Abstract
Purpose of Review
The role of tissue-specific stem cells, differentiation, and the resulting phenotypic heterogeneity in evolution of new mutations has attracted the interest of theoreticians and experimentalists. In the current paper, we first summarize the literature on population dynamics of stem cell and differentiated populations, in the absence of somatic mutations, with focus on applications to cancer therapeutics. We then review recent experimental and theoretical works on the evolutionary dynamics of stem cells and their progenitors in the presence of rare somatic mutation.
Recent Findings
The recent experiments show that the selection process among intestinal stem cells closely follows the previously proposed Moran model in finite populations. We also overview recent theoretical developments in the literature that generalizes selection processes inside the tissue with variable level differentiation and de-differentiation.
Summary
This review tries to give a mathematical description on how phenotypic heterogeneity due to stem cell hierarchy influences growth dynamics and natural selection, inside an epithelial tissue or hematopoietic system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many tissues in human body consist of a heterogeneous population of cells where different phenotypes commit to performing specific functions. At epithelial layers, an isogenic population of cells, i.e., cells with same genetic makeup, is commonly presented in a variety of phenotypically distinct cells performing different functions. Such populations are usually structured in hierarchical multi-component forms. Different cells can be identified by their morphology, (epi) genetic information, and/or functional features within the micro-environment [1,2,3]. A particular surface protein can be associated with a particular functional property of the cell. An important functional property of the cell is its repopulating capacity. This is represented by maturation or differentiation levels in the cell. Tissue-specific somatic (adult) stem cells are a phenotype that has complete proliferation potential. They replenish the tissue cell population and are responsible for regeneration after damage to the tissue. Adult stem cells can be multiipotent, that is, they can undergo various differentiation programs to create functionally distinguished fully committed lineages [1,2,3,4]. The stem cell proliferation scheme inside different tissues can be modeled using mathematical approaches. During mitosis, each stem cell divides (self-renews) into two daughter stem cells. It can also divide asymmetrically into one daughter stem cell and one partially differentiated progeny or symmetrically differentiate into two partially differentiated progenies. Partially differentiated cells (or progenitors) undergo a hierarchical division scheme, during which each cell divides into a more mature progeny. After a finite number of divisions, which is a tissue-dependent factor, the progeny reaches a fully committed stage (Fig. 1). This differentiation hierarchy enables one stem cell to produce a large number of functionally differentiated cells [4,5,6,7]. Unlike adult stem cells, partially differentiated cells, or transient amplifiers (TA), have limited proliferation capacity. There are several examples of mammalian tissues with such hierarchical structure such as hematopoietic system, the epidermis, the intestinal tract, and the mammary gland. Stem cells in other tissues such as liver and pancreas have limited proliferation capacity in steady state and are only activated upon tissue damage [1, 8]. Hematopoietic stem cells, stem cells in the intestinal and skin epithelia are the best understood [9,10,11] (See also [12] and references therein).
The tissue hierarchical organization. Stem cells are a small subpopulation (at far left). Upon successive differentiation events, a stem cell population can create a larger population of fully functional differentiated progeny (far right). The compartments in between represent partially differentiated, or transient amplifier (TA) cells. Upon successive divisions, progenitors express higher maturation level. For brevity, all various lineages of progenitors are colored the same
A similar hierarchical structure exists among tumor cells [13,14,15,16,17]. Similar to adult stem cells, cancer stem cells have self-renewal capacity. Most importantly, it has tumor maintenance capacity. It can also express invasive characteristics, such as higher mesenchymal marker expression levels [18]. Several markers of cancer stem cells are known for various cancers. In breast cancer, the subpopulation of CD44hi/CD24lo has stem-like tumor maintaining capacity [17]. In Glioblastoma, CD133+ is a known cancer stem cell marker [14, 19].
