Abstract
Finding cost-effective and sustainable methods for the removal of hydrogen sulfide (H2S), a highly toxic gas released as a byproduct in many industrial activities, is crucial for environmental health. In this study, the adsorption and electronic sensor properties of Ti, V, Cr and Sc doped graphene nanosheets (GN) for H2S molecule have been investigated using Density Functional Theory (DFT) method. The WB97XD method with 6-31G(d,p)/LanL2DZ basis sets have been utilized in DFT calculations. The charge distribution indicates that the charge transfer occurred between metal doped graphenes and H2S. DFT calculations of H2S molecule adsorption on Ti, V, Cr and Sc doped graphenes demonstrate that the ability to adsorb H2S molecule. The obtained adsorption energy (∆E) values vary in the range of -54.4 to -71.0 kJ/mol. Furthermore, the electrical conductivity of the Cr doped graphene nanosheet (Cr-GN) changed due to the change in the HOMO–LUMO gap (∆Eg = 24.8 kJ/mol). This result indicates that the Cr-GN structure is a potential candidate as an electronic sensor for H2S molecule at room temperature. Through methods like DFT, which are cost-effective and highly compatible with experimental results, predicting suitable adsorbents, understanding their properties, and enhancing them are expected to make substantial contributions to the industrial-scale production of these materials in terms of cost and accuracy in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The advancement in agriculture and industry has augmented the environmental toxic load, attributable to various products employed in industrial processes, including mercury (Hg), pesticides, electronic devices, and batteries [1]. Toxic gas emissions, which are very dangerous for nature, are usually caused by industrial production wastes. These gases are also a significant threat to human life. Because even a very small amount of exposure to some gases can cause permanent damage to human health. For example, H2S which occurs naturally or as a by-product in factory production, is dangerous even at the ppm level. According to OSHA (Occupational Safety and Health Administration) criteria, the safe H2S level was determined as 20 ppm during the daily work (6–8 h) [2, 3]. Therefore, removal of H2S is a necessary process. Especially in industry, it causes corrosion in transport lines and poisoning of any catalysts even at low levels [4].
Known to have serious effects on the eyes and respiratory tract in the range of 20–50 ppm [5]. Moreover, H2S exposure in the range of 100 to 500 ppm for 1 h can cause serious damage such as respiratory paralysis and loss of consciousness, while at higher levels it can cause sudden respiratory arrest and death [5, 6]. As a result, H2S gas in the environment should be detected with appropriate methods, with high accuracy and quickly [6]. It is worth emphasizing that H2S exhibits a higher density than air, leading to its propensity for accumulation within confined environments. Consequently, the utmost vigilance and implementation of appropriate protocols for H2S mitigation are imperative for individuals operating in subterranean workplaces, manholes, and comparably inadequate ventilation settings. Thus far, comprehensive investigations have been conducted to explore diverse desulfurization methodologies encompassing membrane separation, adsorption, catalytic oxidation, and distillation processes [7, 8]. In recently, a prominent research trend has emerged focusing on the utilization of materials possessing at least one dimension within the range of 1 to 100 nm to construct sensors characterized by high sensitivity and economical attributes. Nanomaterials (NMs) are recognized as optimal platforms for applications involving gas adsorption owing to their remarkable surface area to volume ratio and distinctive hollow configuration. İncreased surface area facilitates the augmentation of active sites for molecular interactions, thereby resulting in heightened chemical reactivity and enhanced adsorption capacities. This characteristic confers a distinctive advantage in gas adsorption applications, as nanomaterials demonstrate exceptional proficiency in the efficient sequestration and steadfast retention of gas molecules upon their surfaces [9]. Over the past few decades, carbon-based nanomaterials (C-NMs) have garnered significant attention due to their extraordinary electronic, magnetic, and optical characteristics, alongside their chemical adaptability and biocompatibility [10,11,12,13,14].
