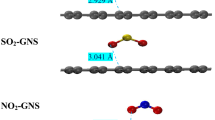
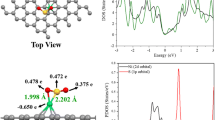
Abstract
Progress of largely selective and sensitive compounds is essential for removing two toxic gases of hydrogen sulfide (H2S) and sulfur dioxide (SO2).The effect of Iron (Fe), Nickel (Ni), and Zinc (Zn) doping of graphene (Gr) nanosheet (NS) on their adsorption for both H2S and SO2 gases has been investigated in this work using first-principles density-functional theory (DFT) computations. In this research, it has been investigated the ability of transition metals of iron, nickel, and zinc doping of Gr@NS for adsorption toxic gas of Sulfur Dioxide and Hydrogen Sulfide Removal. The Langmuir adsorption model with a three-layered ONIOM used CAM-B3LYP functional accompanying LANL2DZ and 6–31 + G (d,p) basis sets due to Gaussian 16 revision C.01 program on the complexes of H2S and SO2 → TM(Fe, Ni, Zn) doping of Gr nanosheet. The changes of charge density have shown the values of ∆QFe-doped = − 0.566 >> ∆QZn-doped = + 0.387 >>> ∆QNi-doped = + 0.605 for H2S adsorption and ∆QFe-doped = − 0.336 >> ∆QZn-doped = + 0.376 >>> ∆QNi-doped = + 0.618 for SO2 adsorption. Based on these amount of changes of charge density, H2S and SO2 have exhibited a significant charge transfer for Fe doping of graphene nanosheet compared to Ni- and Zn-doped Gr@NS. Based on NMR spectroscopy, it has been illustrated that the sharp peaks in the adsorption site are due to the Fe, Ni, and Zn doping on the surface of graphene nanosheet through H2S and SO2 adsorption. However, it has represented some fluctuations in the chemical shielding of isotropic and anisotropy behaviors around Zn-doped on the H2S/ SO2 → Zn-doped/Gr@NS. Moreover, it has exhibited the fluctuation of occupancy of NBO for H2S/SO2 → Fe-doped, H2S/SO2 → Ni-doped, and H2S/SO2 → Zn-doped graphene nanosheet through the Langmuir adsorption process by indicating the active sulfur atom in hydrogen sulfide (H2S) and sulfur dioxide (SO2) becoming close to the nanosheet. The amounts of \({\Delta {G}}_{\mathrm{ads}}^{\mathrm{o}}\) through IR computations based on polarizability have exhibited that \({\Delta {G}}_{\mathrm{ads},\mathrm{SO}2\to \mathrm{ Fe}-\mathrm{C}}^{\mathrm{o}}\) and \({\Delta {G}}_{\mathrm{ads},\mathrm{H}2\mathrm{S}\to \mathrm{ Fe}-\mathrm{C}}^{\mathrm{o}}\) have exhibited the most energy gap because of charge density transfer from sulfur atom in hydrogen sulfide (H2S) and sulfur dioxide (SO2) to Fe doping of Gr@NS, although, \(\Delta {G}_{\mathrm{H}2\mathrm{S}/\mathrm{SO}2 \to \mathrm{ Zn}-\mathrm{C }}^{0}\)> \(\Delta {G}_{\mathrm{H}2\mathrm{S}/\mathrm{SO}2 \to \mathrm{ Ni}-\mathrm{C }}^{0}\)> \(\Delta {G}_{\mathrm{H}2\mathrm{S}/\mathrm{SO}2 \to \mathrm{ Fe}-\mathrm{C }}^{0}\). Frontier molecular orbitals of HOMO, LUMO, and band energy gap accompanying some chemical reactivity parameters have represented the attributes of molecular electrical transport of TM (Fe, Ni, Zn) doping of Gr nanosheet for adsorption of H2S and SO2 gases. Our results have provided a favorable understanding of the interaction between TM doping of Gr@NS nanosheet and H2S and SO2 molecules. A high performance of TM doping of Gr@NS as gas sensor is demonstrated by modeling the material’s transport characteristics by means of the Langmuir adsorption and three-layered ONIOM/DFT method. Furthermore, the results of partial electron density of states (PDOS) have confirmed an obvious charge accumulation between the graphene nanosheet and doped atoms of Fe, Ni, and Zn through adsorption of H2S and SO2 molecules on the surface due to the recognition of the conduction band region. Finally, this research can build up our knowledge about the electronic structure, relative stability, and surface bonding of various metal-doped graphene nanosheets, metal alloy surfaces, and other dependent mechanisms, like heterogeneous catalysis, friction lubrication, and biological systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
There are different practical applications of carbon nanostructures, such as hydrogen adsorption, pollutant molecules adsorption, and gas sensor devices [1,2,3,4,5,6,7,8,9].
Sensing and grabbing toxic and harmful gases like CO, CO2, NO, N2O, CH4, SO2, and H2S can largely help maintain the human health and the ecosystem [10,11,12]. These days many materials like carbon-based materials have been investigated and applied for adsorptive removal of toxic gases [13,14,15,16,17]. Therefore, it is essential to make high‐implement gas sensors for detecting the toxic gases.
