Abstract
Purpose of Review
Cancer is often a complicated and dynamic disease, which makes determining the optimal treatment for a given patient a difficult endeavor. Moreover, even within a particular cancer type, different patients often have varying responses to the same therapies. Bioengineered tumor model systems specific to patients would allow preemptive screening of personalized therapies, facilitating identification of the most effective treatments prior to administration in the patients. Here, we provide an overview of organoid technology, and how these bioengineered tumor models can be harnessed for patient-centric personalized oncology.
Recent Findings
Organoid models have ranged from simple cell spheroids to more complex tumor-on-a-chip systems. The earliest of these models were comprised of easy to culture cell lines, but recent advances in 3D cell culture approaches have facilitated generation of human primary cell-based organoids. Importantly, recent efforts have been made to employ tumor biospecimens from human patients to create personalized tumor models for patient-specific predictive drug screening.
Summary
Bioengineering and tissue engineering technologies have advanced significantly in recent years, culminating in the capability to biofabricate tissue and tumor organoids derived from individual human patients. In the near future, we anticipate such models being implemented in parallel with clinical practice as patient-oriented screening tools, thereby improving the success rates of oncology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: 3D Organoids
The use of bioengineered three-dimensional (3D) tissue and tumor organoids is common across numerous fields, as it is becoming the gold standard for organ and tissue replication ex vivo [1,2,3]. Organoids are generally small-scale cell constructs—much smaller than their in vivo counterparts—that are fabricated in the laboratory to serve as 3D representations of in vivo tissues and organs. These bioengineered platforms support a variety of applications with implications in both research and the clinic. Many of the earliest organoid models take the shape of cell spheroids ranging from simple single-cell line aggregates to heterogeneous multi-cell population spheroids. More advanced organoids include hydrogel-based matrices and components relevant to individual tissue environments. The use of 3D organoid constructs allow for advancement of studies utilizing 3D environments in comparison to traditional two-dimensional (2D) cultures, which can be limiting when trying to replicate tissue-level physiology [4]. 3D culture allows for more nuanced control of cell–cell and cell–matrix interactions, stiffness, addition of biochemical factors, and modulation of tissue density—altogether allowing for tailored extracellular matrix (ECM) to fit the tissue or organ of interest [3]. For applications such as drug development and precision medicine, it is increasingly important that 3D culture systems be utilized to incorporate the many aspects of an in vivo tissue. Aspects like multi-organ communications through organ-on-a-chip technologies and the addition of external factors such as flow or physical forces can be incorporated to better replicate physiologically relevant microenvironments [5, 6]. In recent years, studies suggest that drug development has seen significant improvement in the diversity of assays available due to organoid systems and their in vivo-like properties [7].
Organoids are commonly used for dose- and time-dependent drug compound toxicity studies. Such studies are being conducted on the targeted organ or tissue type, as well as major organs such as the liver and heart that often experience toxicity from drug treatments that are beneficial to target organs or tissue. These studies can be done through connection of organs and tissues to allow for more advanced understanding of system wide behavior or independently to study single organ drug response. Recently published studies have shown evidence that the connection of multiple different tissue type organoids through microfluidic and on-a-chip devices has allowed for a more complete understanding of how the body as a whole may respond to drug [3, 8•, 9•]. Such multi-organoid “body-on-a-chip” platforms have begun to be realized, and while many of the individual of the organ systems housed within these platforms have been characterized, additional work will be necessary for widespread translation to commercial or clinical use. Each of the tissue types within the system can be studied in depth to understand their individual responses to insults, but more importantly, these multi-organoid platforms can be employed to assess integrated responses of other organoids [10]. An example of an application that is feasible already is screening of recalled drugs, which has provided validation of organoid systems in that the organoid platform exhibits negative side effects similar to those reported in human patients by the FDA [3]. These systems are becoming more common among researchers investigating drugs that are being advanced into Phase I clinical trials as doses for administration and their effects over time on multiple human-derived organ systems can be studied before in vivo testing. These current multi-organ systems are in a sense “generic” in that they are comprised of commercially available cells that are sourced from a variety of originating human subjects. As such, the resulting system is not truly personalized but represents a generic human being. The next stage is the development of organ-on-a-chip platforms is to personalize these models. With the engineering and biological expertise gained in the development of the aforementioned body-on-a-chip platforms, researchers can now more confidently pursue development of patient-specific organ-on-a-chip systems as predictive tools for individual patients. Our group, as well as others, are currently moving in this direction using cancer organoids alone or integrated with several other tissue types.