For instance, carbon-based organic polymers modified with heteroatoms such as S, P, O, N are favored due to their advantages in surface area, porosity, and cost-effectiveness [15]. Nanotubes [16], activated carbons [17,18,19,20], and graphene [21, 22] are among the carbon-based materials that have demonstrated their efficacy as adsorbents and catalyst enhancers. In general, graphene nanoparticles (Gnp) can be toxic to living organisms by bypassing physiological barriers to varying degrees. Different application methods and routes of entry, along with varying tissue distribution and clearance, can determine the degree of toxicity of Gnp. For instance, graphene oxide (GO) can interfere with the normal physiological functions of vital organs, leading to acute inflammatory responses and chronic damage [23]. Nevertheless, in industrial fields, graphene has emerged as an exceptionally promising material in various applications, especially in gas sensors, due to its outstanding mechanical properties, electrical conductivity, and thermal conductivity. Graphene, a two-dimensional material comprising a monolayer of carbon atoms organized in a hexagonal lattice, has garnered considerable interest since its experimental synthesis in 2004 [24,25,26]. Its unique atomic structure and remarkable properties make it a compelling candidate for gas sensing applications, where its high sensitivity and selectivity are crucial factors to consider. The extensive research conducted on graphene-based gas sensors underscores its potential in advancing the field and addressing various sensing challenges [3]. In recent years, graphene has been the subject of numerous research as a promising adsorbent for H2S, a prevalent industrial pollutant [27, 28]. Additionally, it can undergo uniform doping with metals/non-metals or relevant functional groups, thereby enhance its adsorption capability [29,30,31,32,33,34,35]. Natan and co-workers, the adsorption mechanism of H2S molecules on pristine and Pt-decorated graphene sheets was investigated using density functional theory calculations based on local density approximation and generalized gradient approximation methods. It has been found that graphene sheets with a Pt atom on each side can stably bind up to seven H2S molecules, each exhibiting the desired binding energy [32]. In another study, the adsorption mechanism, electrical conductance, and binding energy of H2S gas on pure graphene sheets (GS) as well as Cu-decorated and CuO-decorated GS were investigated. The findings revealed that the CuO-GS structure exhibited a higher binding energy with H2S compared to Cu-GS and GS, and important orbital hybridization was observed between H2S and Cu-GS as well as H2S and CuO-GS, whereas no hybridization was detected between H2S gas and GS [36]. The adsorption performance of graphene structures exposed to H2S and CH4 molecules was investigated. It was found that Ni-doped graphene (Ni-G) structures exhibited critical effectiveness in H2S adsorption. Combining vacancy defect graphene (DG) structures with Ni-doped graphene showed promise as a viable option for H2S gas detection [37]. In the literature, it was observed that a graphene sheet decorated with Ni atoms serves as a more favorable adsorbent for H2S molecules compared to undecorated graphene or graphene decorated with BeO or ZnO [38]. Due to differences in chemical potentials, the geometric configuration and chemical bonding of a single metal atom dopant on the support produce unique electronic structures that cause charge transfer between the metal atoms and the support. When acting as active centers, single metal atoms doped into carbon-based materials with distinct and atomically distributed metal atoms on supports can maximize metal utilization efficiency. Carbon-based materials doped with a single metal atom can also be used as a simple model system to compare theoretical predictions with experimental results [39]. According to Qiu et al. atomic nickel doped graphene exhibits good HER activity with a minimal over potential and Tafel slope in an acidic electrolyte when compared to commercial Pt/C and other reported catalysts. It turns out that the strong interaction between the Ni substituents in the carbon framework is the cause of this performance [40]. In a review published by Sun et al. it was stated that element doping into the carbon matrix can reduce residual stress, increase adhesion strength, and improve tribological, corrosion resistance, hydrophobicity, biocompatibility and optical properties [41].
In this study, the adsorption and detection of H2S gas were conducted on graphene surfaces doped with Ti, V, Cr, and Sc metal atoms at room temperature, some of which have not been encountered in the literature before. In addition, the comparison of results aimed to identify the atom that provides the most efficient outcomes when combined with graphene.
Computational method
In this study, DFT [42] calculations were used via Gaussian 09 software [43] to obtain optimized geometries and electrical properties. As the method, the WB97XD (including distribution) hybrid method was used, which takes into account the effects of exchange and correlation [44]. The WB97XD functional augments the WB97X functional with a parameterized classical dispersion term and a van der Waals correction [45]. Additionally, dipole correction was not taken into account in the DFT calculations because its effect is less significant (between 3.8 and 6.7 kJ/mol) [46]. In this study, a graphene sheet structure with 73 carbon atoms was used and a cluster model was created by attaching 21 hydrogen atoms to the ends of the bonds between the free carbon atoms in the sheet. There are 27 hexagonal honeycomb rings in the structure. The graphene structure used in this study is large enough. Smaller graphene clusters have been used in the literature [47]. All atoms were kept at a relaxed state throughout the theoretical computations. Metal atom was replaced with a carbon atom in the center of the graphene structure to form Ti, V, Cr, and Sc doped graphene sheets. The 6-31G (d,p) basis set was employed for carbon, oxygen, hydrogen, and sulfur atoms, whereas LanL2DZ was employed for Ti, V, Cr, and Sc atoms. In DFT calculations, first of all, Spin Multiplicity (SM) calculations are made and optimized geometries for the most stable SM value are obtained. Optimized geometries were used to obtain the equilibrium geometry (EG) and adsorption energies. The energy difference values account for the zero-point energy (ZPE) corrections. The following equations were utilized to calculate the energy values for H2S adsorption on Ti, V, Cr, and Sc doped graphene structures:
Here, E is the total of the thermal, electronic, and zero-point energies, H is the enthalpy, G is the Gibbs free energy, S is the entropy and T is the absolute temperature (298.15 K). The values of the relative adsorption energy (ΔE), enthalpy (ΔH) and Gibbs free energy (ΔG) were determined using Eq. (4).