Therefore, remarkable surface of carbon nanostructures is a privileged factor for gas sensing and gas adsorption devices. In addition, the enough doping of these compounds with transition metals might enhance their adsorption ability and adjust their selective adsorption as the excellent dopant applicants [18,19,20,21,22].
Moreover, the recent researches have shown that vacancy defects on the metal surface have an important effect on the incidence of hydrogen breaking. So, the adsorption and dissociation mechanism of H2S and the diffusion action of hydrogen atoms can gently enhance the reactivity of the vacancy defective metal surface [23,24,25]. Besides, the study on the surface has depicted that the different dissociation behaviors of H2S on the different surfaces depend not only on the electronic properties of the surfaces, but also on the adsorption configurations of the transition states [26, 27].
Thus, this research wants to investigate the adsorption of hazardous gases such as H2S and SO2 using carbon nanostructures decorated by transition metals of iron, nickel, and zinc based on the density-functional theory (DFT) to discover the adsorption parameters of the various TM doping of Gr@NS.
2 Materials, Theoretical Background, and Computational Method
2.1 Adsorptive Removal of Toxic Gases
This article discusses Sulfur Dioxide and Hydrogen Sulfide adsorption on the transition metals. The chapter illustrates the principal mechanisms of bonding occurring during H2S and SO2 chemisorption and reviews the results obtained for H2S and SO2 adsorption on tungsten. The data on tungsten surfaces are compared with the few results available for H2S and SO2 adsorption on molybdenum and chromium. Bonding of the H2S and SO2 molecules to a transition metal atom, either in a carbonyl complex or possibly on a metal surface, can be visualized first proceeding by the donation of the lone pair on the carbon atom into vacant d orbitals of the metal atom. The donor ability of H2S and SO2 in this manner is known to be extremely small and stabilization of the metal–carbon bond is believed to be obtained by back-donation of electrons from filled d orbitals on the metal into vacant antibonding π* orbitals on the H2S and SO2 molecule. It is thought that the two mechanisms, donation and back-donation, tend to enhance each other in a synergic manner.
2.2 Langmuir Adsorption Model and Charge Density Analysis
It can be defined the Langmuir adsorption through a physico-chemical interaction on the area of the homogeneous solid state that adsorb compounds without any interactions with each other making a monolayer of molecules on the surface of the solid state. The Langmuir adsorption equation is as the following [28]:
where \({\theta }_{A}\) is the fractional occupancy of the adsorption sites; the ratio of\(V\), the volume of gas adsorbed onto the solid, to\({V}_{m}\), the volume of a gas molecules monolayer coating the entire surface of the solid and totally filled by the adsorbate. A continuous monolayer of adsorbate molecules coating a homogeneous solid surface is the conceptual basis for this adsorption system [29, 30]. Different studies have concentrated on the gas adsorption susceptibilities of carbon nanosurfaces which denote a good agreement with the Langmuir adsorption model. The adsorption of toxic H2S and SO2 gases on the Fe, Ni, and Zn doping of Gr@NS has been assigned by the most suitable Langmuir isotherm, which exhibits the chemisorptive nature of the bond between \(\mathrm{H}-\ddot{\mathrm{S}}:-\mathrm{H}\) and \(\ddot{:\mathrm{O}}=\ddot{\mathrm{S}}=\ddot{\mathrm{O}:}\) molecules and TM doping of Gr@NS, the equilibrium electron distribution of the adsorbing compound between the solid and gas phases, and a monolayer attribute. The adsorbed H2S and SO2 molecules are kept on TM doping of Gr@NS with Langmuir chemisorption (Scheme 1).
In fact, the nature of the gas sensing mechanism in TM (Fe, Ni, Zn) doping of Gr@NS would be due to charge transfer between surface and H 2 S- and SO 2-adsorbed molecules. The changes of charge density analysis in the adsorption process have illustrated that Fe, Ni, and Zn doping of Gr@NS shows the Mulliken charge of − 1.345, − 2.087, and − 1.416, respectively, before adsorption of hydrogen sulfide (H2S) and − 1.911, − 1.482, and − 1.029, respectively, after adsorption of H2S. In addition, Fe, Ni, and Zn doping of Gr@NS shows the Mulliken charge of − 1.681, − 1.469, and − 1.040, respectively, after adsorption of sulfur dioxide (SO2).
Therefore, the changes of charge density for Langmuir adsorption of H2S on Fe, Ni, and Zn doping of Gr@NS alternatively are ∆ENi-doped = + 0.605 >> ∆EZn-doped = + 0.387 >>> ∆EFe-doped = − 0.566. The values of changes of charge density have shown a more significant charge transfer for Fe doping of Gr@NS, while H2S is adsorbed by the metal-doped graphene surface. Furthermore, the changes of charge density for Langmuir adsorption of SO2 on Fe, Ni, and Zn doping of Gr@NS alternatively are ∆ENi-doped = + 0.618 >> ∆EZn-doped = + 0.376 >>> ∆EFe-doped = − 0.336. In fact, the values of changes of charge density have also indicated a more distinct charge transfer for Fe doping of Gr@NS through SO2 adsorption.
