Abstract
On base of the content of Pb in the soil under different land use patterns in Lanping Lead-zinc mining area, Yunnan in southwest China, the root morphology and leaf traits of maize in different concentration Pb (20, 40, 60, 80, 100, 150, 200, 500, 1000, 2000, 3000 mg/L) were analyzed. The results showed that maize germination rate, germination vigor and growth index decreased with the increase of Pb concentration. The root length, surface area of maize increased by 0.21%–81.58%, 8.99%–73.43%, 1.50%–77.37%, respectively, under 20–500 mg/L Pb concentration. However, these parameters under 1000–3000 mg/L Pb concentration decreased by 37.86%–553.54%, 44.99%–766.16%, 55.99%–92.81%, respectively, and these lowest value appeared in 3000 mg/L Pb treatment. The root volume of maize increased by 4.57%–89.25% in 20–80 mg/L Pb concentration, and it decreased with the increase of Pb concentration when the Pb concentration was higher than 80 mg/L and decreased by 94.13% in 3000 mg/L Pb. The root surface area and length of 0.50–1.00 diameter class were higher than those of other diameter classes, and these value of maize under 500 mg/L Pb were higher than those of other concentrations. The length and perimeter of maize leaves with the highest value of 220.36 and 962.68 mm, respectively appeared in 60 mg/L Pb treatment. The leaf width and area of maize with the highest value of 15.68 mm and 2448.31 mm2, respectively, appeared in 40 mg/L Pb treatment, which indicated that the leaf traits of maize were promoted by low concentration Pb and inhibited by high concentration Pb.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lanping County, Yunnan Province, located in Southwest China, is rich in Lead-zinc deposit resources, ranking first in Asia and second in the world. The exploitation of Lead-zinc mine resources has become an important support for the local economic development of Yunnan (Liu et al. 2005). The development and smelting of non-ferrous metals have caused soil heavy metal pollution and water pollution, which has seriously endangered the health of residents and the safe production of farmland and crops around the mining area (Estival et al. 2012). In recent years, a large number of studies have been carried out on the treatment of heavy metal pollution in mining areas, mainly focusing on the temporal and spatial distribution characteristics of different forms of heavy metals (Bai et al. 2009), the enrichment characteristics of hyperaccumulators (Chen et al. 2005; Guo et al. 2008), soil heavy metal pollution evaluation (Chen et al. 2006). Some progress has been made in phytoremediation of heavy metal pollution and screening of hyperaccumulators in mining areas (Li et al. 2019; Qing et al. 2013; Tan et al. 2016). However, the soil polluted by heavy metals will not only reduce the yield and quality of crops, but also endanger human health through the food chain. Therefore, the safe production of crops in the farmland around Lanping Lead-zinc mine has gradually become the focus of attention.
Plant roots and leaves are the key organs for plants to absorb and transport nutrients and water. Their morphology and spatial distribution are important factors affecting plant nutrient absorption of underground resources and aboveground biomass accumulation (De Costa et al. 2007). The morphological characteristics of roots were changed by heavy metal stress (Dai et al. 1988; Lynch 1995). Most of studies focused on the changes of root morphology of hyperaccumulators (Arabidopsis minor) and tolerant plant (Lolium perenne) under heavy metal stress (Chaplygin and Chernikova et al. 2021; Jiang et al. 2022). For example, the root length, surface area, and volume of Arabis alpine become less affected by heavy metals with the increase of root diameter class (Li et al. 2020), but less attention is paid to farmland crops. Maize is the main food crop in Jinding Town, Lanping County. The response mechanism of its roots and leaves to heavy metals can effectively evaluate the impact of heavy metals on farmland crops. The response of leaf traits and seed germination of maize to heavy metals plays an important role in plant survival and productivity, and have great correlation with the growth and development of plant aboveground organs and photosynthesis (Qi et al. 2006; Dong et al. 2005). Based on the investigation of soil heavy metal pollution of different land use types in mining area, wasteland and farmland in Lanping lead zinc mining area, this project studies the response of maize root morphology, leaf traits and seed germination to lead pollution stress.