Organoids in Cancer
Implementation of Organoids as Cancer Models
To this point, animal models have been primarily used to study cancer behavior and response to drugs beyond 2D models. Initially, animal models seem attractive, because they can provide complexity reminiscent of the in vivo tumor physiology. However, even beyond infrastructure requirements and ethical questions that accompany the use of animals, the power of these models to predict outcomes in humans is tenuous. Patient-derived tumor xenografts (PDX) technology has recently been introduced to predict how a patient’s tumor will respond to drugs [11, 12]. Small fragments of a patient’s tumor are implanted in immune-deficient mice. Once a tumor fragment has grown to adequate size, the tumor is removed, split into several pieces, and re-implanted into new mice. The main advantages of PDX technology are the following: (1) the patient’s cancer cells are expanded in vivo recreating components of the tumor microenvironment that make significant contributions to the pathobiology of the cancer and (2) the ability to test drugs on the patient’s growing tumor prior to clinical treatment of the patient. However, the PDX technology fails to grow tumors that are below a certain size, and the most successful PDXs come from very aggressive tumors, making this technology applicable to some, but not all, cancer patients [13]. The time in which it takes to grow out the tumors and re-implant then can also take extended periods during which patient need to receive treatment and are unable to benefit from personalized decisions. Such models are additionally used for cancer cell and cancer stem cell isolation and disease modeling with relation to behavior and drug response [14]. Furthermore, the tumor microenvironment for PDX is of murine origin and thus, lacking human-specific stromal elements which often leads to behavioral changes [13].
Cancer research has been limited by the inability to accurately model tumor progression and signaling mechanisms in a controlled, in vitro environment. Beyond the problems with PDX models mentioned above, animal models allow only limited manipulation and study, and are not necessarily predictive of results in humans. Traditional in vitro 2D cultures fail to recapitulate the 3D in vivo microenvironment, importantly by lacking spatio-temporal cues created through matrices [15]. Measurably, drug kinetics vary dramatically between the culture types. Doses effective in 2D are often ineffective in patients, and cell–cell/cell–matrix interactions are inaccurate [16, 17]. Tissue culture dishes differ from in vivo tumors with respect to topography, stiffness, and 2D versus 3D architecture. Furthermore, 2D culture can place selective pressure on cells which can alter molecular and phenotypic properties. We have recently demonstrated that on 2D dishes, metastatic colon carcinoma cells appeared epithelial, but when transitioned into a 3D organoid environment, they “switched” to a more appropriate metastatic phenotype [18, 19]. 3D platforms can mimic in vivo structure, cellular heterogeneity, roles of cell–cell, cell–extracellular matrix, and mechanical interactions [20]. Further advances in microfluidics and microfabrication have led to organ/tumor-on-a-chip platforms with additional functionality [19, 21,22,23].
To date, a wide variety of cancer organoids and tumor-on-a-chip systems have been developed. Organoids for tumor applications are developed utilizing spheroids or biofabrication techniques. Spheroids offer a simple platform for development in which homogenous or heterogeneous cell cultures are allowed to aggregate in hanging drop or U-bottom plastic culture plates (Fig. 1a). Cancer studies can then be done directly on the spheroids in culture, or they can be further removed and placed into more complex or larger systems. Homogenous cultures are of value in that direct tumor cell behavior and interactions between tumor cells of a single origin can be studied. Heterogeneous cultures however offer insight into how a more complete tumor environment may respond to external stimuli. These studies can show how both the tumor and stromal cells respond and further interact with each other under stress. Spheroids can be challenging to use within tumor study applications, as they can grow quickly and develop necrotic cores which are not always representative of the in vivo environment and may inappropriately represent drug efficacy or cell response to stimuli. Outside of academic research, pharmaceutical companies utilize spheroids in high-throughput (384 well or greater) screening of compounds before they are advanced to Phase I clinical trials. 3D is used over 2D culture, as it is universally recognized that it better emulates the in vivo environment. Within research, both homo- and heterogeneous spheroids have been used for the advanced study of tumor cells and their response to treatment. They are now being further employed in applications such as body- and organ-on-a-chip in which they are placed in fabricated devices and studied in connection with other tissue and organ cell types to determine systemic drug response. Despite their simplistic nature, because of the ease at which they can be formed in large numbers, spheroids continue to be widely used in research applications.