Stem cell “plasticity” is a controversial subject. In the drosophila germ line, after ablation of stem cells, progenitor cells can become plastic and transform back into a stem cell phenotype. A similar de-differentiation mechanism is observed among tumor cells in vitro. Chaffer et al and Gupta et al [20, 21] observed that a sorted population of tumor cells with differentiated cell markers can grow back into a mixed population with stem and differentiated markers. (These observations have been debated to be caused by imperfect markers, [22]). More recently, de-differentiation in tumor cell lines has been reported in vivo by Tetteh et al. [23••] (See also [24]) They observed that enterocytes in intestinal crypts can dedifferentiate to replace lost Lgr5+ stem cells and dedifferentiating enterocytes generate proliferative stem cells. They concluded that stemness is not “hard-wired” in the intestinal crypt but rather progenitor can regain stemness upon loss of stem cell niche population. There has been a great deal of literature on the notion of plasticity and phenotypic heterogeneity in cancer cells [12, 25, 26]. (For discussion of adult stem cell plasticity in normal tissues see [27, 28]).
Understanding the underlying mechanisms of growth and homeostasis in tissue structures is greatly enriched by having a quantitative understanding of stem cell-progenitor cell dynamics. Tissue responses to environmental stresses are determined by this phenotypic structure. For example in cancer therapeutics, tumor response to the therapy, post-treatment, and fraction of cancer stem cells surviving the treatment and not total number the tumor cells, is an important factor to quantify the tumor response to therapies.
In the presence of somatic mutations, we have to consider evolutionary dynamics in the stem cell hierarchy. If an “unwanted” somatic mutation appears in the tissue, its progeny can spread across the tissue if the original mutation started among the stem cell population and keeps indefinite proliferation capacity. If the cell that is mutated is not a stem cell (and does not become transformed by the mutation) then its progeny will be lost from the tissue after a limited number of divisions. This reduces the chance of successful malignant mutations in a tissue by limiting them to stem cells which are a much smaller subpopulation inside the tissue. The evolutionary dynamical models for a hierarchical structure are the most relevant in modeling cancer evolution. It has been hypothesized that tissues with high cell turnover suppress the chance of malignant mutation and cancer due to the hierarchical structure [29,30,31,32]. More recent generalized modeling approaches [33] prove this to be accurate and independent of details of the cell differentiation processes inside the tissue.
Recently, Tomasetti and Vogelstein [34•] argued that lifetime cancer risk in different organs is related to stem cell turnover rate in those tissues by a simple power law. They further concluded that this implies that environmental effects have much less role in carcinogenesis than previously believed. The latter conclusion has been debated by other authors [35, 36].
Hierarchical Population Dynamics
The growth dynamics of stem cells and their progenies are discussed to some extent in the literature [37,38,39,40,41,42,43,44]. One can begin with a system of ordinary differential equations to describe the population of stem cells and the progenitors and terminally differentiated cells. Most models differ on how differentiation and maturation processes are introduced into the dynamics; however, asymmetric division of stem cells is mostly common among different models. Discrete-time growth dynamics is also discussed in the literature [23••, 45].
Each stem cell divides with a rate representing its fitness, r S. With probability, w, the mitosis event is a symmetric proliferation with two stem cell offspring. With probability, v divides asymmetrically into one daughter progenitor and one daughter stem cell, and with probability, u divides into two daughter progenitors (see Fig. 2). Progenitor cells are categorized into several compartments based on their maturation level. The cell inside ith compartment is denoted by P i . Commonly, early progenitors such as P 1 show low levels of differentiation markers and as they divide into more mature cells, the differentiation expression levels increase.
Stem cell asymmetric division scheme. Each adult stem cell can divide into a) two daughter stem cells or b) into one daughter stem cell and one daughter progenitor or c) two progenitor offspring. The partially differentiated cells (TA cells) divide into d) cells with higher maturation levels. e) They reach full maturation after K generations. The division rate of stem cells and progenitors are denoted by r S and r P, respectively. The probabilities for stem cell self-renewal and asymmetric and symmetric differentiations are w, u, and v (w + u + v = 1)
We denote the stem cell population at time t with N 0(t) and differentiated progeny as N i (t), where i ≥ 1 represents the ith progenitor compartment. The population corresponding to =K , N K (t), denotes population of terminally differentiated cells. Any cell with 1 ≤ i < K is a partially differentiated subtype. A system of ordinary differential equations can describe such population dynamics (see Figs. 1 and 3). Parameter p is u + 2v while 1 − p = w. The equations in Fig. 2 are exact when asymmetric divisions happen at a much smaller rate. Werner et al. [43] solved this system of equations and derived analytical solutions for the population of each compartment at a given time t (see also [42]). They obtained distinct exponential/power-law behavior for short- and long-terms. The time to extinction for a seeding cell—such as a single malignant mutant—can be expressed analytically. As expected, the initial condition for such growth is, to begin with an initial number of stem cells. The above formalism is used to model tumor response to chemo- or radiation therapies. Most noticeably, the two-phase behavior observed by Werner et al. [44] can be used to model tumor growth dynamics after a treatment.