2 Materials and methods
2.1 The situation of lead (Pb) pollution in Lanping county of Yunnan Province
The Jinding town, Lanping county of Yunnan Province, southwest of China, is one of the super-large Lead-zinc mining areas in the world. The climate type is temperate climate, which the annual average temperature is 10.4–11.8 ℃, the altitude is about 2380 m, and the annual average precipitation is 1088.43 mm. Jinding Lead-zinc mine in Lanping County was discovered and developed early, with large reserves, long development time and wide area, which caused great damage to the surrounding farmland ecosystem, forest ecosystem and river ecosystem, and caused serious Pb pollution in soil. The Lanping Jinding Lead-zinc mine is located in the upper reaches of the Pijiang River, and the zinc smelter is located at the foot of the mountain in the mining area. There are many farmlands along the Pijiang River. In these land uses, 23 sampling sites were set up, and soil samples were collected by chessboard sampling method. These crops are mainly rice, maize, wheat and so on. The soil heavy metal Pb content of different land use types is shown in Table 1 (He et al. 2021).
2.2 Determination of seed germination, root and leaf traits of Maize
From the heavy metal lead (Pb) content of different land use types in the Jinding Lead-zinc mining area in Lanping county, Yunnan Province (Table 1), Pb content in the soil of farmland around the Pijiang River and the 30-year-old mining area (with vegetation) showed the minimum and maximum values with 59.74 and 2564.72 mg/kg, respectively. Therefore, the maximum value of Pb stress concentration is set to 3000 mg/kg, and other concentration gradient of 0, 20, 40, 60, 80, 100, 150, 200 mg/kg was set because the majority of land varies in range of 59.74–188.44 mg/kg to observe the influence of small changes in concentration of Pb on the germination of maize and growth of seedlings. So 12 concentration gradients (0, 20, 40, 60, 80, 100, 150, 200, 500, 1000, 2000, 3000 mg/L) of Pb were set up, and the concentration of Pb was 0 mg/L as control (CK) in this experiment. Qiandan 88, a maize variety, was selected to study the changes of seed germination index, root morphology and leaf traits changes under different concentrations of Pb. Each treatment group were performed in triplicate in every concentration of Pb. Firstly, 10 maize seeds (Qiandan 88) are sterilized (75% ethyl C2H5OH and 30% H2O2 solution is prepared in a volume ratio of 4:1, soaked for 30s and then washed with distilled water). Then, these seeds were transferred to the nutrition solution (Hogland’s) with PbNO3 pollution gradient of 0, 20, 40, 60, 80, 100, 150, 200, 500, 1000, 2000, 3000 mg/L for water cultivation and each Pb treatment group was set with three parallel groups. The germination process of maize was monitored in the laboratory, and the germination number was counted every 24 h in period of 10 days to calculate the germination rate, germination vigor and other germination indexes (calculated by the followings).
-
(1)
Germination rate = number of germinated seeds /total number of seeds×100%.
-
(2)
Germination index = ΣGt/Dt (Gt refers to the number of germination within Dt, Dt is the corresponding number of germination days).
-
(3)
Germination vigor = number of seeds germinated during the peak period of germination/total number of seeds ×100%.
The root length, diameter, projected area, surface area, diameter class, leaf area, leaf length, and leaf width of maize were measured by Wanshen plant analysis system (LA-S) after 20 days. Firstly, 3 plants of maize were randomly selected in each treatment group, the maize roots of 3 plant were cleaned by distilled water. The cleaned maize roots were soaked in a beaker containing distilled water for 30 min to make the root structure softly. Then, the root branches and hairs of the root tissue were expanded with tweezers to avoid the crisscross of the branches and root hairs and the water of these tissue were absorbed by absorbent paper. After that, the root branches and hairs of the root tissue were placed flat in the root fixation device. Finally, the root morphology was scanned with Wanshen plant image analyzer. A series of root morphological parameters such as root length, root surface area, root volume, root diameter and root projected area were obtained by analyzing root images with Wanshen plant analysis system. Maize leaves were sampled from the end of leaf vein, and water and impurities on the surface leaf were wiped off with absorbent paper. Tweezers were used to spread out on the absorbent paper, press gently, and then spread in the scanner to obtain the image of leaf traits. A series of leaf traits parameters, such as length, width, area and perimeter, were obtained by Wanshen plant analysis system. SPSS software was used to analyze the data, ANOVA test method was used to analyze the differences of leaf and root indexes in different treatment groups.
In order to clarify the response of maize root and leaf train to Pb stress, we used the inhibition rate to evaluate the inhibition of metal heavy Pb on root morphology and leaf trait (calculated by the following formula).
