Abstract
In this work, we report on polyaniline (PANI)–silver nanoparticles (AgNPs) coatings prepared by in-situ polymerization technique. These composites show an excellent corrosion resistance behavior in aggressive environments much superior to PANI. The corrosion studies were carried out on 6061 aluminum alloy substrates coated with PANI-AgNPs composites with different concentrations of silver nanoparticles. The PANI-AgNPs composites were characterized by Scanning and Transmission electron microscopes (SEM/TEM), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and Thermogravimetric analysis (TGA). The conductivity of these composites strongly depends on AgNPs concentration with percolation maxima at 20 wt% of AgNPs in PANI. The composite coatings were evaluated for anti-corrosion performance by using standard corrosion testing method, open-circuit potential analysis, potentiodynamic polarization curve, and electrochemical impedance spectroscopy. The increase in diffusion barrier, prevention of charge transport by the AgNPs, redox properties of polyaniline as well as the large surface area available for the liberation of dopant due to nano-size additive appear to be associated with the exceptional improvement in anti-corrosion performance of elaborated coatings. Due to ease of preparation, better thermal stability, enhanced conductivity, and excellent corrosion inhibition properties, this PANI-AgNPs composite could be used as a potential anti-corrosion coating material in protecting aluminum infrastructures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There is a growing attention towards harvesting energy using solar power in the past decades [1,2,3,4]. However, the life span and durability of the aluminum-based frameworks used in solar power generation panels are greatly affected by the environmental corrosion [5,6,7,8], and there is a growing demand to protect these frameworks using suitable self-healing anti-corrosion coatings. Metallic structures in modern industry rely on organic coatings for corrosion protection [9, 10]. Intrinsically, conducting polymers have become a landmark for new generation research and innovation due to their interesting physical, chemical, electrical, and electrochemical properties [11]. Provision of corrosion protection using nanocomposites with active components and protective systems with “self-healing” properties has high priorities in organic coating systems [12]. The most commonly used barrier coatings in industry are solvent-based epoxy coatings which are cost-effective and show superior adhesion to substrate and chemical resistance [13, 14]. However, these are prone to crack propagation and suffer from poor resistance and brittleness [15, 16]. Moreover, the structures of these epoxy coatings contain free volumes of hydrophilic and hydroxyl groups that can be penetrated by aggressive agents such as water, oxygen, and chlorine, which accelerate the corrosion process of a metallic elements over a period of time [17, 18].
To overcome these issues of epoxy coatings, nanocomposite coatings are very promising and powerful tool for increasing their corrosion resistance [19, 20]. Polymeric nanocomposites have recently attracted more attention as organic–inorganic coatings due to their superior properties [21]. The organic component provides an excellent conductivity and high mechanical flexibility and improved adhesion to the metallic surface, whereas the inorganic nano-filler provides high aspect ratio and improves the electrical and electrochemical performances by creating the active sites for reaction [22,23,24]. Polyaniline (PANI) is one of the most interesting conducting polymers due to ease of chemistry, redox behavior and excellent anti-corrosion properties [25,26,27]. Owing to its high conductivity and thermal stability, silver is a good filler for polymer nanocomposites [28]. Incorporation of silver nanoparticles into polymer matrices can drastically improve their thermal and electrical conductivity, as well as optical and mechanical properties leading to a new class of materials that are of technological importance [29, 30].
PANI-Ag nanocomposite is one that retains the original intrinsic performances of PANI but also exhibits coadjutant effect to synergetic interactions between the two components. Even though some research has been focused on exploring the electrical performances of these composites in various technological applications, the reports on exploring the anti-corrosion performance of these novel composites are very rare. In the present work, we report on the preparation of novel nanocomposites coatings which comprise PANI and Ag nanoparticles with an epoxy resin to improve the electrochemical performance and anti-corrosion behavior towards protecting aluminum alloys used in solar energy frameworks. The prepared nanocomposite coatings on these frameworks were tested for their robust corrosion inhibition under different environmental exposures over a period of time.
2 Experimental
2.1 Materials and Methods
Ultra-pure research grade chemicals such Aniline monomer (Mw = 93.15), Hydrochloric acid (HCl), Ammonium peroxodisulfate (APS), tetraethylenepentamine (TEPA), N-methyl-2-pyrrolidone (NMP), polyvinyl butyl (PVB), Silver nanoparticles (50–100 nm), Ethanol, Methanol, Acetone, and DI water were purchased from Sigma-Aldrich (India) and used as received.
