interaction between fluorine and silica in quenched melts on the joins Sio2-Aif3 and SiO2-Naf determined by Raman spectroscopy. Phys Chem Miner 12:77–85. https://doi.org/10.1007/BF01046830
Zhao YC, Xiao HN, Tan W (2002) Effect of different amount of Caf2 added on crystallization and properties of a glass ceraic. China Ceram Ind. https://doi.org/10.13958/j.cnki.ztcg.2002.06.009(In Chinese)
Wang YK, Wei YG, Peng B, Li B, Zhou SW (2019) Thermal decomposition of high-magnesium low-nickel laterite: theoretical calculation and experimental study. Mater Rep 3(4):1406–1411 (In Chinese)
Lu SY, Yong C, Yan JH, Li XD, Ni MJ, Cen K (2003) Effects of initial Ph value and solid/liquid ratio on heavy metals leaching of ash from an incinerator. Acta Sci Circum. https://doi.org/10.13671/j.hjkxxb.2003.01.010 ((In Chinese))
Souza CR, Vaughan J, Rocha SDF, Birchal VS (2021) Manufacturing reactive magnesia from nickel laterite waste solution via nesquehonite precipitation. Hydrometallurgy. https://doi.org/10.1016/j.hydromet.2021.105725
Zhu J, Ye N, Liu JW, Yang JK (2013) Evaluation on hydration reactivity of reactive magnesium oxide prepared by calcining magnesite at lower temperatures. Ind Eng Chem Res 52(19):6430–6437. https://doi.org/10.1021/ie303361u
Sutradhar N, Sinhamahapatra A, Pahari SK, Pal P, Bajaj HC, Mukhopadhyay I, Panda AB (2011) Controlled synthesis of different morphologies of MgO and their use as solid base catalysts. J Phys Chem C 115:12308–12316. https://doi.org/10.1021/jp2022314
Funding
Financial support for this study was supplied by the Yunnan Provincial Key Research and Development Program—International Science and Technology Cooperation Special Project (Project Nos.2018IA055) and the National Natural Science Foundation of China (Project Nos. 52074140).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
The contributing editor for this article was Atsushi Shibayama.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, H., Li, B. et al. Magnesium Extraction from Magnetic Separation Tailings of Laterite Ore by Sulfuric Acid Leaching and Ammonium Carbonate Precipitation. J. Sustain. Metall. 8, 370–385 (2022). https://doi.org/10.1007/s40831-022-00496-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-022-00496-x