-
(4)
Inhibition rate= (value of treatment-CK)/value of treatment×100%.
3 Results and analysis
3.1 The effect of lead stress on seed germination
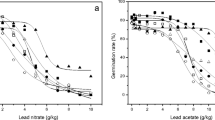
Pb is a non-essential element for plants, and different concentration Pb treatment groups have significant differences in the germination of maize seeds. The germination rate of maize seeds is shown in Fig. 2 under different heavy metal Pb concentration gradient treatments. The germination rate of seeds showed an S-shaped growth trend with time. In the low concentration of Pb treatment group (20, 40, 80, 150 mg/L), the maize germination rate continued to increase with time, reaching the highest value on the 9th day, and the germination rate value ranged from 80% to 95%. In the high concentration treatment groups (200, 500, 2000, 3000 mg/L), the germination rate of maize reached the highest value on the 6th day, and the lowest value of germination rate was 50%, and the germination rate gradually decreased with the increase of Pb pollution. In the Pb treatment concentration of 20, 40 mg/L, the maize germination rate was not significantly different from the control group, which indicated that the maize germination rate did not change significantly in the low concentration of Pb polluted environment. In the high concentration of Pb treatment group, the germination rate of maize was significantly lower than that of the control group, especially in the Pb concentration treatment groups of 2000, 3000 mg/L, the germination rate was only about 50%, indicating that the high concentration of Pb significantly inhibited the germination of maize.
In the Pb concentration treatment group, the germination vigor and germination index were calculated on the 5th, 10th day respectively, as shown in Fig. 3. From the perspective of germination vigor and germination index, it can be found that the germination index of maize under Pb stress decreases with the increase of Pb concentration. In the Pb treatment group with a concentration of 20–500 mg/L, there was no significant difference, and the value range was 13.79–17.22. The maize germination index decreased significantly under 1000–3000 mg/L concentration of Pb, which decreased by 7.45, 9.43, and 9.76 respectively compared with the control, indicating that the high concentration of heavy metal Pb severely inhibited maize germination. Germination vigor is an indicator of seed germination quality, which can better characterize the effect of heavy metal Pb on maize seed germination. The germination vigor of maize seeds showed a fluctuating and rising trend under the 0–200 mg/L concentration of Pb, and the variation range was 60.00%–86.67%, and reached the maximum at 100, 200 mg/L concentration of Pb. Under the stress of 1000, 2000, 3000 mg/L concentration of Pb, the germination vigor of maize gradually decreased, and the range of variation was 33.33%–53.33%, which indicated that the high concentration of Pb had the strongest inhibitory effect on maize seeds.
3.2 Inhibition effect on root system under lead stress
After the seed germinates, the root system directly contacts the heavy metal solution and will be the first to be poisoned. Therefore, the observation of the seedling root morphology can directly reflect the degree of heavy metal toxicity to the seeding. It can see from Fig. 3 that in the treatment groups with the Pb concentration of 20, 40, 60, 80, 100, 150, 200 mg/L, the root projected area (Fig. 4a), root surface area (Fig. 4d), and root length (Fig. 4e) are higher than that of other concentration treatment groups, and the highest values of these treatment groups were 19.94 cm2, 62.80 cm2, and 291.75 cm respectively, which increased by 46.99%, 43.63%, and 44.93% respectively compared with the control group. These results showed that the low concentration of Pb (< 200 mg/L) promoted the growth of the root projected area of maize; In the 20, 40, 60, and 80 mg/L Pb treatment groups, the root volume showed an increasing trend compared with the control group, the highest value was 1.55 cm3, which appeared in the 40 mg/L treatment group (Fig. 4b). It showed that the 40 mg/L concentration of Pb can promote the root elongation of seedlings. In the 1000, 2000, and 3000 mg/L concentration of Pb treatment groups, the root projected area, volume, surface area, and length decreased with the increase of the Pb concentration, and the lowest values were 0.82 cm2, 0.05 cm3, 2.55 cm2, and 11.38 cm respectively, which were only 7.76%, 5.87%, 7.19%, 7.08% of the control group. This showed that the inhibition effect on root of maize appeared under the high concentration treatment and the inhibition degree of root elongation morphology was more seriously with the increase of Pb concentration. Compared with the control group, the average root diameter of maize among these Pb treatment groups decreased, and the minimum and maximum values of root diameter were 0.46 mm and the 0.79 mm, accounting for 53.22% and 91.55% of the control respectively (Fig. 4c).