Here, System is H2S adsorbed metal doped graphene, Adsorptive is adsorbing molecule (H2S) and cluster is metal doped graphene structures. For the theoretical calculations used in this study, the convergence criteria are 12 × 10–4 rad for gradients of root-mean-square (rms) displacement, 18 × 10–4 bohr for maximum displacement, 3 × 10–4 hartree/radian for rms force, and 45 × 10–5 hartree/bohr for maximum force. Additionally, the RMS change in the density matrix and the maximum change in the density matrix had SCF convergence criterion of 1 × 10–8 and 1 × 10–6, respectively. Natural Bond Orbital (NBO) atomic charges of atoms [48] has been found by using the NBO population analysis. In addition, the graphs for Reduced Density Gradient (RDG) scatter graphs and isosurfaces of RDGs, Total Density of States (TDOS) and Partial Density of States (PDOS) were obtained by using Multiwfn software [49].
Results and discussions
For the adsorption of H2S gas on metal-doped graphene structures by DFT method, firstly, the graphene sheet was modeled as a cluster and doped with metal atoms. Initially, pristine graphene structure was optimized and H2S adsorption was performed. The aim here is to see the effects of metal doping and graphene support material on adsorption. Figure 1 shows the optimized geometries for pristine graphene and H2S adsorbed pristine graphene structures. H2S adsorption energies of pristine graphene; ΔE, ΔH and ΔG values were calculated as -9.0, -11.5 and 18.4 kJ/mol, respectively. A metal atom was replaced with a central carbon atom. The SM values of the structures and the H2S molecule are given in Table 1. Also, the formation energies (EF) of metal-doped graphene structures were calculated according to Eq. (5) [50, 51]. The EF value for all structures was calculated as ~ -7.5 eV. Accordingly, the fact that the formation energies remain negative indicates that metal-doped graphene remains stable.
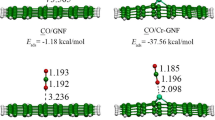
Here, ESystem is thermal energy of metal-doped graphene, x, y and z are number of C, H and metal atoms respectively. N is the number of total atoms. EC, EH and EM are the thermal energies of C, H and metal atoms respectively. The optimized geometry of the H2S molecule and metal doped graphenes are shown in Fig. 2. For the optimized H2S molecule, the H–S bond length was calculated as 1.34 Å and the H–S-H bond angle as 93.0o. In the literature, these values were reported as 1.38 Å and 94o, respectively, by the B3LYP (including dispersion effects) method [52]. Experimental data for H2S are as follows; the H–S bond length is 1.33 Å and the H–S-H bond angle is 92.1o [53]. Accordingly, we have confirmed that WB97XD functional gives very close results to do experimental data and more accurate compared to B3LYP functional. Adsorption geometries were obtained by position the H2S molecule close to the surface of all metal doped graphene structures and releasing it. Optimized geometries are shown in Fig. 3.
The obtained adsorption energies are listed in Table 2. According to the results, it is seen that all metal doped graphene structures can adsorb H2S gas. The lowest ΔE was calculated as -71.0 kJ/mol. The fact that these values are not too low means that the amount of energy required for desorption is not too high. Considering the ΔH and ΔG values, all processes are exothermic and occur spontaneously. Compared to the adsorption energies of the pristine graphene structure, metal atoms both increased the H2S adsorption ability and ensured spontaneous adsorption. The changes in bond distances between metal atoms and carbon atoms before and after H2S adsorption were investigated. The calculated values are listed in Table 3. According to the values in the table, it can be said that all metal atoms are in a movement towards the H2S molecule. In proportion to this, the bond distance with some of the carbon atoms increased. Figures 4 and 5 show the bond angles of metal doped graphene structures before and after adsorption. The reason why the Sc-graphene structure has the lowest bond angle is that it is the structure with the highest C51-, C52- and C58-M bond length values in Table 3. Also, atom labels are shown in Figs. 4 and 5. These labels were used in both Tables 3 and 7.
In order to compare the H2S adsorption on the materials used in this study with in the literature, the ΔE values of the H2S molecule are listed in Table 4. According to these values, it is seen that the graphene structure is a better H2S adsorbent material compared to other carbon structures such as CNT and fullerene. The very low energy of Fe and Pt doped graphene structures is undesirable in terms of desorption. In addition, considering the ΔE values of pristine graphene and Sc doped graphene, it is shown that Sc metal is not effective in adsorption.