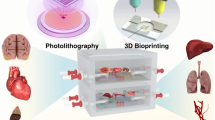
Organoid form factors and use of patient-derived tumor organoids in precision oncology through drug screening assays. Tissue and tumor exist in a number of forms, including a spheroids (homogeneous or heterogeneous) that can be formed by the hanging drop method; b organoids that are formed through aggregation to a biomaterial microcarrier bead in a rotating wall vessel (RWV) bioreactor (arrows represent rotation of the bioreactor vessel); In 3D constructs in which cells are housed in extracellular matrix-based hydrogels that are c bioprinted (arrows indicate X-Y-Z stage movement) or d photopatterned in situ within microfluidic device chambers (indicated by dashed arrow). e By connecting multiple organoids within perfused microfluidic systems, more complex phenomena, such as metastasis from one site to another, can be observed in vitro. Blue arrows: direction of recirculating flow; red arrows: metastasis of tumor cells from a gut organoid to a liver organoid. f A workflow of how patient-derived organoids can be deployed for precision oncology. Blue arrows: the current state of the art precision medicine pipeline, in which therapies are designed for patients based on their tumor genetic profiles. However, in practice, even after identification of key mutations, oncologists are often left with several potential drug options, resulting in a best guess of the optimal treatment. Moreover, statistically, precision medicine efforts have only benefited a small subset of cancer patients to date. Red arrows: Implementation of organoids created with patient cells can supplement genetic screens of biopsied tumor cells, ultimately predicting the optimal therapies for patients. g An example of a drug panel screen, assessing viability of patient-derived appendiceal cancer tumor organoids subjected to a variety of drugs
Biofabricated organoids can be created using a wide range of methods related to placing cells in 3D suspension. Such methods include rotating wall vessels, 3D bioprinting, photopatterning, and by hand suspension. Each of these methods offers a range of benefits and are ultimately selected based on which has ideal properties for the intended application. Rotating wall vessels allow for cells to self-aggregate around microcarrier beads (Fig. 1b) in which various materials can be used for bead formation to vary cell adherence and behavior in 3D [24]. This method prevents necrotic cores commonly seen in spheroids from developing and allows for many structures to be created in parallel for experimentation. 3D bioprinting, which has made substantial advancement in the past 15 years, is commonly used to create complex, multi-zoned 3D organoids (Fig. 1c). Many different bioprinter modalities exist including use of inkjet-like printers, extrusion-based devices, and laser-assisted devices. Of these methods, each has ideal print speeds, resolution, cell densities, and cell viability after printing which are considered when selecting print type [25,26,27]. Photopatterning strategies have been implemented to integrate 3D tissue and tumor constructs within microfluidic devices. Through UV or blue light exposure, biomaterials with added crosslinking components are able to form solid structures through photomasks to yield defined shapes and locations in situ within microfluidic tumor-on-a-chip devices (Fig. 1d) [28]. Such methods allow for designs and locations that would be previously challenging to make possible. Additional complexity and physiological relevance can be realized by creating multiple tissue and tumor organoids and combining them in a single closed system. This facilitates study of phenomena such as metastasis, where events take place in two locations—a primary tumor site and a downstream tissue. Notably, we recently demonstrated such a system using a metastasis-on-a-chip device to model metastasis of colorectal cancer cells from a gut organoid to a liver organoid (Fig. 1e). Biomaterials are utilized for each fabrication technique highlighted which allows for more physiologically relevant components to be added to culture as previously described. Components commonly used for the study of tumor cells specifically include collagen, hyaluronic acid, laminin, and fibronectin among many others [29].