The inhibitory rate of different concentrations of heavy metal Pb on maize roots is shown in the Figs. 4f and 5 that the length, projected area and surface area of the maize root was promoted in the 20–200 mg/L Pb concentration treatment groups with the highest values of promotion rates 0.45, 0.47, and 0.44, respectively. But the 500–3000 mg/L Pb concentration significantly inhibited root length, projected area and surface area of maize root, and the inhibitory effect increased with the increase of Pb concentration. The highest inhibition rates of three parameters were 13.67%, 12.07% and 13.26%, respectively. In the treatment groups with Pb concentration of 20, 40, 60 and 80 mg/L, Pb had a slight promoting effect on maize root volume, and the highest promoting rate value was 0.47. Under the concentrations of 100, 150, 200, 500, 1000, 2000 and 3000 mg/L of Pb, Pb significantly inhibited the root volume of maize, and the inhibition rate increased significantly with the higher Pb concentration. In the 3000 mg/L of Pb treatment group, the inhibition rate of root volume of maize reached the maximum with the value of 16.08%. In each Pb treatment group, Pb had a significant inhibitory effect on root diameter of maize.
The length of the root system mainly reflects the growth status of the plant root system and its colonization ability in the soil. It can be seen from Table 2 that there are differences in the root length of the same diameter class maize in different concentration Pb stress treatment groups, and there are also differences in the root length of different diameter classes at the same concentration of Pb. Diameter class 0 ≤ d1 ≤ 0.50, the range of maize root length between different concentration Pb treatment groups is 0.15–0.62 cm, and the root length of diameter class is mostly higher than that of the control, which shows that Pb stress has no obvious inhibitory effect on the length of fine roots. The diameter class 0.5 ≤ d1 ≤ 1.0, in the 40, 60, 80 mg/L Pb stress treatment groups, the root length values were 0.64, 0.65, and 0.63 cm, respectively, which were significantly higher than those of the control group; With the increase of stress concentration (> 80 mg/L), the root system length showed a certain downward trend, and the minimum value of root system was 0.31 cm, which appeared in the 2000 mg/L Pb stress treatment group. Diameter class 1 ≤ d1 ≤ 1.5, the Pb stress concentration is 20, the root length of the 40 mg/L treatment group has no significant difference with the control, when the Pb concentration is 60 mg/L, the root length shows the maximum value of 0.41 cm, which is significantly higher group; With the increase of Pb concentration (> 80 mg/L), the root system length decreased significantly. In the diameter class 1.5 ≤ d1 ≤ 2.0, 2.0 ≤ d1 ≤ 2.5, the Pb concentration (> 100 mg/L) treatment group, the root length was significantly lower than the control, and the root length value approached 0 cm. Diameter class 2.50 ≤ d1 ≤ 3.0, 3.0 ≤ d1 ≤ 3.5, 3.50 ≤ d1 ≤ 4.0, 4.0 ≤ d1 ≤ 4.5, the root length decreases rapidly to 0 cm with the increase of Pb concentration, which indicates that heavy metal Pb stress has an effect on the length of thick roots it has obvious inhibitory effect. According to the Wanshen Root System Analysis Software, the root system is divided into 3 grades of fine roots (0.05–0.25 mm), medium roots (0.25–0.50 mm) and thick roots (> 0.5 mm) according to the diameter. From the data in Table 2, it is found that no matter the concentration of heavy metal Pb2+ is high or low, the inhibitory intensity of heavy metal Pb on thick roots is higher than that of fine roots.