The electronic characteristics of the structures were determined by analyzing the HOMO–LUMO gap energy (Eg) values with the difference between the calculated HOMO and LUMO energies. The HOMO, LUMO, Eg and ΔEg values of the metal doped graphenes before and after adsorption are tabulated in Table 5. The Eg has been shown to be a good indicator for determining the sensitivity of nanosensors on numerous times [67]. There is also a relation between Eg and electrical conductivity (σ), which has also been mentioned in [68, 69]:
here κ is the Boltzmann’s constant, A (electrons/m3K3/2) is a constant and T is absolute temperature. There are a number of papers that verifies the use of this formula [70, 71]. Equation (6) indicates when Eg changes, the population of conduction electrons changes exponentially. As a result, the change in electrical conductivity indicates the presence of the chemical (H2S for this study) in the environment. According to the results in Table 5, there is a significant change only for the Cr-graphene structure. A change of 24.8 kJ/mol was observed in the Eg of this structure. There was no significant change in other structures. NBO population analysis was performed to analyze the atomic charge distributions in the interactions. In Table 6, the atomic charges of metal atoms in all structures and the total charge of the H2S compound are given. When the NBO charges in the table are examined, electron transfer occurred from the H2S molecule to the metal atom in all processes. Table 7 lists the energies calculated from the second-order perturbation theory analysis. This table lists the stabilization energy E(2) between electron donor orbital i, electron acceptor orbital j, and their interactions. The larger E(2), the stronger the interaction between them, and the greater the tendency for i to provide electrons to j, meaning the more charge will be transferred [72]. The stabilization energy E(2) associated with electron delocalization between donor and acceptor is calculated by the following equation [73].
For each donor NBO (i) and acceptor NBO (j), where qi is the orbital occupancy, Ei – Ej are the diagonal elements, and Fi,j is the off-diagonal NBO fock matrix element. E(2) values between Lewis type (donor) NBOs and non-Lewis (acceptor) type NBOs show that the electron density is significantly delocalized from carbon atoms to metal atoms. Here there are molecular LP-LP* and BD-LP* interactions. The LP-LP* transition exhibited higher stabilization energy. Moreover, BD-LP* stands out as an important transition, although it exhibits lower stability. In Table 6, the most positive charge among the metal atoms belongs to the Sc atom. This means that Sc-C bonds are more ionic than other metal-C bonds. Therefore, the energy difference between the acceptor Sc atom and the donor C atoms is larger than the energy difference between the other metal atoms and the donor C atoms.
Chemical hardness (η), chemical potential (µ) and electronegativity (χ) were calculated for metal doped graphenes and tabulated in Table 8. Detailed information on the calculation of these parameters is given in Supplementary Material. There was no significant change in chemical hardness, chemical potential and electronegativity of Ti, V and Sc doped graphene structures after H2S adsorption. In other words, the stability and chemical reactivity of the structures is preserved. However, the chemical hardness of the Cr-graphene structure (especially for αMOs) increased and the structure became harder.
Figure 6 shows the HOMO and LUMO illustrations of Cr doped graphene and H2S molecule adsorbed Cr doped graphenes. When the HOMO LUMO analysis is examined, HOMOs are spread on the graphene sheet, while LUMOs are located around the metal atom. The most obvious result here is the reduction of LUMOs around the metal atom after adsorption, especially in a molecular orbitals. This refers to the increase in the total charge of the H2S molecule, that is, it donates electrons. HOMO–LUMO representations of other structures are presented in Figs. 7, 8 and 9. Accordingly, for the Ti doped graphene structure, βMOs HOMOs and αMOS LUMOs are located around the metal atom. Only for αMOs LUMOs, orbital displacements occurred before and after adsorption. For the V doped graphene structure, a decrease in αMOs LUMOs occurred after adsorption. According to the HOMO and LUMO analysis for the Sc doped graphene structure, there is an increase in the HOMOs around the metal after adsorption.
The TDOS and PDOS of Cr doped graphene and H2S adsorbed Cr doped graphene have been presented in Fig. 10. As can be seen the Fig. 10, we see that the HOMO–LUMO gap increases in αMOs. Thus, it can be concluded that there is a change in electrical conductivity. It is seen in Figs. S1–S3 that the TDOS and PDOS plots of other structures do not change significantly. Moreover, the electrostatic potential (ESP) distributions for Cr doped graphene before and after H2S adsorption have been shown in Fig. 11. Blue and red colors were respectively defined on the ESP maps by the Van der Waals surface’s positive and negative areas [74, 75]. In Fig. 11, blue colors are seen in the metal atom before adsorption, but the blue colors decrease after adsorption. In this case, we can say that the positively charged H2S molecule is adsorbed in blue and the blue colors are reduced due to the electron gain from the metal atom. ESP maps of other structures are presented in Figs. S4–S6.
Johnson et al. group [76] has proposed the RDG analysis, which enables determining the kind of interactions that take place between two species. By examining the reduced gradient of the density as a function of the electron density multiplied by the sign of the second eigenvalue of the Hessian matrix, this analysis has been utilized to look at the many non-covalent interactions that are still present in the molecules. The greatest eigenvalue is known to be λ2 in Hessian matrix. Based on this, one could create a distinction between the dissimilar kinds of noncovalent interactions. Figure 12 presents RDG scatter map and isosurface map for H2S/V-graphene system (since it is the system with the lowest adsorption energy). RDG maps of other structures are presented in Figs. S7–S9.
