Abstract
In this study, extraction of aluminum-, iron-, and titanium-bearing constituents from diaspore-type bauxite ores was investigated by stepwise treatment consisting of pre-desilication via alkali-leaching of bauxite ore, extraction of alumina via Bayer process, and recovery of iron from red mud via magnetic separation. The pre-desilication results showed that the removal of silica reached 73.92% and that the mass ratio of alumina to silica (A/S) of bauxite concentrate increased from 2.92 to 9.25 under the conditions of sodium hydroxide concentration of 50 wt.%, leaching temperature of 95°C, leaching time of 30 min, and liquid-to-solid ratio of 5 mL/g. A total of 96.31% alumina was extracted from the bauxite concentrate via the Bayer process. Subsequently, by using two-step magnetic separation (intensity: 0.8 T and 0.2 T), TiO2-bearing iron concentrate with total iron grade of 56.39% and TiO2 grade of 8.66% was obtained with recoveries of iron and TiO2 of 55.79% and 17.37%, respectively. The grade of TiO2 reached 21.22% in the nonmagnetic fraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the U.S. Geological Survey (USGS),1 the global proven bauxite ore reserves were 28 billion tons at the end of 2015. The diaspore-type bauxite ore spreads over many countries, such as China, Yugoslavia, and Greece. It mainly consists of diaspore, hematite, kaolinite, anatase, and rare earths as trace elements. The diaspore-type bauxite is characterized by a complex disseminated mineral relationship; high contents of iron, titanium, and silicon; and a low mass ratio of alumina to silica (A/S).2 The Bayer process is inappropriate for this kind of bauxite despite its high utilization value for comprehensive recovery of alumina, iron, titanium, etc.
Silicon-bearing minerals are the most harmful impurities in alkali production of alumina. The generated hydrated sodium aluminum silicate causes the loss of Al2O3 and Na2O, which lowers the quality of the alumina product. To eliminate the adverse impact of silica on alumina production, pre-desilication processes have been proposed.3,4 The pre-desilication methods mainly include physical separation methods,5 chemical methods,6,7,8 and biological methods.9 As a result of most silicon in the form of aluminosilicates, which restrains mineral liberation, it is difficult to achieve satisfying desilication results by physical separation. In addition, the biological methods demand a long cycle to desilication. According to the dissolving properties of diaspore and kaolinite under different reaction conditions, Yang et al.6 used sodium hydroxide solution to leach bauxite ore directly. The removal of SiO2 reached 42.5%, and the A/S of the bauxite concentrate increased from 7.6 to 12. The roasting-alkali leaching process can also achieve good desilication from bauxite ore with low iron content. But it is inefficient for iron-rich bauxite because iron promoted the generation of mullite under the roasting conditions, which is harmful for the desilication, and the formation of hercynite through reactions with alumina, leading to difficult comprehensive utilization of iron and alumina from bauxite ore.
To achieve comprehensive recovery of iron-bearing constituents from bauxite ore with high iron content, many studies have been carried out.10,11,12 There are three main methods for the utilization of iron in the alumina industry: direct magnetic separation,13 pyro-metallurgical,14,15,16 and hydrometallurgical17,18 methods. Pyro-metallurgical methods are the main choice for iron recovery but suffer high-energy consumption. Hydrometallurgical methods are of less energy consumption but have other shortcomings, including a low reaction rate and secondary pollution. Compared with pyro-metallurgical and hydrometallurgical methods, recovering iron from red mud by direct magnetic separation has the advantage of low-energy consumption and does not produce secondary pollution.
In this study, the comprehensive utilization of aluminum-, iron-, titanium-bearing diaspore bauxite ore was investigated by stepwise treatment, consisting of pre-desilication for improving A/S of bauxite ore, extraction of alumina via Bayer process from bauxite concentrate, and then recovery of iron and titanium from red mud generated via magnetic separation.
Experimental
Materials
Bauxite Ore
The bauxite ore sample was taken from the Yunnan province of China, where reserves of more than 145 million tons of this kind of bauxite ore exist. Its chemical composition is shown in Table I. The sample is characterized by high silica content and total iron grade. The x-ray diffraction (XRD) pattern (Fig. 6) shows that the bauxite ore mainly consists of diaspore, kaolinite, hematite, anatase, and titanomagnetite.