The root surface area is determined by the root length and root diameter, which reflects the combined area of the root system and the soil. It can be seen from Table 3 that there were certain differences in the root surface area of maize roots with different diameter class. The root surface area with the diameter class of 0 ≤ d1 ≤ 0.50 was different among the Pb treatment groups, and the root surface area of 40 and 60 mg/L concentration of Pb treatment group was significantly lower than that in the control group. When Pb concentration was > 80 mg/L, the root surface area increased with the increase of the Pb concentration and its values were between 0.17 and 0.38 cm2. The root surface area with the diameter class of 0.5 ≤ d1 ≤ 1.0 varied between 0.45 and 0.73 cm2, and in the Pb concentration (> 40 mg/L) treatment group, except for the 2000 mg/L of Pb concentration treatment group, the root surface area increased with the increase of Pb concentration, which was significantly higher than that of the control group, and the highest value was 0.73 cm2 in the 500 mg/L concentration of Pb treatment group. Under the 20 and 40 mg/L concentration of Pb treatment groups, the root surface area with the diameter class of 1 ≤ d1 ≤ 1.5 were 0.26, 0.24 cm2, respectively, which were significantly higher than those of the control group, and its values under the 60–2000 mg/L concentration of Pb treatment group decreased with the increase of Pb concentration with the range of 0.02–0.16 cm2. The root surface area with the diameter class of 1.5 ≤ d1 ≤ 2.0 under the 40, 60, 80 mg/L concentration of Pb was significantly higher than that of the control group, but its values under the 100, 150, 200 mg/L concentration of Pb were significantly lower than the control group. When the Pb concentration was 500, 1000, 2000 mg/L, the root surface area of maize increased. Under each Pb concentration groups, the root surface area with the diameter class of 2.0 ≤ d1 ≤ 2.5 was significantly lower than that in the control group. In each Pb treatment group, the root surface area with the diameter class of 2.50 ≤ d1 ≤ 3.0, 3.0 ≤ d1 ≤ 3.5, 3.50 ≤ d1 ≤ 4.0, 4.0 ≤ d1 ≤ 4.5 gradually decreased with the increase of diameter class.
3.3 The effect of Pb on maize leaf
Leaves are important organs for plants to resist stress environments. Under the different Pb concentrations, the shape of maize leaves was shown in Table 4. In the treatment groups with Pb concentration of 20, 40, 60, 80 mg/L, the leaf lengths of maize with the values of 211.36, 217.42, 220.36, and 205.69 mm respectively were significantly higher than the control group. In the 3000 mg/L concentration of Pb treatment group, the leaf length was the lowest with the value of 146.12 mm, indicating that the low concentration of Pb promoted leaf elongation to some extent, and the high concentration of Pb significantly inhibited the leaf elongation. The leaf area and leaf length showed similar changes, and the value was the lowest of 1501.46 mm2 in the 3000 mg/L concentration of Pb treatment group. Compared with the control, the variation of maize leaf width in different concentration of Pb treatment groups was not obvious. In the 40, 60 mg/L concentration of Pb treatment groups, the leaf perimeter values of maize were 945.48 and 962.68 mm, and it increased by 6.57% and 7.84% respectively, compared with the control group. The leaf perimeter gradually decreased with the increase of Pb concentration, and maize at the 3000 mg/L concentration of Pb treatment groups had the lowest leaf perimeter with the value of 515.26 mm.
Inhibition rate of maize leaves the concentration of heavy metal Pb is shown in the Fig. 6: at the 20, 40, 60, and 80 mg/L concentration of Pb treatment groups, the leaf length of maize was promoted with the promotion rate of 1.40%–7.91%. At the 100–3000 mg/L concentration of Pb treatment group, the leaf length inhibition rate gradually increased with the increase of the heavy metal Pb concentration, and the highest inhibition rate reached the maximum with the value of 39.00% at the 3000 mg/L concentration of Pb. The low Pb concentration of 20, 40, and 60 mg/L promoted the increase of leaf circumference and leaf area of maize, but the high Pb concentration of 80–3000 mg/L gradually inhibited the leaf circumference and leaf area of maize. In the 3000 mg/L concentration of Pb, the inhibition rate of maize leaf area and perimeter were the most serious with the value of 51.28% and 72.19% respectively. The effect of Pb on maize leaf width showed the same trend as other parameters.