2.3 ONIOM/DFT Method
The combination of three levels in decreasing order of accuracy has been defined, including high, medium, and low levels of theory. High level has been done using the DFT method of Cam-B3LYP with 6–31 + G (d,p) basis set for oxygen, sulfur, and LANL2DZ for transition metals of iron, nickel, and zinc in the adsorption sites. Medium level has been done on some carbon atoms of graphene in the adsorption site due to semi-empirical methods. Finally, a low level has been performed on the other carbon atoms of graphene with MM2 force fields, \({E}_{ONIOM}\)= \({E}_{High}+ {E}_{Medium}+ {E}_{Low}\), (Scheme 2) [31].
On the other hand, the three-layered method of ONIOM lets us to discover a larger system more exactly than the one-layered model which could behave a medium-size system precisely like a ground system with acceptable accuracy [32, 33]. In this article, the structures have been calculated using the density-functional theory (DFT) on the mechanisms of adsorption of H2S and SO2 by TM (Fe, Ni, Zn) doping of Gr@NS through bonding between transition metals (Fe, Ni, Zn) and gas molecules of \(\mathrm{H}-\ddot{\mathrm{S}}:-\mathrm{H}\) and \(\ddot{:\mathrm{O}}=\ddot{\mathrm{S}}=\ddot{\mathrm{O}:}\). It has been found that the surface binding site preference of S-atom of H2S and SO2 in adsorption site is largely affected by the presence of neighboring atoms in the graphene sheet. The calculated H2S/SO2 → Fe-doped/Gr, H2S/ SO2 → Ni-doped/Gr, and H2S/SO2 → Zn-doped/Gr pair distribution functions have indicated that the formation of clusters directs to shorter S → Fe, S → Ni, and S → Zn bond lengths when compared to the homogeneous growth (Scheme 2).
Hybrid functional is a group of approximations for the exchange–correlation energy functional in DFT (Density-Functional Theory) which combines a part of exact exchange from HF (Hartree–Fock theory) method with the rest of the exchange–correlation energy from other information such as empirical or ab initio methodologies. Therefore, the exact exchange energy functional is illustrated by the Kohn–Sham orbitals instead of the density, so is placed as the indirect density functional. This study has applied the influence of the hybrid functional of three-parameter basis set of B3LYP (Becke, Lee, Yang, Parr) within the framework of DFT upon theoretical calculations [34, 35]. Transition metal doping of Gr@NS has been built by rigid system and Z-Matrix format of which a blank line has been placed and after that the following information has been illustrated. The rigid PES has been performed at CAM-B3LYP functional [36] and employing LANL2DZ /6–31 + G (d,p) basis sets to assign HOMO, LUMO, Mulliken charges, nuclear magnetic resonance properties, dipole moment, thermodynamic characteristics, and other quantum properties for this study [37] for H2S and SO2 adsorbed onto TM doping of Gr@NS, including H2S/SO2 → Fe-doped/Gr, NO → Ni-doped/Gr, and H2S/ SO2 → Zn-doped/Gr using Gaussian 16 program package [38].
2.4 PDOS Graphs and Electronic Properties Analysis
To further study the influence of the adsorbed transition metal atoms on the electronic properties of the TM (Fe, Ni, Zn)-doped graphene nanosheet, the projected density of states (PDOS) have been calculated and plotted in Scheme 3a–f. A distinct metallic feature can be observed in the TM (Fe, Ni, Zn)-doped graphene nanosheet because of the strong interaction between the p states of C in graphene sheet and the d state of Fe, Ni, and Zn near the Fermi energy. Moreover, the existence of covalent features for these alloys has exhibited the identical energy amount and figure of the PDOS for the p orbitals of C and d orbitals of Fe, Ni, and Zn (Scheme3a–f).
Scheme 3a–c shows that the Fe, Ni, and Zn states doping of the graphene nanosheet through H2S adsorption have more contributions at the middle of the conduction band between − 5 eV and − 15 eV, while contributions of Fe states are expanded (Scheme 3a), but Ni states (Scheme 3b) and Zn states (Scheme 3c) have minor contributions.
Besides, Fig. 3d–f indicates that the Fe, Ni, and Zn states doping of the graphene nanosheet through SO2 adsorption have more contributions at the middle of the conduction band between − 5 eV and − 15 eV, while contribution of Fe states (Scheme 3d), but Ni states (Scheme 3e) and Zn states (Scheme 3f) have major contributions.
Moreover, the results of the projected density of states (PDOS) have showed a certain charge association between the graphene nanosheet and doped elements of Fe, Ni, and Zn. In other words, the Fe states have large contributions in the valence band, while Ni and Zn states have fewer contributions. Thus, the cluster dominant of non-metallic and metallic features and a certain degree of covalent features can illustrate the increasing of the semiconducting direct band gap of (Fe, Ni, Zn)-doped graphene nanosheet.