Reagents
Sodium hydroxide and aluminum hydroxide of analytical grade were used in the experiments. The lime used in the experiments was obtained by calcination the analytical grade of calcium carbonate in a muffle furnace at 1000°C for 3 h.
Methods
The discussed mineralogical analysis indicates that pre-desilication of the bauxite ore is feasible by removing kaolinite based on its reaction with sodium hydroxide. After pre-desilication, which increases the A/S ratio, alumina can be extracted from bauxite concentrate via the Bayer process. Then, iron and TiO2 can be recovered from the Bayer red mud via magnetic separation as a result of high magnetic susceptibility of titanomagnetite. The anatase was enriched in the magnetic tailing.
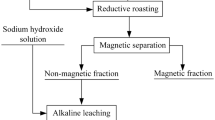
Therefore, an experimental flowsheet in principle is proposed in Fig. 1. The experimental procedure mainly includes (I) pre-desilication by leaching the bauxite ore with sodium hydroxide liquor; (II) Bayer digestion of the bauxite concentrate to extract Al2O3; and (III) magnetic separation to recover iron from red mud.
Pre-desilication of Bauxite Ore
Leaching experiments were carried out in a DY-8 autoclave equipped with eight 150-mL stainless steel pots rotating in a bath of glycerine. At the beginning of each trail, bauxite ore was added into the pots and mixed with an appropriate concentration sodium hydroxide solution at a given liquid-to-solid ratio. Then, the sealed pots were soaked in the bath at a specific temperature and agitated at 60 rpm. After leaching for a given period of time, the slurry was cooled and separated by filtration. The leaching residue was washed with hot water and dried at 105°C. Thereafter, it was collected for determination of chemical composition by x-ray fluorescence analysis. The A/S, removal of SiO2 (\( \eta_{\text{S}} \)), and leaching of Al2O3 (\( \eta_{\text{A}} \)) were determined as follows:
where W (A) and W (S) are the weight contents of Al2O3 and SiO2, respectively; \( W_{{\text{(A}_{\text{1}} \text{)}}} \) and \( W_{{\text{(A}_{\text{2}} \text{)}}} \) are Al2O3 contents of bauxite ore and bauxite concentrate, respectively; M 1 and M 2 are the mass of bauxite ore and bauxite concentrate, respectively; and \( W_{{\text{(S}_{\text{1}} \text{)}}} \) and \( W_{{\text{(S}_{\text{2}} \text{)}}} \) are SiO2 contents of bauxite ore and bauxite concentrate, respectively.
Bayer Digestion of Bauxite Concentrate
The digestion experiments were carried out in a DY-4 autoclave. The autoclave was heated by molten salt, and the pot was rotated at 60 rpm.19 Lots of research was conducted on the Bayer process, and the conditions of Bayer digestion were referred from previous studies.20 The slurry (20-g bauxite concentrate in 100-mL liquor) was added into the pots and mixed with an appropriate concentration of sodium aluminate solution [240 g/L Na2O, 131.6 g/L Al2O3, α k = 3(α k is the mole ratio of Na2O to Al2O3 in the liquor)]. Then, the sealed pots were soaked and rotated in the molten salts bath at a given temperature for a given time. The slurry was then cooled and separated by filtration. The residues were washed and dried. The extraction alumina was calculated using the following formula:
where η is the leaching of Al2O3; \( W_{{\text{(A}_{\text{2}} \text{)}}} \) and \( W_{{\text{(A}_{\text{3}} \text{)}}} \) are the Al2O3 contents of bauxite concentrate and red mud, respectively; and M 2 and M 3 are the mass of bauxite concentrate and red mud, respectively.
Magnetic Separation of Red Mud
The red mud obtained from the Bayer process were ground to 98% passing 74 μm for magnetic separation. The recovery of iron by magnetic separation and TFe and TiO2 grade of magnetic fraction and nonmagnetic fraction were analyzed, respectively. The iron recovery was calculated as follows:
where ε is the recovery of iron or titanium; γ is the yield; and α and β are the TFe or TiO2 grade of the sample before and after magnetic separation, respectively.