According to the RDG analysis, for metal doped graphenes, the Van der Waals interactions are indicated by values near zero. All scatter maps and isosurface maps of RDG show us that the interactions are typically VdW-type interactions for H2S adsorbed metal doped graphenes. It is important to note that extremely strong interactions do not detect molecules since they show that the adsorbate is difficult to desorb and that the system requires more time to recover. The traditional transition theory forecasts a significantly longer recovery time in the event that the energy of adsorption rises significantly equation is employed to elucidate this situation:
Here τ is the recovery time, ΔE is adsorption energy, T is adsorption temperature (298.15 K), k is the Boltzmann constant (~8.368 × 10–3 kJ/mol) and the ν0 is attempt frequency (~1012 s−1) [77]. According to this equation, the greater the ΔE value, resulting in progressively longer recovery times. In other words, the material adsorbed to the surface takes longer to separate from the surface. The recovery time of the Cr-graphene structure, which has an electronic sensor feature, is 0.26 s. The surface recovery times of Ti-, V- and Sc-graphene structures were calculated as 0.54, 2.28 and 0.003 s, respectively.
Conclusions
In this study, Ti, V, Cr and Sc doped graphene nanosheets, along with the molecular system formed by hydrogen sulfide, were investigated for their adsorption and sensor properties using the DFT method. As a result of the thermodynamic data, it was revealed that all metal-doped graphene structures adsorbed the H2S molecule spontaneously. Compared to the literature, the ΔE values are not found to be enough to make desorption difficult. In the light of RDG analyses, all interactions were realized as Van der Waals interactions. In addition, in all interactions, there is charge transfer between the H2S molecule and metal atoms. The HOMO–LUMO gap change of the Cr doped graphene structure before and after adsorption was calculated as 24.8 kJ/mol. For other structures, this change remained insignificant. Consequently, this finding confirms that the structure in question could be used as an electronic sensor material for the H2S molecule.
Data availability
There is no data availability.
References
Zeng H, Wang L, Zhang D, Yan P, Nie J, Sharma VK, Wang C (2019) Highly efficient and selective removal of mercury ions using hyperbranched polyethylenimine functionalized carboxymethyl chitosan composite adsorbent. Chem Eng J. https://doi.org/10.1016/j.cej.2018.10.001
Wang S, Nam H, Lee D, Nam H (2022) H2S gas adsorption study using copper impregnated on KOH activated carbon from coffee residue for indoor air purification. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2022.108797
Salih E, Ayesh AI (2020) DFT investigation of H2S adsorption on graphenenanosheets and nanoribbons: Comparative study. Superlattices Microstruct. https://doi.org/10.1016/j.spmi.2020.106650
Ozekmekci M, Salkic G, Fellah MF (2015) Use of zeolites for the removal of H2S: A mini-review. Fuel Process Technol. https://doi.org/10.1016/j.fuproc.2015.08.015
Agbroko OW, Piler K, Benson TJ (2017) A comprehensive review of H2S scavenger technologies from oil and gas streams. ChemBioEng Rev. https://doi.org/10.1002/cben.201600026
Nam H, Wang S, Jeong HR (2018) TMA and H2S gas removals using metal loaded on rice husk activated carbon for indoor air purification. Fuel. https://doi.org/10.1016/j.fuel.2017.10.089
Shah MS, Tsapatsis M, Siepmann JI (2017) Hydrogen sulfide capture: From absorption in polar liquids to oxide, zeolite, and metal-organic framework adsorbents and membranes. Chem Rev. https://doi.org/10.1021/acs.chemrev.7b00095
Habeeb O, Ramesh K, Ali GA, Yunus R, Thanusha T, Olalere O (2016) Modeling and optimization for H2S adsorption from wastewater using coconut shell based activated carbon. Aust J Basic Appl Sci 10:136–147
Srivastava R, Suman H, Shrivastava S, Srivastava A (2019) DFT Analysis of Pristine and functionalized Zigzag CNT: A case of H2S sensing. Chem Phys Lett. https://doi.org/10.1016/j.cplett.2019.07.003
El-Sherbiny I, Salih E (2018) In: Kanchi S, Ahmed S (ed) Green synthesis of metallic nanoparticles using biopolymers and plant extracts: Synthesis, characterization and their applications. Wiley, New York. https://doi.org/10.1002/9781119418900.ch10