The dynamics of stem cell and its progenitors. Stem cell population at time, t, is denoted by N 0(t). N i (t), the ith progenitor population. N K (t) denotes committed tissue cells. Stem cells differentiate with rate p and self-renew with rate 1-p . Parameters r s r p is the division rate of the progenitor population. d is the death rate. The exact solutions for the system of ordinary differential equations can be obtained. It describes two growth phases for short term and long term. (See the main text and also Werner et al. [44])
For small populations, stochasticity in cell division times needs to be taken into account. For example, this is the case for sphere formation assays where a limiting dilution of stem cells is used to create aggregates of cells. (Notice that the total number of cells in assay might be large but the characteristic population number is that of each sphere or aggregate of cells). The survival probability of a stem cell (or a progenitor) can be calculated using branching process variations of the system of equations in Fig. 2 [40, 46, 47]. tumor control probability (TCP) as a measure of treatment success in cancer therapeutics can be modeled using a similar approach (see [46] and references therein).
Stem Cell Evolutionary Dynamics Inside the Niche
Stem cells can be the target of malignant mutations leading to diseases such as cancer. A mutation can affect reproductive fitness of stem cells. Populations of stem cells inside a niche tend to be small and therefore the clonal expansion of mutant cells is sensitive to drift and stochastic fluctuations. The evolutionary dynamics for stem cells should be modeled with drift and finite population effects in mind. To measure the effect of initial genetic mutations in intestinal cancers, Vermeulen et al. [48] (also [49••, 50]) visualized and quantified stem cell dynamics in the mouse intestine. The model focuses on the evolutionary dynamics inside the stem cell niche and ignores differentiation. The authors demonstrated that their measurements for fixation probability of mutant stem cells inside stem cell niche are in very excellent agreement with Moran process on a cycle of size N = 5 [31, 51]. In this model, at each time step, a mutant stem cell replaces a neighboring cell—upon a reproduction event, with rate r, while a wild type (WT) resident stem cell replaces a neighboring cell with rate 1. The sequence of events leading to fixation or extinction of mutant stem cells and the analytic result for the fixation probability as a function of relative fitness, r, are presented in Fig. 4.
Clonal expansion of a mutant stem cell in intestinal crypt base. Crypt base stem cells divide with relative fitness r (mutant) and 1 (WT) and the offspring replaces a neighboring cell in the base. The probability of fixation of a single mutant stem cell is given by the Moran result (in the middle). The mutant cells are depicted with red and normal cells are in blue. The transition rates for gain or loss of mutant are denoted in the figure. Vermeulen et al. showed that the results is in excellent agreement with measurements of fixation probability for Apc, Kras, and p53 mutants in mice with stem cell base population of N = 5
The initiating genetic mutations in colorectal cancers often include inactivation of the Apc tumor suppressor gene, Kras activation, and p53 inactivation. Vermeulen and coworkers [48, 49••] traced the mutant and WT stem cell populations in the niche over time until one of the types reaches fixation. By comparing the fixation probability from mice intestinal models and that of Moran process, the fitness of an Apc −/+ mutant cell in a background of Apc −/− wild-type cells are measured to be 1.63. Similarly, the fitness of an Apc +/+ mutant cell in a background of Apc −/+ cells is r = 2.22. Activation of Kras oncogene has the most pronounced effect, with the relative fitness of r = 3.54. These results are particularly interesting as they denote high fitness gains. Inactivation of p53 tumor suppressor gene, however, had very small fitness gains/loss and only resulted in selective advantage in inflammatory environments.