Each of these organoid formation modalities has allowed for advancement of tumor-on-a-chip devices. The tumor organoids are created using biomaterials with single-cell or spheroid suspensions and that are placed within micro-devices or systems. Being open or closed loop, they allow for cross organ and tissue communication while supplying the system with adequate nutrients, oxygen, and external factors which may include drug treatment. These technologies have advanced to reduce the overall size of the devices used to allow for them to be produced in both research and industrial capacities on plates as small as 384 wells which allow for a large number of studies to be conducted in parallel.
Organoids in Precision Medicine
Most often, a drug or drug combination is administered based on statistical likelihood of success in the broader population, and actual effectiveness in a particular patient is assessed only after the fact. In patients with intrinsic or acquired resistance to the treatment, this results in further growth of the tumor and a loss of critical treatment time. Additional drugs can then be investigated, but only serially and with each one still being a “best guess” with diminishing probabilities of success. An ideal solution would be a method by which a tumor could be probed outside of the patient, where multiple candidate treatments could be investigated in parallel to determine effectiveness without loss of time or potential harm to the patient. The central challenge in conventional cancer treatment design is that there is only one reliable test bed: the patients themselves.
Currently, it is preemptively unknown which patients will benefit from systemic chemotherapy. It is also unknown if systemic chemotherapy should be delivered prior to surgery or after surgery. This is a crucial decision that has to balance the risk of disease progression, in the case of a chemotherapy-resistant tumor, against the benefits from reducing the volume and therefore extent of surgical resection, for a potentially chemotherapy-sensitive tumor. Precision medicine currently identifies tumor mutations and correlates them with available drugs without being able to predetermine, if those mutations for the individual patient, represent downstream actionable targets for the proposed drug. Therefore, the efficacy of the proposed therapy is being determined only by the outcome.
Precision medicine can be defined as individualized diagnosis and treatment using diagnostic and therapeutic strategies targeting patient- or disease-specific genetic, proteomic, and phenotypic characteristics [30]. Such innovations have become vital for the advancement of patient-oriented prognosis, diagnosis, and treatment. Organoids have become a tool within precision medicine efforts for a number of reasons; for instance, they require a minimal number of cells to replicate the in vivo microenvironment, and they can be used for many precision applications to determine specific primary cell and patient outcomes [9•].
As previously described, many organ systems have been the basis for both healthy and diseased organoid models; however, they incorporate commercially available cells that may not represent a patient’s unique physiology. Integrating patient cells into organoid models brings a patient’s biology to the bench for diagnosis and prognosis. These studies are advantageous over cell–line, or even commercial primary cell disease studies, as they offer insight into natural genetic variation, cell-type mixture, and patient-specific behavior. Precision medicine can broadly concern methods, techniques, and analyses that yield exact results for individual patients and their disease state. Such studies no longer generalize disease but seek to more precisely understand its behavior and response to treatment to benefit the patient as well as gain greater insight into disease.
Precision medicine strategies require the isolation of patient cells, integration into a model system, and subsequent experimental study. For personalized organoid development, tissue is isolated directly from the patient and processed for single-cell culture use. Isolation is commonly carried out with diseased tissue resections or biopsies. Models may also use human induced pluripotent cells (hiPSC) techniques by gathering easy to isolate cells from patients, dedifferentiating the cells into hiPSCs, before differentiating the cultures into desired cell types for study. This type of culture can create its own challenges; however, due to the nature of hiPSC, there is often variability in the differentiation process, and results can be unpredictable or unrepresentative of the disease state [31].