Dresselhaus M, Terrones M (2013) Carbon-based nanomaterials from a historical perspective. Proc IEEE. https://doi.org/10.1109/JPROC.2013.2261271
Iijima S, Ichihashi T (1993) Single-shell carbon nanotubes of 1-nm diameter. Nature. https://doi.org/10.1038/363603a0
Baptista F, Belhout S, Giordani S, Quinn S (2015) Recent developments in carbon nanomaterial sensors. Chem Soc Rev. https://doi.org/10.1039/C4CS00379A
Lan G, Tang L, Dong J, Nong J, Luo P, Li X, Li Z, Zhang Y, Dai Y, Wang W, Shi H, Wei W (2023) Enhanced asymmetric light-plasmon coupling in graphene nanoribbons for high-efficiency transmissive infrared modulation. Laser Photonics Rev. https://doi.org/10.1002/lpor.202300469
Tang T, Zhou M, Lv J, Cheng H, Wang H, Qin D, Hu G, Liu X (2022) Sensitive and selective electrochemical determination of uric acid in urine based on ultrasmall iron oxide nanoparticles decorated urchin-like nitrogen-doped carbon. Colloids Surf B. https://doi.org/10.1016/j.colsurfb.2022.112538
Mubeen S, Zhang T, Chartuprayoon N, Rheem Y, Mulchandani A, Myung NV, Deshusses MA (2010) Sensitive detection of H2S using gold nanoparticle decorated single-walled carbon nanotubes. Anal Chem. https://doi.org/10.1021/ac901871d
Tsai JH, Jeng FT, Chiang HL (2001) Removal of H2S from exhaust gas by use of alkaline activated carbon. Adsorption. https://doi.org/10.1023/A:1013142405297
Bandosz TJ (2002) On the adsorption/oxidation of hydrogen sulfide on activated carbons at ambient temperatures. J Colloid Interface Sci. https://doi.org/10.1006/jcis.2001.7952
Yan R, Liang DT, Tsen L, Tay JH (2002) Kinetics and mechanisms of H2S adsorption by alkaline activated carbon. Environ Sci Technol. https://doi.org/10.1021/es0205840
Kaliva AN, Smith JW (1983) Oxidation of low concentrations of hydrogen sulfide by air on a fixed activated carbon bed. Can J Chem Eng. https://doi.org/10.1002/cjce.5450610210
Águila JEC, Cocoletzi HH, Cocoletzi GH (2013) A theoretical analysis of the role of defects in the adsorption of hydrogen sulfide on graphene. AIP Adv. https://doi.org/10.1063/1.4794953
Wang S, Sun H, Ang HM, Tadé MO (2013) Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem Eng J. https://doi.org/10.1016/j.cej.2013.04.070
Ou L, Song B, Liang H, Liu J, Feng X, Deng B, Sun T, Shao L (2016) Toxicity of graphene-family nanoparticles: a general review of the origins and mechanisms. Part Fibre Toxicol. https://doi.org/10.1186/s12989-016-0168-y
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater. https://doi.org/10.1038/nmat1849
Dutta S, Manna A, Pati S (2009) Intrinsic half-metallicity in modified graphene nanoribbons. Phys Rev Lett. https://doi.org/10.1103/PhysRevLett.102.096601
Ren H, Li Q, Luo Y, Yang J (2009) Graphene nanoribbon as a negative differential resistance device. Appl Phys Lett doi 10(1063/1):3126451
Hernandez Y, Nicolosi V, Lotya M, Blighe FM, Sun Z, De S, McGovern IT, Holland B, Byrne M, Gun’Ko YK, Boland JJ, Niraj P, Duesberg G, Krishnamurthy S, Goodhue R, Hutchison J, Scardaci V, Ferrari AC, Coleman JN (2008) High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol. https://doi.org/10.1038/nnano.2008.215
Li X, Zhang G, Bai X, Sun X, Wang X, Wang E, Dai H (2008) Highly conducting graphene sheets and Langmuir-Blodgett films. Nat Nanotechnol. https://doi.org/10.1038/nnano.2008.210
Tang Y, Yang Z, Dai X, Ma D, Fu Z (2013) Formation, stabilities, and electronic and catalytic performance of platinum catalyst supported on non-metal-doped graphene. J Phys Chem C. https://doi.org/10.1021/jp400202e
Ganji M, Sharifi N, Ahangari M (2014) Adsorption of H2S molecules on non-carbonic and decorated carbonic graphenes: A van der Waals density functional study. Comput Mater Sci. https://doi.org/10.1016/j.commatsci.2014.05.035
Zhang YH, Han LF, Xiao YH, Jia DZ, Guo ZH, Li F (2013) Understanding dopant and defect effect on H2S sensing performances of graphene: A first-principles study. Comput Mater Sci. https://doi.org/10.1016/j.commatsci.2012.11.048
Ganji MD, Sharifi N, Ardjmand M, Ahangari MG (2012) Pt-decorated graphene as superior media for H2S adsorption: a first-principles study. App Surf Sci. https://doi.org/10.1016/j.apsusc.2012.08.083
Zhang HP, Luo XG, Song HT, Lin XY, Lu X, Tang Y (2014) DFT study of adsorption and dissociation behavior of H2S on Fe-doped graphene. App Surf Sci. https://doi.org/10.1016/j.apsusc.2014.08.141
Borisova D, Antonov V, Proykova A (2013) Hydrogen sulfide adsorption on a defective graphene. Int J Quantum Chem. https://doi.org/10.1002/qua.24077