3 Results and Discussion
In this investigation, transition metals such as iron, nickel, and zinc doped on the graphene nanosheet have been investigated as the efficient surface for adsorption of toxic gases H2S and SO2 causing air pollution. These experiments have been conducted by spectroscopy analysis through some physical and chemical properties.
3.1 NMR Spectroscopy and NBO Analysis
Isotropic (σiso) and anisotropy (σaniso) shielding tensors of NMR spectroscopy for certain atoms in the active site of H2S and SO2 adsorbed by the Fe, Ni, and Zn doping of Gr@NS through the formation of the binding between gas molecule and solid surface have been calculated using Gaussian 16 program software and reported in Tables 1 and 2 [38, 39].
In Fig. 1, it has been indicated the chemical shielding (ppm) of NMR graphs versus atom type through adsorption of H2S and SO2 onto the Fe-doped/Gr, Ni-doped/Gr, and Zn-doped/Gr nanosheet.
The graphs of NMR spectroscopy in Fig. 1a–c and aʹ–cʹ have shown approximately the identical chemical shielding behavior of isotropic and anisotropy parameters for H2S/ SO2 → Fe-doped/Gr@NS (Fig. 1a, aʹ) and H2S/SO2 → Ni-doped/Gr@NS (Fig. 1b, bʹ), with a sharp peak close to linkage of Fe-doped and Ni-doped on the surface of graphene with sulfur atom of H2S and SO2, respectively. Although, in the NMR spectroscopy, it has been observed the sharp peak around Zn-doped on the H2S/SO2 → Zn-doped/Gr@NS, there are some fluctuations in the chemical shielding behaviors of isotropic and anisotropy parameters (Fig. 1c, cʹ).
Furthermore, the Natural Bond Orbital (NBO) analysis of H2S and SO2 adsorbed on the TM (Fe, Ni, Zn) doping of Gr@NS has illustrated the character of electronic conjugation between bonds in the gas molecules and TM doping of Gr@NS (Table3; Fig. 2).
Occupancy fluctuation extracted of NBO method for bond orbitals of C–Fe, C–Ni, and C–Zn in Table 3 through adsorption of H2S and SO2 on the TM (Fe, Ni, Zn) doping of Gr@NS
In Fig. 2, it has been observed the fluctuation of occupancy of natural bond orbitals for H2S/SO2 → Fe-doped, H2S/SO2 → Ni-doped, and H2S/SO2 → Zn-doped graphene nanosheet through the Langmuir adsorption process by indicating the active sulfur atom in hydrogen sulfide (H 2 S) and sulfur dioxide (SO 2 ) becoming close to the nanosheet. Bond orbitals of C14—Ni 16 and C9—Ni in the adsorption of H2S and SO2, respectively, in the Ni doping of Gr@NS have shown the maximum occupancy.
3.2 Thermodynamic Properties and IR Spectroscopy Analysis
Thermodynamic parameters have been estimated for adsorption of Sulfur Dioxide \(\ddot{(:\mathrm{O}}=\ddot{\mathrm{S}}=\ddot{\mathrm{O}:}\)) and Hydrogen Sulfide (\(\mathrm{H}-\ddot{\mathrm{S}}:-\mathrm{H}\)) on the surfaces of (Fe, Ni, Zn) doping of Gr@NS as the gas sensors which can be used as the selective detectors for toxic gases (Table 4).
Moreover, the infrared spectra for adsorption of H2S and SO2 by (Fe, Ni, Zn) doping of Gr@NS have been reported in Fig. 3a–c and aʹ–cʹ. Each of these graphs has been seen in the frequency range around 500–2000 cm−1 for the complexes of \(\mathrm{H}-\ddot{\mathrm{S}}:-\mathrm{H}\)→ Fe–C, \(\mathrm{H}-\ddot{\mathrm{S}}:-\mathrm{H}\)→ Ni–C, \(\mathrm{H}-\ddot{\mathrm{S}}:-\mathrm{H}\)→ Zn–C and \(\ddot{:\mathrm{O}}=\ddot{\mathrm{S}}=\ddot{\mathrm{O}:}\)→ Fe–C, \(\ddot{:\mathrm{O}}=\ddot{\mathrm{S}}=\ddot{\mathrm{O}:}\)→ Ni–C, and \(\ddot{:\mathrm{O}}=\ddot{\mathrm{S}}=\ddot{\mathrm{O}:}\)→ Zn–C.