More recently, Kaveh et al. [51] discussed a more generalized model with variable birth rate, r, and death rate, d, as well as birth-death versus death-birth update rules. The authors derived a range of values for r and d that correspond to the same selection dynamics and measured fixation probabilities by Vermeulen et al. They were also able to show that a birth-death model can fit the measurements while a seemingly equivalent death-birth process gives rise to unrealistic death rate values.
Differentiation and Plasticity
So far, we discussed that how asymmetric division scheme of stem cells and potential presence of de-differentiation events affect growth dynamics (Fig. 3). Also, we summarized novel observation that shows mutant stem cell dynamics in the crypt base accurately follow finite population evolutionary models that has been hypothesized in the literature. For evolutionary dynamics, the question is how differentiation and plasticity [20, 21, 23••, 24] affect the selective advantage (or disadvantage) of a mutant raised in the population. To elaborate this, consider only differentiation events in the absence of any de-differentiation. Now the fitness advantage of a mutant stem cell is not determined by how it can produce a new mutant stem in a given time. One genotype (say mutant) might divides more often than the competing type (WT) but instead produces more differentiated progenies instead of stem cell offspring. This means that while mutant stem cells might be disadvantaged inside a niche, their differentiated progenies might be evolutionarily advantageous. Introduction of de-differentiation and plasticity makes it even more complicated. To resolve this seemingly contradictory issue, we need to generalize quantitative model of natural selection in this population structures with hierarchy and plasticity.
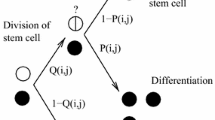
Consider two populations of wild type and mutant cells. Each genotype is divided into a phenotypically different subpopulation of stem cell and progenitors. Stem cells self-renew and replenish their own population or differentiate and increase the differentiated cell population (Fig. 5). Due to homeostasis, stem cell and differentiated cell populations have almost constant sizes, denoted by N S and N P, respectively. In the absence of de-differentiation, fixation probability of a random mutant is given by equation in Fig. 5 [52•] (See also [53, 54]). This is the generalization of Moran result (Fig. 3). If one or both types are plastic, there is a chance that a differentiated cell can transform back into a stem-like state. Kaveh et al. [55] modeled de-differentiation using the replicator equation formalism for the selection dynamics and estimated selective advantage of the plastic mutant against WT population. The authors estimated average time to fixation as a function of stem cell niche size and plasticity rate as well as the relative fitness of WT and mutant stem cells [55, 56]. It is observed that higher de-differentiation rates can elevate a deleterious mutant to an advantageous one. Mahdipour et al. used a generalized framework of Kaveh et al and Jilkine et al [55, 56] to obtain exact results for the fixation probability of a mutant starting in either stem cell or differentiated populations as a function of stem cell and progenitor/differentiated cell proliferation rates as well as differentiation and de-differentiation rates.
Evolutionary dynamics of stem cell-differentiated population in the presence of differentiation and plasticity. Mutant (red) and normal stem cell (blue) compete inside a niche of size N S . Their differentiated progeny (orange for mutant and light blue for WT) compete in the differentiated compartment of size N P . Differentiation and de-differentiation link two selection processes. The differentiation probabilities of mutant and WT types are p 2 and p 1, respectively. Relative division rate of mutant stem cell to WT is r S . In the absence of plasticity the fixation probability of a random mutation is given by the formula on the left. (Dashed circles (blue or red) represent the offsprings of an asymmetric differentiation or de-differentiation event where the offpsring is moved to the corresponding compartment)
Discussion and Conclusions
Quantitative modeling of tissue hierarchy where a stem cell population is responsible for sustaining the cell turnover inside the tissue is of substantial importance in understanding the underlying biology. While almost all cells in the tissue are genetically identical, variation in phenotypes presents itself in varying proliferation potential across tissue. Stem cells are the subpopulation with long-term (generally for the life of the organism) proliferation capacity. They generate the functional differentiated cell population by a continuous process of producing short-lived partially differentiated cells that divide a finite number of times until reaching maturation. From the evolutionary dynamics point of view, this hierarchical structure lets a small population of cells maintain and replenish a large tissue population while avoiding the risk of unwanted mutations [29, 30, 33]. Stochastic evolutionary models that account for drift and selection predict the probability and time for a random mutant to undergo successful clonal expansion. These models can explain clonal evolution of a mutant stem cell inside its niche [48,49,50]. The variable proliferation potentials of stem cells lead to complex dynamics that blur the hierarchy and presumed distinction between stem cells and their progeny. More recent studies [52•, 55, 56] have generalized the well-known evolutionary dynamical model for natural selection of somatic mutants inside the tissue by taking into account of the details of proliferation dynamics that sustain tissues phenotypic heterogeneity. These mechanisms are asymmetric division of stem cell population in the tissue and hierarchical differentiation or maturation process. In addition to the potential de-differentiation that returns a partially differentiated cell into a stem cell. The above models are particularly important to describe cancer progression. This is also important in cancer therapeutics where variable selective pressures appear as TIC cells (stem-like cells) have different treatment response than tumor population.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Visvader JE, Clevers H. Tissue-specific designs of stem cell hierarchies. Nat Cell Biol. 2016;18(4):349–55.