Thus far, few patient-oriented personalized organoid models have been developed for the study of disease behavior or their response to external stimuli. Although it has been found that 3D models yield different and potentially more in vivo-like results than 2D culture, most personalized models to this point have been in 2D [32]. The gap between the use of patient-derived cells for personalized medicine and organoid culture is closing. Models related to cancer have started to integrate patient isolated cells to recapitulate the in vivo microenvironment for drug screening and behavior prediction [33, 34]. Notably, we recently published a study in which we created mesothelioma tumor-on-a-chip (TOC) devices, and performed drug screens to demonstrate that like patients, their organoids and TOC demonstrate matched selective responses to drugs and can be targeted via PM data from our Cancer Center [35••]. Looking forward, precision medicine applications are being designed to bridge the gap between research and clinical space. Such technologies have been implied to have use in clinical trials for testing drug treatments previous to or in conjunction with animal models with the intentions of better replicating the in vivo patient response [36,37,38]. These screenings would allow for many different patient types to be tested such as those with other disease states or conditions that may affect drug efficacy previous to actual patient trials [39, 40]. Results would be able to indicate groups that may be at higher or lower risk for adverse events and aid in pre-selecting patient populations that would be most responsive to treatment [41]. Additionally, results may expose adverse events related to treatments that were not identified in animal models and thus determine that treatments should not be administered to human patients [42]. Importantly, such technologies, if FDA approved and implemented in clinical trials, may be able to lower pharmaceutical industry attrition rates by representing a more comprehensive range of the human population in a low-cost manner and preventing drugs with negative side effects from being advanced to phase III clinical trials.
Patient-Specific Organoids From Tumor Biospecimens
To solve this problem within the framework of precision medicine, we have micro-engineered in our lab tumor organoids directly from fresh tumors [43]. These organoids replicate the tumor microenvironment, allowing for cellular biomarker recognition, biomarker expression quantification, and real-time testing of chemotherapy drug efficacy. These systems are human, 3D, and replicate in vivo conditions, resulting in a platform that more accurately models human tumor physiology than other models. Our tumor organoids respond to chemotherapy agents can be manipulated mechanistically and are housed in microfluidic devices—resulting in personalized tumor-on-a-chip systems [19, 28]—enabling parallel screening of multiple drugs and linking of multiple tissues and tumor together in a systems biology approach. Implementation of organoids or tumor-on-a-chip platforms created with patient cells can supplement genetic screens, ultimately predicting the extent of malignancy and allowing robust prediction of optimal therapies for individual patients prior to administration to the patient (Fig. 1f). Importantly, we can biofabricate patient-specific organoids quickly and cheaply, and perform drug screens of varieties of drug types and concentrations, obtaining results within less than a week from the surgical event. In comparison, typical genetic sequencing services do not result in actionable data sets for 3–4 weeks on average.
To date, we have generated sets of patient tumor-derived organoids from a variety of tumor biopsies, including lung, mesothelioma, melanoma, colorectal, appendiceal, and sarcoma tumors, with above an 80% take rate in vitro (versus approximately 20–30% in traditional 2D cell cultures and 30–40% in patient-derived xenograft models) and have employed them in preliminary drug screens to demonstrate that like patients, their tumor organoids demonstrate selective response to different drugs. While personalized tumor organoid technology is in its infancy, it holds incredible clinical potential. Once validated through correlation, wide adoption of such systems may be able to significantly improve outcomes of cancer patients and reduce unnecessary health care costs through quick matching with the best available effective drugs at the single patient level. These 3D tumor organoids can be biofabricated in high-throughput fashion, resulting in viable sets of patient-specific models that can be implemented in drug screens. These drug screens can be designed to replicate the actual therapy the patient is receiving, thus providing insight and potentially a predictive element as to the likelihood of a successful patient response. Moreover, as we can create large numbers of organoids from a single, relatively small tumor biospecimen, we can not only screen the treatment in question, but nearly any other potential drug or drug cocktail (Fig. 1g). Our goal is to demonstrate tight correlation between the organoid platform drug responses and patient treatment responses, thus providing a reliable platform that allows accurate prediction of which therapy will be most effective for a given patient. Following screening of prospective drug treatment options, the results from the screens can then aid oncologists in designing optimal therapies for the patient.
Conclusion
Tumor organoid platform technologies for personalized medicine face many commercialization challenges as the methods for cell isolation and characterization, organoids fabrication, drug screening, and efficacy testing must all be standardized. Many of these methods, while commonplace within research, have not been used in FDA-regulated settings and would need modification and approval to be used as part of the FDA regulatory process. Additionally, hospital staff, training, and facilities would have to be adapted to accommodate the systems being used. However, there are already companies built on organoid and organ-on-a-chip technology that are already working with the FDA to receive the necessary approval to use such systems in the drug development pipeline. Once these technologies are vetted appropriately, these systems will be a gateway for personalized medicine applications to be more easily commercialized and could easily be seen in use as a clinical resource in the next 5 to 10 years.