Figure 3a and aʹ shows the strongest IR peaks for \(\mathrm{H}-\ddot{\mathrm{S}}:-\mathrm{H}\)→ Fe–C and \(\ddot{:\mathrm{O}}=\ddot{\mathrm{S}}=\ddot{\mathrm{O}:}\)→ Fe–C approximately between 800 and 1000 cm−1. Besides, it has seen the frequencies of 1600 cm−1 and 700 cm−1 for strongest peaks of \(\mathrm{H}-\ddot{\mathrm{S}}:-\mathrm{H}\)→ Ni–C and \(\ddot{:\mathrm{O}}=\ddot{\mathrm{S}}=\ddot{\mathrm{O}:}\)→ Ni–C, respectively (Fig. 3b, bʹ). Furthermore, the most frequencies of sharp peak for \(\mathrm{H}-\ddot{\mathrm{S}}:-\mathrm{H}\)→ Zn–C (two peaks) and \(\ddot{:\mathrm{O}}=\ddot{\mathrm{S}}=\ddot{\mathrm{O}:}\)→ Zn–C have been represented around 750 cm−1, 1600 cm−1, and 750 cm−1, respectively (Fig. 3c, cʹ).
From Fig. 4, it could be found that the maximum of the Langmuir adsorption isotherm plots based on \({\Delta {G}}_{\mathrm{ads}}^{\mathrm{o}}\) versus dipole moment may depend on the interactions between the H2S and SO2 and the TM doping of Gr@NS. The order of Gibbs free energy changes of SO2 adsorption on TM-doped Gr@NS is \(\Delta {G}_{\mathrm{SO}2\to \mathrm{ Zn}-\mathrm{C }}^{0}\) > \(\Delta {G}_{\mathrm{SO}2\to \mathrm{ Ni}-\mathrm{C }}^{0}\) > \(\Delta {G}_{\mathrm{SO}2\to \mathrm{ Fe}-\mathrm{C }}^{0}\) and also the order of Gibbs free energy changes of the clusters of H2S → TM doping of Gr@NS is \(\Delta {G}_{\mathrm{H}2\mathrm{S}\to \mathrm{ Zn}-\mathrm{C }}^{0}\) > \(\Delta {G}_{\mathrm{H}2\mathrm{S}\to \mathrm{ Ni}-\mathrm{C }}^{0}\) > \(\Delta {G}_{\mathrm{H}2\mathrm{S}\to \mathrm{ Fe}-\mathrm{C }}^{0}\) (Fig. 4).
The adsorptive capacity of H2S and SO2 on the TM doping of Gr@NS is approved by the \({\Delta G}_{ads}^{o}\) amounts. \({\Delta {G}}_{\mathrm{ads}}^{\mathrm{o}}= {\Delta {G}}_{\mathrm{NO}\to \mathrm{TM}-\mathrm{C}}^{\mathrm{o}}-\left({\Delta {G}}_{\mathrm{NO }}^{\mathrm{o}}+ {\Delta {G}}_{\mathrm{TM}-\mathrm{C}}^{\mathrm{o}}\right);\) \(\mathrm{TM}=\mathrm{Fe},\mathrm{ Ni},\mathrm{Zn}.\)
On the basis of data in Table 4, it is predicted that the adsorption of H2S and SO2 on the TM doping of Gr@NS must be physical and chemical nature. As shown in Fig. 4, all the computed \({\Delta G}_{ads}^{o}\) amounts are very close, which exhibits the agreement of the evaluated data by all methods and the validity of the computations.
Fig. 4 indicates that \({\Delta {G}}_{\mathrm{ads},\mathrm{ H}2\mathrm{S}\to \mathrm{ Fe}-\mathrm{C}}^{\mathrm{o}}\) and \({\Delta {G}}_{\mathrm{ads},\mathrm{ SO}2\to \mathrm{ Fe}-\mathrm{C}}^{\mathrm{o}}\) have the largest gap of Gibbs free energy adsorption with dipole moment which defines the changes between Gibbs free energy of initial compounds (\({\Delta {G}}_{\mathrm{H}2\mathrm{S }}^{\mathrm{o}}\)/\({\Delta {G}}_{\mathrm{Fe}-\mathrm{C}}^{\mathrm{o}}\)) and (\({\Delta {G}}_{\mathrm{SO}2 }^{\mathrm{o}}\)/\({\Delta {G}}_{\mathrm{Fe}-\mathrm{C}}^{\mathrm{o}}\)) and product compounds (\({\Delta {G}}_{\mathrm{H}2\mathrm{S}\to \mathrm{Fe}-\mathrm{C}}^{\mathrm{o}}\)) and (\({\Delta {G}}_{\mathrm{SO}2\to \mathrm{Fe}-\mathrm{C}}^{\mathrm{o}}\)) through polarizability. In fact, TM-doped/Gr can possess enough efficiency for adsorption toxic gases of hydrogen sulfide and sulfur dioxide through charge transfer from sulfur to the transition metal.
3.3 Frontier Molecular Orbitals of HOMO, LUMO, and UV–VIS Analysis
The highest occupied molecular orbital (HOMO) energy is generated by ionization and the lowest unoccupied molecular orbital (LUMO) energy is observed by the electron affinity. These parameters have been evaluated for adsorption of hydrogen sulfide and sulfur dioxide on the TM (Fe, Ni, Zn) doping of Gr@NS as the gas detector in Table 5. The HOMO (au), LUMO (au), and band energy gap (∆E = ELUMO − EHOMO) (ev) have exhibited the pictorial explanation of the frontier molecular orbitals and their respective positive and negative areas which are a significant parameter for discovering the molecular properties of efficient compounds in the adsorption of H2S and SO2 on the TM doping of Gr@NS (Table 5).