Results and Discussion
Extraction of Alumina
Pre-desilication of Bauxite Ore by NaOH Liquor Leaching
Effects of Sodium Hydroxide Concentration
The effects of sodium hydroxide concentration on the A/S of bauxite concentrate, removal of silica, and leaching of alumina were studied by fixing the leaching temperature of 95°C, leaching time of 30 min, and liquid-to-solid ratio of 5 mL/g. Figure 2 shows that the A/S is gradually increased with the increasing concentration of NaOH. When the sodium hydroxide concentration increases to 50%, the A/S reaches 10, which meets the requirements of Bayer process. Meanwhile, the removal of silica and leaching of alumina is raised. To obtain appropriate silica removal ratio and to reduce the loss of alumina, the suitable sodium hydroxide concentration is about 50%.
Effects of Leaching Temperature
The effects of leaching temperature on the A/S of bauxite concentrate, the removal of silica, and the leaching of the alumina were studied. Parameters were kept constant during the experiments at the NaOH concentration of 50%, leaching time of 30 min, and liquid to solid ratio of 5 mL/g. It is shown from Fig. 3 that with the leaching temperature increased, the A/S, the removal of silica, and the leaching of alumina increase first, and then decline. At 115°C, the A/S, the removal of silica, and the leaching of alumina attain a maximum. Nevertheless, as the temperature is increased further, the removal of silica and the leaching of alumina decrease. This is because as the leaching temperature increases, the reaction accelerates. In this case, kaolinite can react with sodium hydroxide faster. To achieve the appropriate silica removal ratio and to reduce the loss of alumina, the optimum leaching temperature is 95°C.
Effects of Leaching Time
The effects of leaching time on the pre-desilication of bauxite ore were investigated at the NaOH concentration of 50%, leaching temperature of 95°C, and liquid to solid ratio of 5 mL/g. From Fig. 4, in the first 30 min, the A/S increased sharply because of more free hydroxyl ions involved in the reaction. After reaching the maximum, A/S declined slightly. As time is prolonged, the removal of silica and leaching of alumina are increased with different rates. The optimum leaching temperature is 30 min.
Effects of Leaching Liquid-to-Solid Ratio
The effects of the liquid-to-solid ratio on the A/S, the removal of silica and the leaching of the alumina were studied. Parameters were kept constant during the experiments at the NaOH concentration of 50%, leaching temperature of 95°C, and leaching time of 30 min. It is observed from Fig. 5 that with the L/S ratio increased, A/S, the removal of silica, and the leaching of the alumina increased. When the L/S ratio is too low, there is incomplete reaction between kaolinite with sodium hydroxide solution, which resulted in a low removal ratio of silica. By contrast, an excessively high L/S ratio increases the loss of alumina. Thus, the appropriate liquid-to-solid ratio is 5 mL/g.
It can be concluded from the previous alkali-leaching results that the removal of silica reached 73.92% and A/S increased from 2.92 to 9.25 under optimum pre-desilication conditions of sodium hydroxide concentration of 50%, leaching temperature of 95°C, leaching time of 30 min, and liquid-to-solid ratio of 5 mL/g.
Mechanisms on Pre-desilication via Alkali-Leaching
The kaolinite reacts with sodium hydroxide as the following reactions:
Dissolution of kaolinite:
Formation of sodium aluminosilicate hydrate:
At the beginning of the pre-desilication process, the sodium hydroxide concentration is the biggest and concentration of silica and alumina is the smallest in liquor; the reaction velocity of kaolinite dissolved is much greater than that of sodium aluminosilicate hydrate formed. Therefore, the desilication rate of bauxite ore quickly increases. Reaction 6 is predominant over reaction 7. Nevertheless, with the lapse of time, the sodium hydroxide concentration in liquor and quantity of kaolinite in bauxite ore decreases gradually. On the other hand, sodium silicate concentration increases quickly, and sodium aluminate concentration also increases gradually at the same time. As a result, the reaction rate of sodium aluminasilicate hydrate formation rises when the rate of reaction 6 is equal to the rate of reaction 7; the desilication rate of bauxite ore does not increase any more, as shown in Fig. 3. To achieve good desilication, it is required to control the leaching time and reaction 6 must predominant over reaction 7.