4 Discussion
Based on heavy metal Pb pollution in the soil of different types of land use in Jinding Lead-zinc mining area, which it exceeded GB15618–2018 “Soil Environmental Quality Agricultural Land Soil Pollution Risk Control Standard” (Table 1), the fluctuation ranges of soil Pb in farmlands is high, indicating that the farmland in Jinding Town may cause large fluctuation of Pb. According to the situation of Pb pollution soil of this area, the different Pb concentration gradients were designed to determine the changes of maize seed germination, root morphology and leaf traits. It was found that there were differences in the response of each parameter to heavy metal Pb toxicity. Seed germination rate is an important index to reflect heavy metal toxicity. Germination vigor and germination index characterize the effect of heavy metal stress on seed germination (Wei et al. 2021; Pu et al. 2021; Wen et al. 2022; Duan & Liu 2021; Tang et al. 2021). The germination rate, germination vigor and germination index of maize seeds decreased with the increase of Pb concentration. In the seed germination cycle, in the high Pb concentration treatment group, the seed germination rate reached the maximum on the 6th day of culture, and in the low Pb concentration treatment group, the seed germination rate reached the maximum on the 9th day of culture. This is similar to the seed germination results of Pogonatherum crinitum (Thunb.) Kunth (Hou et al. 2013), Solanum nigrum L. (Liu et al. 2012), Elsholtzia splendens Nakai ex F. Maek. (Zhang et al. 2010), Festucaelata Kengex E. Alexeev (Du and Yang et al. 2010), and Phyllostachys edulis (Carrière) J. Houz. (Chen 2016) under heavy metal stress. Generally speaking, the heavy metal Pb destroys the mineral nutrient balance in the plant, especially the Na+/K+ balance is broken, and a large amount of Pb ions accumulate in the plant (Hou et al. 2013). Pb2+ is an unnecessary element in plant growth. It can be accumulated in plants by root absorption, inhibit seed germination, destroy cell structure and reduce photosynthesis, thus affecting the growth and development of plants (Tao et al. 2007). Seed germination and seedling growth are the most sensitive periods of plant to environmental stress (Li et al. 2006). Therefore, the study on the characteristics of germination and seedling growth of seeds under stress can reflect the tolerance of plants to environmental stress to some extent (Zhang et al. 2011).
Plant roots are the first barrier to contact with heavy metal pollution soil. The absorption of nutrients by the roots of plant depends on the length, diameter and surface area of the root, and the root growth is significant in determining nutrient supply to the shoot and crop yield (Rout et al. 2014). Compared with aboveground parts, roots of plants are the first to be poisoned by heavy metals. There is a large amount of Pb2+ around the root, so that there are a large number of exchange sites on the root cell wall that can fix heavy metal ions (Zhang et al. 2016). When heavy metals are absorbed by the root tip, they are induced to produce free radicals. When the free radicals exceed the scavenging capacity of enzymes of the plant’s own antioxidant system, the excess free radicals will damage succinate dehydrogenase in root metabolism and reduce root activity. Thus, the transfer of heavy metals from roots to aboveground parts was inhibited (Ma et al. 2012; Nuzaaiti et al. 2010). In this study, in the low concentration Pb2+ treatment groups of 20, 40, 60, 80 and 100 mg/L, the trait indexes of maize root area, root surface area, and root length showed an increasing trend compared with the control group. But in the high concentration treatment groups of Pb > 100 mg/L, it had a significant inhibitory effect on the root index, showing an obvious phenomenon of “low concentration Pb promotes root growth and high concentration Pb inhibits root growth “. These results were consistent with the change of root traits of Wheat (Wang et al. 2020) and Phyllostachys edulis (Peng 2015) under heavy metal stress. No matter the concentration of Pb stress is high or low, the root length and root surface area of root diameter 0 ≤ d1 ≤ 0.50 and 0.5 ≤ d1 ≤ 1.0 are higher than other diameter classes, which indicates that heavy metal pollution has an obvious inhibitory effect on maize thick rhizome. By comparing the root length and root surface area of maize in different Pb concentration treatment groups, it was found that these two root morphology indexes decreased with the increase of Pb concentration in each root diameter class, indicating that high concentration of Pb significantly inhibited the increase of root elongation and surface area.
5 Summary and conclusions
The high germination of seedlings and seed vigor determine the reproduction and survival of the population. The high germination of seedlings and seed vigor not only effect on the initial stage of life cycle of plant, but also the growth of other period. This study showed that the germination of seedlings and seed vigor in maize were obviously inhibited under the 1000, 2000, 3000 mg/L Pb concentration groups.
When the Pb concentration is lower than 100 mg/L, the length, diameter, surface area of maize root showed an increasing trend compared with the control. However, with the increase of the Pb concentration, the length, projected area, surface area and volume of the maize root system gradually decreased and were significantly inhibited, indicating an obvious “low concentration Pb promotes root growth and high concentration Pb inhibits root growth”.