Moreover, for getting more conclusive approval in identifying the compound characteristics of adsorption complexes of H2S/ SO2 → TM (Fe, Ni, Zn) doping of Gr@NS, a series of chemical reactivity parameters such as chemical potential (µ), electronegativity (χ), hardness (η), softness (ζ), and electrophilicity index (ψ) have been carried out (Table 5) [41,42,43].
On the other hand, the HOMO shows the capability for giving an electron, while the LUMO as an electron acceptor exhibits the capability for achieving an electron. Therefore, the energy gap (∆E = ELUMO − EHOMO) indicates the energy difference between frontier HOMO and LUMO orbital introducing the stability for the structure and unravels the chemical activity of the molecule. In this work, energy gap establishes how toxic gas of H2S and SO2 can be adsorbed on the TM (Fe, Ni, Zn) doping of Gr@NS as the gas sensor at B3LYP/LANL2DZ, 6–311 + G (2d, p) quantum method. Besides, frontier molecular orbitals play an important function in the optical and electrical properties, like in UV–VIS spectra [44].
The energy gap between HOMO and LUMO has distinguished the attributes of molecular electrical transport [45]. Through Frank–Condon principle, the maximum absorption peak (max) depends on an UV–visible spectrum to vertical excitation.
The negative values of the chemical potential (μ) versus the positive values of other amounts have displayed an appropriate efficiency of scavenging H2S and SO2 by TM (Fe, Ni, Zn) doping of Gr@NS.
In addition, TD-DFT/ LANL2DZ, 6–31 + G (2d, p) computations have been done to identify the low-lying excited states of H2S and SO2 adsorbed on the TM (Fe, Ni, Zn) doping of Gr@NS. The results consist of the vertical excitation energies, oscillator strength, and wavelength which have been introduced in Fig. 5a–c and a′–c′.
Figure 5a–c and a′–c′ have shown UV–VIS spectra for H2S → Fe-doped, H2S → Ni-doped, and H2S → Zn-doped graphene sheet with maximum adsorption bands between 2000 and 5000 nm. Moreover, it has observed the maximum adsorption around 2000 and 10000 nm for SO2 → Fe-doped, SO2 → Ni-doped, and SO2 → Zn-doped graphene sheet (Fig. 5a–c, a′–c′).
4 Conclusion
This research aimed to remark the study of polluting gases such as hydrogen sulfide and sulfur dioxide adsorption employing graphene nanosheet. The graphene sheet reviewed in general physically adsorb many of the pollutant gas molecules considered and the interaction can usually enhance by transition metal doping which ameliorates their detecting properties through chemisorption study. This article has reported the trends for H2S and SO2 chemisorption on transition metal (iron, nickel, and zinc) doping of Gr@NS. In particular, the energetic, structural, and infrared adsorption characteristics of linearly (atop) H2S and SO2 adsorbed on (Fe, Ni, Zn)-doped graphene nanosheet have been discovered. Spin-unrestricted density-functional theory (DFT) calculations were applied to verdict the tendency of H2S and SO2 adsorption energy (H2S/SO2 → Fe-doped, H2S/SO2 → Ni-doped, and H2S/SO2 → Zn-doped on the Gr nanosheet) and N–O vibrational frequency (νNO) for clusters composed of Fe, Ni, and Zn. The effects of the transition metal electronic structure on the adsorption energy of H2S and SO2, and how these chemical factors might be related to the catalytic activity of transition-supported metal catalysts that deal with the adsorption, and surface diffusion, have been investigated. Therefore, the cluster dominant of non-metallic and metallic features and a certain degree of covalent features can indicate the enhancement of the semiconducting direct band gap of transition metal doping of graphene nanosheet which can conduct us toward the electronic structure, relative stability, and surface bonding of various metal-doped graphene nanosheet, metal alloy surfaces, and other related mechanisms of friction lubrication and biological systems.
Data Availability
It is not applicable.