Marjanovic ND, Weinberg RA, Chaffer CL. Cell plasticity and heterogeneity in cancer. Clin Chem. 2013;59(1):168–79.
Lin SA, Barker N. Gastrointestinal stem cells in self-renewal and cancer. J Gastroenterol. 2011;46(9):1039–55.
Visvader JE, Stingl J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev. 2014;28:1143–58.
Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441(7097):1068.
Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132(4):583–97.
Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012;11(4):452–60.
Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63.
Fuchs E, Horsley V. Ferreting out stem cells from their niches. Nat Cell Biol. 2011;13:513–8.
Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318–26.
Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145(6):851–62.
Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22(3):457.
Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17(3):313.
Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14(3):275–91.
O'Brien CA, Kreso A, Dick JE. Cancer stem cells in solid tumors: an overview. In: Seminars in radiation oncology. vol. 19. Elsevier; 2009. p. 71–77.
Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99.
Al-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. 2003;100(7):3983–8.
Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;1334:704–15.
Singh SK, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;6318:5821–8.
Chaffer CL, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci. 2011;108(19):7950–5.
Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146(4):633–44.
Zapperi S, La Porta CAM. Do cancer cells undergo phenotypic switching? The case for imperfect cancer stem cell markers. Sci Rep. 2012;2:441.
•• Tetteh PW, et al. Replacement of lost Lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell. 2016;18(2):203–13. This study shows evidence for de-differentiation of enterocyte cells into proliferative stem cells in vivo. They argue stemness is not hard-wired in intestinal crypts and many progenitors can regain stemness property.
Merrell AJ, Stanger BZ. Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat Rev Mol Cell Biol. 2016;17(7):413–25.
Marusyk A, Polyak K. Cancer cell phenotypes, in fifty shades of grey. Science. 2013;339:528–9.
Nguyen LV, et al. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12(2):133.
Raff M. Adult stem cell plasticity: fact or artifact? Annu Rev Cell Dev Biol. 2003;19(1):1–22.
Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116(5):639–48.
Pepper JW, Sprouffske K, Maley CC. Animal cell differentiation patterns suppress somatic evolution. PLoS Comput Biol. 2007;3(12):e250.
Nowak MA, Michor F, Iwasa Y. The linear process of somatic evolution. Proc Natl Acad Sci. 2003;100(25):14966–9.
Nowak MA. Evolutionary dynamics: exploring the equations of life. Harvard University Press, 2006.
Michor F, Iwasa Y, Nowak MA. Dynamics of cancer progression. Nat Rev Cancer. 2004;4(3):197.
Derényi I, Szöllősi GJ. Hierarchical tissue organization as a general mechanism to limit the accumulation of somatic mutations. Nat Commun. 2017;8:14545.
• Tomasetti C, Vogelstein B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347(6217):78–81. Original study that shows life time cancer risk is strongly correlated with number of stem cell divisions.
Nowak MA, Waclaw B. Genes, environment, and “bad luck”. Science. 2017;355(6331):1266–7.