2.2 Materials Characterization
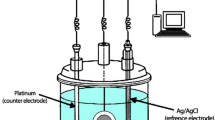
The surface morphologies of pure PANI, AgNPs, and PANI-AgNPs composites were recorded using Zeiss Ultra-60 (Japan). SEM and TEM images were obtained using JEM-2100 (China). Different functional groups and chemical compositions of the films were investigated by using Thermo-Nicolet 6700 FTIR (Japan) spectrophotometer. The thermal stability of the samples was carried out using a NETSCH STA-409PC thermal analyzer. X-ray diffraction patterns of the powdered samples were performed using X’Pert pro X-ray diffractometer with nickel filter. Diffraction data were obtained by exposing the samples to Cu-Kα radiation of wavelength 1.5414 Å. The temperature-dependent conductivity of the samples was studied by 4 probe method using Keithley 6487 picoammeter/voltmeter I-V characterization unit. Electrochemical behavior of the composites was studied in 1 M/L HCl by using Platinum as counter electrode, a saturated calomel electrode as reference electrode, and PANI composite as working electrode. The impedance spectrum of the samples was recorded under open-circuit potential for frequencies ranging from 100 kHz to 5 MHz using 10 mV ac amplitude of sinusoidal voltage signal. The electrochemical performances were recorded on an electrochemical workstation (Chenhua CHI-660C) at ambient conditions. The oxygen gas permeability of PANI and PANI-CNT nanocomposites was investigated using a Yanaco GTR-31 gas permeability analyzer (GPA). The oxygen transmission rate and the oxygen permeability of the samples were estimated by gas chromatography technique.
2.3 Synthesis of PANI
In-situ chemical oxidative polymerization technique which is regarded as the most universal method for PANI preparation was adapted. During the synthesis process, 0.1 M aniline solution (0.93 \(\approx \) 1 ml of aniline added to 100 ml of distilled water) and 1 M HCl were used to obtain aniline hydrochloride. Around 100 ml of 0.1 M APS (2.28 g of APS dissolved in 100 ml of distilled water) was added dropwise to the aniline hydrochloride solution along with stirring for about 3 h to initiate polymerization [31]. Oxidative polymerization of aniline using an oxidizing agent in the presence of acidic aqueous solution for the formation of PANI is a rapid exothermic reaction releasing heat energy in the process of oxidizing aniline to PANI [32]. To avoid the decomposition of PANI by heat energy produced in the reaction medium, oxidizing agent is being added dropwise and the polymerization reaction was carried out in a cold environment (0–5 °C). The polymerized product was filtered to obtain PANI which was then subjected to washing and then vacuum dried.
2.4 Synthesis of PANI-AgNPs Composites
PANI-AgNPs composites with different wt% of AgNPs (5, 10, 15, 20, and 25) in PANI were synthesized by in-situ oxidative polymerization of aniline in the presence of AgNPs. In a typical procedure, 0.1 M aniline hydrochloride was added to AgNPs dispersions. The mixture was continuously stirred for about an hour to produce a homogenous dispersion of AgNPs in aniline hydrochloride solution. The oxidizing agent APS (0.1 M) was added dropwise to the above mixture to initiate polymerization of aniline in a stirred reactor which was maintained between 0 and 5 °C. After the process is complete, the solution was filtered and the obtained product was washed with deionized water and finally dried.
2.5 Preparation of PANI-AgNPs Composite Thin films
The synthesized PANI powder (0.3 g) was added to 5 ml of tetra ethylene pentamine (TEPA) and the mixture was magnetically stirred for about 12 h under room temperature conditions to obtain a homogenous dispersion of PANI in TEPA. The reaction mixture was then filtered using Whatman filter paper of pore size 0.5 μm. Also, AgNPs (0.4 g) were added to 10 ml of N-methyl-2-pyrrolidone (NMP) solvent and the resulting mixture was sonicated for three hours to attain uniform dispersal of AgNPs in NMP. After the sonication process is complete, the solution was filtered using same Whatman filter paper to obtain a pure solution of AgNPs. In parallel, polyvinyl butyral (PVB, 5 g) was dissolved in methanol (50 ml) and the mixture was stirred for six hours and then sonicated for three hours to obtain a homogenous solution. Then for the preparation of thin films, pure PANI solution and PANI solution doped with different weight percentages of AgNPs (5 wt.%, 10 wt.%, 15 wt.%, 20 wt.%, and 25 wt.%) were made into a slurry by adding to a mixture of methanol-PVB solution. This was further sonicated for 12 h to achieve uniform distribution of PANI-AgNPs. The aluminum-6061 substrates were polished using emery paper and then washed with acetone. Thin films of pure PANI and PANI containing AgNPs were prepared by using a dip-coating unit (model no.: HO-TH-02) for 50 s on the dried aluminum substrates. Finally, in order to remove the moisture, the prepared thin films were cured at room temperature for a week followed by post-curing at 90° for five hours. The thickness of the coatings on aluminum substrates was 160 ± 5 µm.