In the low-concentration Pb (< 80 mg/L) environment, the length of root in diameter class root (0 ≤ d ≤ 1.0) is higher than the control, and in diameter class root (2.50 ≤ d ≤ 4.50) it reduces with the increase of Pb concentration. We concluded that high concentration Pb interferes with the plant’s ability to obtain nutrients by reducing the structure of roots to absorb, transport and store nutrients from the soil.
Leaf traits, including the length, area and circumference and so on, may reflect the adaptability of plant to environmental stress. The low concentration of Pb (< 60 mg/L) in circumstance may promote the length, area and circumference of maize leaves compared to the control. But the lowest values of these parameters appeared in 3000 mg/L Pb concentration treatment group with the value of 146.12 mm, 1501.46 mm2, and 515.26 mm, respectively. The effect of Pb on leaf traits is consistent with the change trend of root morphology.
References
Bai ZH, Zhang XL, Duan YF et al (2009) Review on the influence of Lang Use/Cover changes on Soil Quality. World Sci-tech R&D 31(4):682–685
Chaplygin V, Chernikova N, Fedorenko G et al (2021) Influence of soil pollution on the morphology of roots and leaves of Verbascum thapsus L. Environmental geochemistry and health. https://doi.org/10.1007/S10653-021-00975-2
Chen JR (2016) The characters of root tolerance and bioactivity of Moso Bamboo (Phyllostachys pubescens) under the heavy metals stress. Zhejiang Agriculture & Forestry University Master Degree Thesis
Chen TB, Song B, Zhen YM et al (2006) A survey of arsenic concentrations in vegetables and soils in Beijing and the vigor risks to Human Health. Acta Geogr Sin 61(3):297–310
Chen TB, Zheng YM, Chen H et al (2005) Arsenic accumulation in soils for different land use types in Beijing. Geographical Res 24(2):229–235
Chen TB, Zheng YM, Lei M et al (2005) Assessment of heavy metal pollution in surface soils of urban parks in Beijing, China. Chemosphere 60(4):542–551
De Costa W, ZÖrb C, Hartung W et al (2007) Salt resistance is determined by osmotic adjustment and abscisic acid in newly developed maize hybrids in the first phase of salt stress. Physiol Plant 131(2):311–321
Dong XH, Duan LS, He ZP et al (2005) Effects of 30%Diethyl-Amino-Ethyle-Hexanoate·Ethephon soluble concentrate on roots bleeding sap and its components of zea mays. Acta Bot Boreal Occident Sin 25(3):587–591
Du TQ, Yang JZ, He JP et al (2010) The pollution monitoring index system of wheat at different growth stages under the stress of cd, cr and pb. Acta Ecol Sin 30(7):1845–1852
Duan DX, Liu JH (2021) Inhibitory effect of heavy metal lead stress on seed germination and Seedling Growth of Mung Bean. Seed 40(1):84–87
Dai EYJ, Gu JY WL et al (1988) Study on the Growth Law of Maize Root System and its relationship with Yield I. The relationship between the growth and absorption capacity of maize root system and the above-ground parts. Acta Agron Sinica 14(2):149–154
Estival JR, Barasona JA, Mateo R (2012) Blood pb and 6-ALAD inhibition in cattle and sheep from a Pb-polluted mining area. Environ Pollution 160:118–124
Guo CH, Xiao XY, Chen TB et al (2008) Heavy Metal Pollution of Soils and vegetables from Midstream and downstream of Xiangjiang River. J Geog Sci 63(1):3–11
Hou XL, Liu AQ, Cai LP et al (2013) Effects of Pb stress on seed germination and seedlings growth of Pogonatherum crinitum as Pb Accumulator Plant. J Southwest Forestry Univ 33(5):54–58
Jiang R, Liu P, He Y et al (2022) Characteristics of heavy metals in soil of lead-zinc mining areas and changes of antioxidant enzyme systems in maize leaf under pb stress. Int J Coal Sci Technol 9:79. https://doi.org/10.1007/s40789-022-00538-5
Li B, Yuan XC, Zhan FD et al (2019) Effects of heterogeneous cd stress on root growth of Vicia faba and Sonchus asper. J Agro-Environment Sci 36(1):62–70
Li HH Effects of Sulfur on Chlorophyll contents and Root morphology in Sedum alfredii Hance. Environ Sci Technol 36(1):81–84
Li Q, Zu YQ, Wang JX et al (2020) Effects of heavy metal stress on root characteristics of wild Arabis alpina in lead-zinc Ming Area. Guizhou Agricultural Sciences 48(4):148–152