Wu S, et al. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529(7584):43–7.
Michor F, Hughes TP, Iwasa Y, Branford S, et al. Dynamics of chronic myeloid leukaemia. Nature. 2005;435(7046):1267.
Johnston MD, et al. Mathematical modeling of cell population dynamics in the colonic crypt and in colorectal cancer. Proc Natl Acad Sci. 2007;104(10):4008–13.
Turner C, Stinchcombe A, Kohandel M, Singh S, Sivaloganathan S. Characterization of brain cancer stem cells: a mathematical approach. Cell Prolif. 2009;42(4):529–40.
Turner C, Kohandel M. Investigating the link between epithelial–mesenchymal transition and the cancer Stem cell phenotype: a mathematical approach. J Theor Biol. 2010;265(3):329–35.
Gao X, McDonald JT, Hlatky L, Enderling H. Acute and fractionated irradiation differentially modulate glioma stem cell division kinetics. Cancer Res. 2013;73(5):1481–90.
Weekes SL, et al. A multicompartment mathematical model of cancer stem cell-driven tumor growth dynamics. Bull Math Biol. 2014;76(7):1762–82.
Werner B, Dingli D, Lenaerts T, Pacheco JM, Traulsen A. Dynamics of mutant cells in hierarchically organized tissues. PLoS Comput Biol. 2011;7(12):e1002290.
Werner B, et al. The cancer stem cell fraction in hierarchically organized tumors can be estimated using mathematical modeling and patient-specific treatment trajectories. Cancer research. 2016;767:1705–13.
Porta L, Caterina AM, Zapperi S, Sethna JP. Senescent cells in growing tumors: population dynamics and cancer stem cells. PLoS Comput Biol. 2012;8(1):e1002316.
Clayton E, Doupé DP, Klein AM, Winton DJ, Simons BD, Jones PH. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446(7132):185–9.
Dhawan A, Kohandel M, Hill R, Sivaloganathan S. Tumour control probability in cancer stem cells hypothesis. PLoS One. 2014;9(5):e96093.
Vermeulen L, Morrissey E, van der Heijden M, Nicholson AM, Sottoriva A, Buczacki S, et al. Defining stem cell dynamics in models of intestinal tumor initiation. Science. 2013;342(6161):995–8.
•• Vermeulen L, Snippert HJ. Stem cell dynamics in homeostasis and cancer of the intestine. Nat Rev Cancer. 2014;14(7):468. Recent study that measures the fixation probability of known driver mutations inside the intestinal crypt.
Bozic I, Nowak MA. Unwanted evolution. Science. 2013;342(6161):938–9.
Kaveh K, Komarova NL, Kohandel M. The duality of spatial death–birth and birth–death processes and limitations of the isothermal theorem. Royal Society open science. 2015;2(4):140465.
• Mahdipour A, Kaveh K, Kohandel K, Sivaloganathan S. Phenotypic heterogeneity in modeling cancer evolution. arXiv preprint arXiv:1610.08163 (2016). Theoretical study that formula the fixation probability in a stem cell-differentiated population in presence of differentiation and de-differentiation.
Dingli D, Traulsen A, Michor F. (A) symmetric stem cell replication and cancer. PLoS Comput Biol. 2007;3(3):e53.
Shahriyari L, Komarova NL. Symmetric vs. asymmetric stem cell divisions: an adaptation against cancer? PLoS One. 2013;8(10):e76195.
Kaveh K, Kohandel M, Sivaloganathan S. Replicator dynamics of cancer stem cell: selection in the presence of differentiation and plasticity. Math Biosci. 2016;272:64–75.
Jilkine A, Gutenkunst RN. Effect of dedifferentiation on time to mutation acquisition in stem cell-driven cancers. PLoS Comput Biol. 2014;10(3):e1003481.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kamran Kaveh declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Mathematical Models of Stem Cell Behavior
Rights and permissions
About this article
Cite this article
Kaveh, K. Stem Cell Evolutionary Dynamics of Differentiation and Plasticity. Curr Stem Cell Rep 3, 366–372 (2017). https://doi.org/10.1007/s40778-017-0109-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-017-0109-1