According to the kaolinite formula, the theoretic leaching ratio of alumina to silica is 0.85, which means that the dissolution of 1-kg kaolinite completely will take 0.425-kg Al2O3 and 0.50-kg SiO2 away. If the ξ is less than 0.85, more SiO2 will be leached than Al2O3. In other words, the silica is removed from the kaolinite. The practical leaching ratio of alumina to silica was calculated as follows:
where ξ is the practical leaching ratio of alumina to silica; α 1 and α 2 is the contents of Al2O3 and SiO2 of the bauxite ore, respectively; δ 1 is the leaching ratio of Al2O3; and δ 2 is the removal ratio of SiO2.
At optimum pre-desilication conditions, ξ = 0.73, which means that more SiO2 had been leached than Al2O3 and the silica is removed from kaolinite. This can be confirmed by the XRD patterns in Fig. 6. It is revealed that at optimum pre-desilication conditions, the kaolinite peaks are significantly weakened, and the other peaks are attributed to the diaspore. It is indicated that the silica is removed from kaolinite at optimum pre-desilication conditions.
Bayer Digestion of Al2O3 from Bauxite Concentrate
The bauxite concentrate used for Bayer digestion experiments was obtained under optimum pre-desilication conditions. The chemical composition is shown in Table II.
Parameters were kept constant during the digestion experiments as 240-g/L Na2O, 131.6-g/L Al2O3, α k = 3, digestion temperature of 280°C, and digestion time of 90 min. It is shown from Fig. 7 that with the lime dosage increases, the leaching of Al2O3 gradually increases. When the lime dosage is 6%, the digestion of Al2O3 reaches 96.31%. The result shows that bauxite concentrate fully meets the Bayer digestion requirements. The chemical composition of red mud in the absence of lime is compared with that in the presence of 6% lime in Table II, which shows the iron content of the red mud increases sharply compared with bauxite ore, from 19.05% to about 36%.
Recovery of Iron Concentrate from Red Mud by Magnetic Separation
Effect of addition of lime during Bayer digestion on magnetic separation recovery of iron and enrichment of TiO2 from the red mud was further examined. The red mud was milled to 98%, and a passing 0.074-mm sieve and two-step magnetic separation (0.8 T and 0.2 T) were used. The magnetic separation efficiency of iron concentrate is shown in Table III. The XRD patterns of red mud and iron concentrate are presented in Fig. 8. The main chemical compositions of iron concentrate and titanium-rich tailing are shown in Table III. The two iron concentrates, derived from magnetic separation of Bayer digestion red mud in the absence and presence of lime, show a great difference. In comparison, with the addition of 6% lime, the total iron grade and iron recovery of iron concentrate increase from 48.33% to 56.39% and from 42.38% to 55.79%, respectively (Table III). The improved magnetic separation efficiency is a result of simply more phase compositions of red mud in the presence of 6% lime. And titanium different phase compositions in red mud contribute to different magnetic separation efficiencies (Fig. 8). As a result of the good efficiency of magnetic separation, the TiO2 is enriched more significantly in the nonmagnetic material, and a titanium-rich material with 21.22% TiO2 is obtained (Table III). The compositions of iron concentrate obtained in the presence of 6% lime are similar to the iron-making raw materials used in Chengde, China.21 And the nonmagnetic material can be used to recover titanium.
XRD patterns of red mud obtained: (a) in the absence of lime and (b) in the presence of 6% lime, XRD patterns of iron concentrate: (c) in the absence of lime and (d) in the presence of 6% lime (A—Andradite, C—Cancrinite, H—Hematite, G—Goethite, M—Magnetite, O—Sodalite, P—Pseudobrookite, S—Sodium tri-titanate, T—Titanomagnetite)
Conclusion
The feasibility of an integrated technological route for stepwise recovery of valuable components from low-quality diasporic bauxite ores was investigated, and the following conclusions were obtained:
-
1.
The pre-desilication results showed that the removal of silica reached 73.92%, and A/S increased to 9.25 under the condition of sodium hydroxide of 50%, leaching temperature of 95°C, leaching time of 30 min, liquid-to-solid ratio of 5 mL/g.