Liu YH, Ding Y, Shi RR (2010) Effects of copper and cadmium pollution on elsholtzia splendens seed germination. J Nanchang Hangkong Univ (Natural Science) 24(1):91–95
Liu JG, Zhang YX, Shi PL et al (2012) Effect of cadmium on seed germination and antioxidative enzymes activities in cotyledon of Solanum nigrum L. J Agro-Environment Sci 31(5):880–884
Ma M, Gong HH, Deng H (2012) Effects of heavy metal stress on seed germination and seedling growth of eight urban pants. Chin Agric Sci Bull 28(22):206–211
Nuzaaiti A, Liu YG, Song HX et al (2010) Effects of Zn and Cu on physiological and biochemical processes and their accumulation characteristics of vetiver. J Agro-Environment Sci 29(1):54–59
Peng DL (2015) Effect on growth and heavy metal accumulation of Moso bamboo (phyllostachys pubescens) under different control measures. Zhejiang Agriculture and Forestry University
Pu QP, Wu YR, Zhu SY et al (2021) Effects of salicylic acid on seed germination and Seedling Growth of Mung Bean under lead nitrate stress.Heilongjiang Agricultural Sciences(3):23–27
Qi J, Song FB, Liu SQ et al (2006) Some physiological response of roots and leaves of zea mays seedling to drought-stress[J]. Ecol Environ 15(6):1264–1268
Qing L, Zu YQ, Li Y et al (2013) Heavy metal contents and Accumulation characteristic of seven wild plants from the Slagheap surrounding of Huize lead-zinc tailings. J Agro-Environment Sci 32(8):1558–1563
Rout GR, Sunita S, Das AB et al (2014) Screening of iron toxicity in rice genotypes on the basis of morphological, physiological and biochemical analysis. J experimental biology agricultural Sci 2(6):561–569
Tan JB, Zhan FD, Liu YY et al (2016) Soil chemical properties and cd form distribution in Vicia faba and Sonchus asper intercropping system. J Agro-Environment Sci 35(1):53–60
Tang GM, Jia YJ, Guo JH et al (2021) Effect of Heavy Metal Pollution on seed germination and seedling growth of peanut and soybean. J Yunnan Normal Univ (Natural Sci Edition) 41(1):64–69
Tao L, Ren J, Zhu GH et al (2007) Advance on the effects of heavy metals on seed germination. J Agro-Environment Sci 26(S1):52–57
Wang YR, Nie MJ, Wang YQ et al (2020) Transcriptome analysis of wheat root in response to heavy metal pb stress. J Henan Agricultural Sci 49(6):8–15
Wei YY, Zhang J, Xie SM et al (2021) The effect of heavy metal cd stress on the seed germination and seedling growth of Dionysus and Phragmites communis. J Anhui Normal Univ (Natural Science) 44(2):145–152
Wen YP, Luo R, Wang ZY et al (2022) Response of castor bean seed germination and seedling growth to heavy metal copper and zinc ion stress. Molecular Plant Breeding
Zhang YB, Liu AR, Dong JG et al (2010) Response of germination and growth of nine Festuca arundinacea Schreb varieties to zinc stress. Chin J Eco-Agriculture 18(2):371–376
Zhang ZJ, Gao J, Cai CJ et al (2011) Absorption and distribution of mineral nutrients in Pleioblastus fortune under lead stress. Scientia Silvae Science 47(1):153–157
Zhang XD, Wang ZW, Han QF et al (2016) Effects of water stress on the root structure and physiological characteristics of early-stage maize. Acta Ecol Sin 36(10):2969–2977
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was supported by special project of Basic Research in Yunnan Local Colleges and Universities (2017FH001-026, 2018FH001-004), the National Natural Science Foundation of China (42167009, 31300349, International Joint Innovation Team for Yunnan Plateau Lakes and Great Lakes of North America which is sponsored by Yunnan Provincial Education Department (to XC), and Scientific and Technological Innovation team Project of Agricultural Resources Utilization of Kunming University.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
He, Y., Jiang, R. & Hou, X. Responses of maize germination, root morphology and leaf trait to characteristics of lead pollution: a case study. Int J Coal Sci Technol 10, 12 (2023). https://doi.org/10.1007/s40789-023-00565-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-023-00565-w