Hegde VI, Shirodkar SN, Tit N, Waghmare UV, Yamani ZH (2014) First principles analysis of graphene and its ability to maintain long-ranged interaction with H2S. Surf Sci. https://doi.org/10.1016/j.susc.2013.11.015
Mohammadi-Manesh E, Vaezzadeh M, Saeidi M (2015) Cu- and CuO-decorated graphene as a nanosensor for H2S detection at room temperature. Surf Sci. https://doi.org/10.1016/j.susc.2015.02.002
Gao X, Zhou Q, Wang J, Xu L, Zeng W (2020) Performance of intrinsic and modified graphene for the adsorption of H2S and CH4: a DFT study. Nanomaterials. https://doi.org/10.3390/nano10020299
Salmankhani A, Karami Z, Mashhadzadeh AH, Ganjali MR, Vatanpour V, Esmaeili A, Habibzadeh S, Saeb MR, Fierro V, Celzard A (2020) New insights into H2S adsorption on graphene and graphene-like structures: a comparative DFT study. C. https://doi.org/10.3390/c6040074
Bayatsarmadi B, Zheng Y, Vasileff A, Qiao SZ (2017) Recent advances in atomic metal doping of carbon-based nanomaterials for energy conversion. Small. https://doi.org/10.1002/smll.201700191
Qiu HJ, Ito Y, Cong W, Tan Y, Liu P, Hirata A, Fujita T, Tang Z, Chen M (2015) Nanoporous graphene with single-atom nickel dopants: an efficient and stable catalyst for electrochemical hydrogen production. Angew Chem Int Ed Engl. https://doi.org/10.1002/anie.201507381
Sun H, Yang L, Wu H, Zhao L (2023) Effects of element doping on the structure and properties of diamond-like carbon films: a review. Lubricants. https://doi.org/10.3390/lubricants11040186
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (2015) Gaussian 09, Revision D. 01; Gaussian, Inc: Wallingford, CT
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev. https://doi.org/10.1103/PhysRev.140.A1133
Chai JD, Head-Gordon M (2008) Systematic optimization of long-range corrected hybrid density functionals. J Chem Phys doi 10(1063/1):2834918
Tsuneda T, Hirao K (2014) Long-range correction for density functional theory. Wiley Interdiscip Rev: Comput Mol Sci. https://doi.org/10.1002/wcms.1178
Natan A, Kronik L, Shapira Y (2006) Computing surface dipoles and potentials of self-assembled monolayers from first principles. App Surf Sci. https://doi.org/10.1016/j.apsusc.2006.03.052
Kumar S, Sharma S, Karmaker R, Sinha D (2021) DFT study on the structural, optical and electronic properties of platinum group doped graphene. Mater Today Commun. https://doi.org/10.1016/j.mtcomm.2020.101755
Wong MW (1996) Vibrational frequency prediction using density functional theory. Chem Phys Lett. https://doi.org/10.1016/0009-2614(96)00483-6
Lu T, Chen F (2012) Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem. https://doi.org/10.1002/jcc.22885
Kumar N, Sharma M, Sharma JD, Ahluwalia PK (2015) Study of magnetism in nano structures of graphene and functionalized graphene: a first principle study. Indian J Phys. https://doi.org/10.1007/s12648-014-0526-2
Nagarajan V, Nivedhana R, Chandiramouli R (2023) Nucleobases adsorption studies on chair graphane nanosheets – A DFT outlook. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2023.110683
Bokhimi X (2019) Atomic and electronic properties of a 155 H2S cluster under pressure. ACS Omega. https://doi.org/10.1021/acsomega.9b00705
Cook RL, De Lucia FC, Helminger P (1975) Molecular force field and structure of hydrogen sulfide: recent microwave results. J Mol Struct. https://doi.org/10.1016/0022-2860(75)80094-9
Yousefian Z, Ghasemy E, Askarieh M, Rashidi A (2019) Theoretical studies on B, N, P, S, and Si doped fullerenes toward H2S sensing and adsorption. Phys E. https://doi.org/10.1016/j.physe.2019.113626
Ayesh AI (2022) H2S and SO2 adsorption on Cu doped MoSe2: DFT investigation. Phys Lett A. https://doi.org/10.1016/j.physleta.2021.127798
Salih E, Ayesh AI (2021) Computational study of metal doped graphene nanoribbon as a potential platform for detection of H2S. Mater Today Commun. https://doi.org/10.1016/j.mtcomm.2020.101823
Bo Z, Guo X, Wei X, Yang H, Yan J, Cen K (2019) Density functional theory calculations of NO2 and H2S adsorption on the group 10 transition metal (Ni, Pd and Pt) decorated graphene. Phys E. https://doi.org/10.1016/j.physe.2019.01.012
Aghaei SM, Monshi MM, Calizo I (2016) A theoretical study of gas adsorption on silicene nanoribbons and its application in a highly sensitive molecule sensor. RSC Adv. https://doi.org/10.1039/C6RA21293J