References
Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE (1985) C60: Buckminsterfullerene. Nature 318:162–163
Nasibulin AG, Pikhitsa PV, Jiang H, Brown DP, Krasheninnikov AV, Anisimov AS, Queipo P, Moisala A, Gonzalez D, Lientschnig G et al (2007) A novel hybrid carbon material. Nat Nanotechnol 2:156–161
Mollaamin F, Shahriari S, Monajjemi M et al (2023) Nanocluster of aluminum lattice via organic inhibitors coating: a study of Freundlich adsorption. J Clust Sci 34(3):1547–1562. https://doi.org/10.1007/s10876-022-02335-1
Moisala A, Nasibulin AG, Shandakov SD, Jiang H, Kauppinen EI (2005) On-line detection of single-walled carbon nanotube formation during aerosol synthesis methods. Carbon 43:2066–2074
Mollaamin F, Monajjemi M (2023) Transition metal (X = Mn, Fe Co, Ni, Cu, Zn)-doped graphene as gas sensor for CO2 and NO2 detection: a molecular modeling framework by DFT perspective. J Mol Model 29:119. https://doi.org/10.1007/s00894-023-05526-3
Delgado JL, Herranz M, Martín N (2008) The nano-forms of carbon. J Mater Chem 18:1417
Falcao EH, Wudl F (2007) Carbon allotropes: beyond graphite and diamond. J Chem Technol Biotechnol 82:524–531
Langenhorst F, Campione M (2019) Ideal and real structures of different forms of carbon, with some remarks on their geological significance. J Geol Soc 176:337–347
Mollaamin F, Monajjemi M (2023) Doping of graphene nanostructure with iron, nickel and zinc as selective detector for the toxic gas removal: a density functional theory study. C 9(1):20.https://doi.org/10.3390/c9010020.
Su Y, Wang J, Wang B, Yang T, Yang B, Xie G, Zhou Y, Zhang S, Tai H, Cai Z et al (2020) Alveolus-inspired active membrane sensors for self-powered wearable chemical sensing and breath analysis. ACS Nano 14:6067–6075. https://doi.org/10.1021/acsnano.0c01804
Ma D, Zhang J, Li X, He C, Lu Z, Lu Z, Lu Z, Yang Z, Wang Y (2018) C3N monolayers as promising candidates for NO2 sensors. Sens Actuators B 266:664–673. https://doi.org/10.1016/j.snb.2018.03.159
Pacheco M, Pacheco J, Valdivia R, Santana A, Tu X, Mendoza D, Frias H, Medina L, Macias J (2017) Green applications of carbon nanostructures produced by plasma techniques. MRS Adv 2:2647–2659
Lee SW, Lee W, Hong Y, Lee G, Yoon DS (2018) Recent advances in carbon material-based NO2 gas sensors. Sens Actuators B 255:1788–1804. https://doi.org/10.1016/j.snb.2017.08.203
Chatterjee SG, Chatterjee S, Ray AK, Chakraborty AK (2015) Graphene–metal oxide nanohybrids for toxic gas sensor: a review. Sens Actuators B 221:1170–1181. https://doi.org/10.1016/j.snb.2015.07.070
Xiao Z, Kong LB, Ruan S, Li X, Yu S, Li X, Jiang Y, Yao Z, Ye S, Wang C et al (2018) Recent development in nanocarbon materials for gas sensor applications. Sens Actuators B 274:235–267. https://doi.org/10.1016/j.snb.2018.07.040
Bakhshi K, Mollaamin F, Monajjemi M (2011) Exchange and correlation effect of hydrogen chemisorption on nano V(100) surface: a DFT study by generalized gradient approximation (GGA). J Comput Theor Nanosci 8:763–768. https://doi.org/10.1166/jctn.2011.1750
Mollaamin F, Monajjemi M (2023) Graphene embedded with transition metals for capturing carbon dioxide: gas detection study using QM methods. Clean Technol 5(1):403–417. https://doi.org/10.3390/cleantechnol5010020
Monajjemi M, Baie MT, Mollaamin F (2010) Interaction between threonine and cadmium cation in [Cd(Thr)] (n = 1–3) complexes: density functional calculations. Russ Chem Bull 59:886–889. https://doi.org/10.1007/s11172-010-0181-5.
Khaleghian M, Zahmatkesh M, Mollaamin F, Monajjemi M (2011) Investigation of solvent effects on armchair single-walled carbon nanotubes: a QM/MD study. Fuller Nanotub Carbon Nanostruct 19:251–261. https://doi.org/10.1080/15363831003721757
Monajjemi M, Khaleghian M, Tadayonpour N, Mollaamin F (2010) The effect of different solvents and temperatures on stability of single-walled carbon nanotube: a QM/MD study. Int J Nanosci 09:517–529. https://doi.org/10.1142/S0219581X10007071
Shahriari S, Mollaamin F, Monajjemi M (2023) Increasing the performance of {[(1-x-y) LiCo0.3Cu0.7] (Al and Mg doped)] O2}, xLi2MnO3, yLiCoO2 composites as cathode material in lithium-ion battery: synthesis and characterization. Micromachines 14:241. https://doi.org/10.3390/mi14020241.