-
2.
The leaching of alumina reached 96.31% when Bayer digestion of the bauxite concentrate was performed under the condition of alkali concentration of 240 g/L, caustic ratio of 3, leaching temperature of 280°C, leaching time of 90 min, and lime dosage of 6%.
-
3.
With the two-step magnetic separation of field intensity 0.8 T and 0.2 T, respectively, magnetic concentrate had a total iron grade of 56.39% and iron recovery of 55.79%; the TiO2 was enriched in nonmagnetic material.
References
U.S. Geological Survey, Bauxite and Alumina Statistics and Information (Mineral Commodity Summaries, 2016). http://minerals.usgs.gov/minerals/pubs/commodity/bauxite/mcs-2016-bauxi.pdf.
C.A. Pickles, T. Lu, B. Chambers, and J. Forster, Can. Metall. Q. 51, 424 (2012).
X.L. Pan, H.U. Yu, K.W. Dong, G.F. Tu, and S.W. Bi, Int. J. Miner. Metall. Mater. 19, 973 (2012).
P. Smith, Hydrometallurgy 98, 162 (2009).
Y.H. Wang, D.X. Sun, L.G. Wang, and Y.L. Zhou, Miner. Eng. 24, 1031 (2011).
B. Yang, J.G. Wang, Y.F. Zhang, and Y. Zhang, Chin. J. Process. Eng. 7, 921 (2007).
T. Jiang, G.H. Li, G.Z. Qiu, X.H. Fan, and Z.C. Huang, Appl. Clay Sci. 40, 8 (2008).
G.H. Li, J.H. Zeng, J. Luo, M.X. Liu, T. Jiang, and G.Z. Qiu, Appl. Clay Sci. 99, 282 (2014).
V.I. Groudeva and S.N. Groudev, Travaux Icsoba 13, 257 (1983).
Y.J. Liu and R. Naidu, Waste Manag 34, 2662 (2014).
W.C. Liu, J.K. Yang, and B. Xiao, J. Hazard. Mater. 161, 474 (2009).
L.C. Venancio, J.A. Souza, E.N. Macedo, J.N. Quaresma, and A.E. Paiva, JOM 62, 9 (2010).
Y.R. Li, J. Wang, X.J. Wang, B.Q. Wang, and Z.K. Luan, J. Phys. Chem. C 471, 91 (2011).
M. Samouhos, M. Taxiarchou, P.E. Tsakiridis, and K. Potiriadis, J. Hazard. Mater. 254, 193 (2013).
G.H. Li, M.X. Liu, J.M. Rao, T. Jiang, J.Q. Zhuang, and Y.B. Zhang, J. Hazard. Mater. 280, 774 (2014).
Z.W. Peng and J.Y. Hwang, Int. Mater. Rev. 60, 30 (2015).
T. Jiang, L. Yang, G.H. Li, J. Luo, J.H. Zeng, Z.W. Peng, and M.X. Liu, Can. Metall. Q. 55, 345 (2016).
D. Debadatta and K. Pramanik, Res. J. Chem. Environ. 17, 50 (2013).
G.H. Li, J. Luo, T. Jiang, Z.X. Li, Z.W. Peng, and Y.B. Zhang, Metals 6, 294 (2016).
R. Zhang, S.L. Zheng, S.H. Ma, and Y. Zhang, J. Hazard. Mater. 189, 827 (2011).
W.S. Wang, J.Y. Sun, G. Ren, F.M. Li, and Q. Lv, Iron and Steel 45, 9 (2010) (in Chinese).
Acknowledgements
The authors wish to express their thanks to the National Natural Science Foundation of China (Nos. 51234008 and 51174230) for financial support. This work was also financially supported by the Co-Innovation Center for Clean and Efficient Utilization of Strategic Metal Mineral Resources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, G., Gu, F., Jiang, T. et al. Beneficiation of Aluminum-, Iron-, and Titanium-Bearing Constituents from Diasporic Bauxite Ores. JOM 69, 315–322 (2017). https://doi.org/10.1007/s11837-016-2215-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-016-2215-4