The continuing goal of the described technology is the implementation of tumor organoids and tumor-on-a-chip platforms that will serve as advanced models applicable to a variety of cancer types, and that will be deployed for individual patients. Patient-centric drug screens will be performed, providing predictive data that will aid oncologists in determining the most effective therapies for each patient. Additional advancements will further expand the capabilities of tumor organoid technology, potentially allowing for assessment of more complicated therapies, such as immunotherapies, and the use of healthy tissue organoids for evaluation of treatment side effects. Ultimately, if successfully deployed, tumor organoid technology has the potential to significantly drive advancements in oncology and change the way patients are treated.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mills M, Estes MK. Physiologically relevant human tissue models for infectious diseases. Drug Discov Today. 2016;21(9):1540–52.
Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125.
Skardal A, Shupe T, Atala A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov Today. 2016;21(9):1399–411.
Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8(10):839–45.
Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng. 2011;13:55–72.
Kang L, Chung BG, Langer R, Khademhosseini A. Microfluidics for drug discovery and development: from target selection to product lifecycle management. Drug Discov Today. 2008;13(1–2):1–13.
Astashkina A, Grainger DW. Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev. 2014;69-70:1–18.
• Zhang YS, Aleman J, Shin SR, Kilic T, Kim D, Mousavi Shaegh SA, et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc Natl Acad Sci U S A. 2017;114(12):E2293–E302. This study describes organ-on-a-chip devices with a sophisticated biosensor suite that allows automated and real-time sensing of organoid health and behaviors
• Skardal A, Murphy SV, Devarasetty M, Mead I, Kang HW, Seol YJ, et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep. 2017;7(1):8837. This study demonstrates the development and implementation of multiple human organoids in a single perfused “body-on-a-chip” platform and showcases several integrated drug studies in which the function of one organoid type influences outcomes of the other organoid types
Skardal A, Devarasetty M, Rodman C, Atala A, Soker S. Liver-tumor hybrid organoids for modeling tumor growth and drug response in vitro. Ann Biomed Eng. 2015;43(10):2361–73.
Rosfjord E, Lucas J, Li G, Gerber HP. Advances in patient-derived tumor xenografts: from target identification to predicting clinical response rates in oncology. Biochem Pharmacol. 2014;91(2):135–43.
Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer discovery. 2014;4(9):998–1013.
Kamb A. What’s wrong with our cancer models? Nat Rev Drug Discov. 2005;4(2):161–5.
Williams SA, Anderson WC, Santaguida MT, Dylla SJ. Patient-derived xenografts, the cancer stem cell paradigm, and cancer pathobiology in the 21st century. Lab Investig. 2013;93(9):970–82.
Kunz-Schughart LA, Freyer JP, Hofstaedter F, Ebner R. The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. J Biomol Screen. 2004;9(4):273–85.
Ho WJ, Pham EA, Kim JW, Ng CW, Kim JH, Kamei DT, et al. Incorporation of multicellular spheroids into 3-D polymeric scaffolds provides an improved tumor model for screening anticancer drugs. Cancer Sci. 2010;101(12):2637–43.
Drewitz M, Helbling M, Fried N, Bieri M, Moritz W, Lichtenberg J, et al. Towards automated production and drug sensitivity testing using scaffold-free spherical tumor microtissues. Biotechnol J. 2011;6(12):1488–96.
Skardal A, Devarasetty M, Rodman C, Atala A, Soker S. Liver-tumor hybrid organoids for modeling tumor growth and drug response in vitro. Ann Biomed Eng. 2015;43:2361–73.
Skardal A, Devarasetty M, Forsythe S, Atala A, Soker S. A reductionist metastasis-on-a-chip platform for in vitro tumor progression modeling and drug screening. Biotechnol Bioeng. 2016;113:2020–32.
Devarasetty M, Wang E, Soker S, Skardal A. Mesenchymal stem cells support growth and organization of host-liver colorectal-tumor organoids and possibly resistance to chemotherapy. Biofabrication. 2017;9:021002.