Mollaamin F, Monajjemi M (2015) Harmonic linear combination and normal mode analysis of semiconductor nanotubes vibrations. J Comput Theor Nanosci 12:1030–1039. https://doi.org/10.1166/jctn.2015.3846
Wen X, Bai P, Han Z, Zheng S, Luo B, Fang T, Song W (2019) Effect of vacancy on adsorption/dissociation and diffusion of H2S on Fe(1 0 0) surfaces: a density functional theory study. Appl Surf Sci 465:833–845. https://doi.org/10.1016/j.apsusc.2018.09.220
Mollaamin F, Monajjemi M (2023) In silico-DFT investigation of nanocluster alloys of Al-(Mg, Ge, Sn) coated by nitrogen heterocyclic carbenes as corrosion inhibitors. J Clust Sci. https://doi.org/10.1007/s10876-023-02436-5
Mollaamin F, Monajjemi M (2023) Molecular modelling framework of metal-organic clusters for conserving surfaces: Langmuir sorption through the TD-DFT/ONIOM approach. Mol Simul 49(4):365–376. https://doi.org/10.1080/08927022.2022.2159996
Wen X, Bai P, Shuqi Zheng Yu, Tian, (2021) Adsorption and dissociation mechanism of hydrogen sulfide on layered FeS surfaces: a dispersion-corrected DFT study. Appl Surf Sci 537:147905. https://doi.org/10.1016/j.apsusc.2020.147905
Wen X, Bai P, Liang J, Zheng S, Tian Y (2022) Slab model studies of H2S adsorption/dissociation and diffusion on pristine FeS(001) surfaces and FeS(001) surfaces with pre-adsorbed X atoms (X = H, O, and S). J Mater Res Technol. https://doi.org/10.1016/j.jmrt.2022.03.047
Hanaor DAH, Ghadiri M, Chrzanowski W, Gan Y (2014) Scalable surface area characterization by electrokinetic analysis of complex anion adsorption (PDF). Langmuir 30(50):15143–15152. https://doi.org/10.1021/la503581e
Boyd A, Dube I, Fedorov G, Paranjape M, Barbara P (2014) Gas sensing mechanism of carbon nanotubes: from single tubes to high-density networks. Carbon 69:417–423
Zhao J, Buldum A, Han J, Lu JP (2002) Gas molecule adsorption in carbon nanotubes and nanotube bundles. Nanotechnology 13:195–200
Svensson M, Humbel S, Froese RDJ, Matsubara T, Sieber S, Morokuma K (1996) ONIOM: a multilayered integrated MO + MM method for geometry optimizations and single point energy predictions. A test for diels−alder reactions and Pt(P(t-Bu)3)2 + H2 oxidative addition. J Phys Chem 100(50):19357–19363. https://doi.org/10.1021/jp962071j
Brandt F, Jacob CR (2022) Systematic QM region construction in QM/MM calculations based on uncertainty quantification. J Chem Theory Comput 18(4):2584–2596. https://doi.org/10.1021/acs.jctc.1c01093
Mollaamin F, Monajjemi M, Salemi S, Baei MT (2011) A dielectric effect on normal mode analysis and symmetry of BNNT nanotube. Fuller Nanotub Carbon Nanostruct 19:182–196. https://doi.org/10.1080/15363831003782932
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Ditchfield R, Hehre WJ, Pople JA (1971) Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54:724–728
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JAJr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT
Tahan A, Mollaamin F, Monajjemi M (2009) Thermochemistry and NBO analysis of peptide bond: Investigation of basis sets and binding energy. Russ J Phys Chem A 83:587–597. https://doi.org/10.1134/S003602440904013X
Novotný J, Jan Vícha J, Bora PL, Repisky M, Straka M, Komorovsky S, Marek R (2017) Linking the character of the metal-ligand bond to the ligand NMR shielding in transition-metal complexes: NMR contributions from spin-orbit coupling. J Chem Theory Comput 13(8):3586–3601. https://doi.org/10.1021/acs.jctc.7b00444
Kohn W, Becke AD, Parr RG (1996) Density functional theory of electronic structure. J Phys Chem 100:12974–12980. https://doi.org/10.1021/jp960669l
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516. https://doi.org/10.1021/ja00364a005
Politzer P, Abu-Awwad F (1998) A comparative analysis of Hartree-Fock and Kohn-Sham orbital energies. Theor Chem Acc 99:83–87. https://doi.org/10.1007/s002140050307
Aihara J (1999) Reduced HOMO−LUMO gap as an index of kinetic stability for polycyclic aromatic hydrocarbons. J Phys Chem A 103(37):7487–7495. https://doi.org/10.1021/jp990092i
Silverstein RM, Bassler GC, Morrill TC (1981) Spectrometric identification of organic compounds, 5th edn. Wiley, New York
Acknowledgements
In successfully completing this paper and its research, the authors are grateful to Kastamonu University for their support through the office, library, and scientific websites.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
FM contributed to conceptualization and idea, methodology, software, validation, formal analysis, investigation, data curation, writing of the original draft preparation, visualization, supervision, and project administration. MM contributed to methodology, software, formal analysis, investigation, data curation, writing, reviewing, and editing of the manuscript, visualization, and resources.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
The authors consent to participate and publish the data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mollaamin, F., Monajjemi, M. Tribocorrosion Framework of (Iron, Nickel, Zinc)-Doped Graphene Nanosheet: New Sights into Sulfur Dioxide and Hydrogen Sulfide Removal Using DFT/TD-DFT Methods. J Bio Tribo Corros 9, 47 (2023). https://doi.org/10.1007/s40735-023-00768-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-023-00768-3