Faye O, Raj A, Mittal V, Beye AC (2016) H2S adsorption on graphene in the presence of sulfur: A density functional theory study. Comput Mater Sci. https://doi.org/10.1016/j.commatsci.2016.01.034
Wei H, Gui Y, Kang J, Wang W, Tang C (2018) A DFT study on the adsorption of H2S and SO2 on Ni doped MoS2 monolayer. Nanomaterials. https://doi.org/10.3390/nano8090646
Zhang X, Yu L, Wu X, Hu W (2015) Experimental sensing and density functional theory study of H2S and SOF2 adsorption on Au-modified graphene. Adv Sci. https://doi.org/10.1002/advs.201500101
Lin C, Qin W, Dong C (2016) H2S adsorption and decomposition on the gradually reduced α-Fe2O3(001) surface: A DFT study. App Surf Sci. https://doi.org/10.1016/j.apsusc.2016.06.104
Peng X, Liu D, Zhao F, Tang C (2022) Gas sensing properties of Mg-doped graphene for H2S, SO2, SOF2, and SO2F2 based on DFT. Int J Quantum Chem. https://doi.org/10.1002/qua.26989
Gecim G, Ozekmekci M (2021) A density functional theory study of molecular H2S adsorption on (4,0) SWCNT doped with Ge, Ga and B. Surf Sci. https://doi.org/10.1016/j.susc.2021.121876
Khodadadi Z (2018) Evaluation of H2S sensing characteristics of metals–doped graphene and metals-decorated graphene: Insights from DFT study. Phys E. https://doi.org/10.1016/j.physe.2018.02.022
Fellah MF (2016) Adsorption of hydrogen sulfide as initial step of H2S removal: A DFT study on metal exchanged ZSM-12 clusters. Fuel Process Technol. https://doi.org/10.1016/j.fuproc.2016.01.003
Fellah MF (2019) Pt doped (8,0) single wall carbon nanotube as hydrogen sensor: A density functional theory study. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2019.08.169
Ahmadi A, Hadipour NL, Kamfiroozi M, Bagheri Z (2012) Theoretical study of aluminum nitride nanotubes for chemical sensing of formaldehyde. Sens Actuators B. https://doi.org/10.1016/j.snb.2011.12.001
Hadipour NL, Peyghan AA, Soleymanabadi H (2015) Theoretical study on the Al-doped ZnO nanoclusters for CO chemical sensors. J Phys Chem C. https://doi.org/10.1021/jp513019z
Peyghan AA, Hadipour NL, Bagheri Z (2013) Effects of Al doping and double-antisite defect on the adsorption of HCN on a BC2N nanotube: Density functional theory studies. J Phys Chem C. https://doi.org/10.1021/jp312503h
Eslami E, Vahabi V, Peyghan AA (2016) Sensing properties of BN nanotube toward carcinogenic 4-chloroaniline: A computational study. Phys E. https://doi.org/10.1016/j.physe.2015.09.043
Khan MS, Khan MS (2012) Comparative theoretical study of iron and magnesium incorporated porphyrin induced carbon nanotubes and their interaction with hydrogen molecule. Phys E. https://doi.org/10.1016/j.physe.2012.05.010
Utsu PM, Gber TE, Nwosa DO, Nwagu AD, Benjamin I, Ikot IJ, Eno EA, Offiong OE, Adeyinka AS, Louis H (2023) Modeling of anthranilhydrazide (HL1) salicylhydrazone and its copper complexes Cu(I) and Cu(II) as a potential antimicrobial and antituberculosis therapeutic candidate. Polycyclic Aromat Compd. https://doi.org/10.1080/10406638.2023.2186444
Sjoberg P, Politzer P (1990) Use of the electrostatic potential at the molecular surface to interpret and predict nucleophilic processes. J Phys Chem. https://doi.org/10.1021/j100373a017
Baydir E, Altun A, Fellah MF (2022) Molecular adsorption of silane on Ge, Ga and Al-doped CNT structures: a density functional theory study. Prot Met Phys Chem Surf. https://doi.org/10.1134/S2070205122050033
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc. https://doi.org/10.1021/ja100936w
Peng S, Cho K, Qi P, Dai H (2004) Ab initio study of CNT NO2 gas sensor. Chem Phys Lett. https://doi.org/10.1016/j.cplett.2004.02.026
Acknowledgements
The numerical calculations reported in this paper were partially performed at TUBITAK ULAKBIM, High Performance and Grid Computing Center (TRUBA resources).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Methodology, Software, Writing—Original Draft were performed by [Ömer Faruk TUNALI] and [Numan YUKSEL]. Validation, Formal analysis, Writing—Review & Editing, Supervision were performed by [Gökhan GECE]. Conceptualization, Validation, Formal analysis, Writing—Review & Editing, Supervision were performed by [Mehmet Ferdi FELLAH]. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tunalı, Ö.F., Yuksel, N., Gece, G. et al. A DFT study of H2S adsorption and sensing on Ti, V, Cr and Sc doped graphene surfaces. Struct Chem 35, 759–775 (2024). https://doi.org/10.1007/s11224-023-02265-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02265-2