Benam KH, Dauth S, Hassell B, Herland A, Jain A, Jang KJ, et al. Engineered in vitro disease models. Annu Rev Pathol. 2015;10:195–262.
Bhise NS, Ribas J, Manoharan V, Zhang YS, Polini A, Massa S, et al. Organ-on-a-chip platforms for studying drug delivery systems. J Control Release. 2014;190:82–93.
Polini A, Prodanov L, Bhise NS, Manoharan V, Dokmeci MR, Khademhosseini A. Organs-on-a-chip: a new tool for drug discovery. Expert Opin Drug Discovery. 2014;9(4):335–52.
Barrila J, Radtke AL, Crabbe A, Sarker SF, Herbst-Kralovetz MM, Ott CM, et al. Organotypic 3D cell culture models: using the rotating wall vessel to study host-pathogen interactions. Nat Rev Microbiol. 2010;8(11):791–801.
Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–85.
Skardal A. Bioprinting essentials of cell and protein viability. In: Atala A, Yoo JJ, editors. Essentials of 3D Biofabrication and Translation: Elsevier; 2015.
Skardal A, Atala A. Biomaterials for integration with 3-d bioprinting. Ann Biomed Eng. 2015;43(3):730–46.
Skardal A, Devarasetty M, Soker S, Hall AR. In situ patterned micro 3D liver constructs for parallel toxicology testing in a fluidic device. Biofabrication. 2015;7(3):031001.
Murphy SV, Skardal A, Atala A. Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A. 2013;101(1):272–84.
Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med. 2015;372(23):2229–34.
Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13(5):497–505.
Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125(Pt 13):3015–24.
Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med. 2015;21(11):1364–71.
Sachs N, Clevers H. Organoid cultures for the analysis of cancer phenotypes. Curr Opin Genet Dev. 2014;24:68–73.
•• Mazzocchi AR, Rajan SAP, Votanopoulos KI, Hall AR, Skardal A. In vitro patient-derived 3D mesothelioma tumor organoids facilitate patient-centric therapeutic screening. Sci Rep. 2018;8(1):2886. This study describes biofabrication of tumor organoids and tumor-on-a-chip devices using actual patient tumor-derived biospecimens, and subsequent drug screening studies that correlate with patient drug responses.
Capulli AK, Tian K, Mehandru N, Bukhta A, Choudhury SF, Suchyta M, et al. Approaching the in vitro clinical trial: engineering organs on chips. Lab Chip. 2014;14(17):3181–6.
Fabre KM, Livingston C, Tagle DA. Organs-on-chips (microphysiological systems): tools to expedite efficacy and toxicity testing in human tissue. Exp Biol Med (Maywood). 2014;239(9):1073–7.
Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20(6):616–23.
Strauss DG, Blinova K. Clinical trials in a dish. Trends Pharmacol Sci. 2017;38(1):4–7.
Carvajal-Vergara X, Sevilla A, D'Souza SL, Ang YS, Schaniel C, Lee DF, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465(7299):808–12.
Mou H, Brazauskas K, Rajagopal J. Personalized medicine for cystic fibrosis: establishing human model systems. Pediatr Pulmonol. 2015;50(Suppl 40):S14–23.
Usta OB, McCarty WJ, Bale S, Hegde M, Jindal R, Bhushan A, et al. Microengineered cell and tissue systems for drug screening and toxicology applications: evolution of in-vitro liver technologies. Technology (Singap World Sci). 2015;3(1):1–26.
Mazzocchi AR, Soker S, Skardal A. Biofabrication technologies for developing in vitro tumor models. In: Soker S, Skardal a, editors. Tumor organoids. Berlin, Germany: Springer Nature; 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Andrea Mazzocchi and Konstantinos Votanopoulos declare that they have no conflict of interest.
Aleksander Skardal reports a pending patent on cancer modeling platforms and methods of using the same.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Artificial Tissues
Rights and permissions
About this article
Cite this article
Mazzocchi, A., Votanopoulos, K. & Skardal, A. Personalizing Cancer Treatments Empirically in the Laboratory: Patient-Specific Tumor Organoids for Optimizing Precision Medicine. Curr Stem Cell Rep 4, 97–104 (2018). https://doi.org/10.1007/s40778-018-0122-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-018-0122-z