3 Results and Discussion
3.1 Scanning Electron Microscopy (SEM)
Surface morphology of PANI, AgNPs, and PANI-AgNPs composite were investigated using SEM analysis. Figure 1a–c illustrates SEM images of pure PANI, silver nanoparticles, and PANI-AgNPs (20 wt.%), respectively. The SEM image of pristine PANI exhibits an agglomerated morphology representing amorphous nature with no specific geometry similar to that previously reported [33]. Though the distribution of polymer chains looks erratic, an arrangement of well-interconnected grains can be seen from the topography of the pure PANI film. The SEM image of silver nanoparticles illustrates a homogeneous distribution of silver nanoparticles of various sizes which are mostly spherical in shape. The SEM image of 20 wt.% of PANI-AgNPs composite represents a layered structure where the features of PANI and AgNPs are fused together obscuring the initial shape of the spherical silver nanoparticles. The morphology clearly indicates the presence of PANI over and in between the conducting channels of AgNPs due to the adsorption of anilinium cations by the channel surfaces. Therefore, it can be concluded that the filler particles serve as backbone in the polymer matrix, thus greatly enhancing the properties of the composite film.
3.2 Tunneling Electron Microscopy (TEM)
Sophisticated microscopic analysis, such as TEM, was carried out to further investigate the structure and morphology of the samples under study. Figure 2a shows a TEM image of pristine PANI. The linking of aniline monomer units during the formation of PANI in the form of polymer chains can be clearly seen as fibrous structures. The TEM image of 20 wt.% of PANI-AgNPs composite in Fig. 2b illustrated a cross-linked structure due to synergistic interaction between PANI and AgNPs. The nanoparticles of Ag served as adsorption sites for the aniline monomers during the process of oxidative polymerization thereby resulting in bonding between the two constituents. The morphology displays particles with average diameters of about 20–30 nm.
3.3 Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectral studies are very useful to identify the functional groups and chemical bonds, thereby confirming the presence of constituents in the sample under study. Figure 3a–c represents the FTIR spectra of pure PANI, silver nanoparticles, and 20 wt.% of PANI-AgNPs composite. The bands observed in the pure PANI film at 3440, 1603, 1385, 1110, and 830 cm−1 correspond to O–H stretching, C = N stretching of quinoid ring, C–C stretching of benzoid ring, in-plane bending vibration of the C–H mode, and out-of-plane C–H bending vibration, respectively, which confirms the formation of PANI [34,35,36]. The FTIR spectra of silver nanoparticles show the broadband characteristic peaks at 3450 cm−1 are due to O–H stretching, 1590 and 1120 cm−1 are attributed to N–H bend and C–O stretch, whereas the ones observed at 950 and 830 cm−1 correspond to C–H stretching [37]. The presence of all these peaks in 20 wt.% of PANI-AgNPs composite with a slight red-shift compared to bare PANI indicates the physiochemical bonding between PANI and AgNPs. Thus, the results confirm the successful formation of PANI over the surface of AgNPs.
3.4 X-ray Diffraction (XRD)
X-ray diffraction characterization technique was adopted to identify the crystalline phases present in the composite at the percolation threshold, thereby analyzing the structural properties of the samples. The X-ray diffraction patterns recorded for pure PANI, AgNPs, and 20 wt% of PANI-AgNPs composite are shown in Fig. 4. The X-ray diffraction profile of pure PANI shows major broadband peaks centered at 2θ = 28°, 34°, 57°, and 62°, which are the characteristic peaks of PANI synthesized via chemical oxidative polymerization [22, 24]. There are various reports in the literature indicating that PANI exhibits semi-crystalline nature with crystallinity of 10 to 30%, which is also evident and consistent in the diffractograms of pure PANI. The X-ray diffractograms of pure AgNPs show the sharp characteristic peaks spread over 2θ = 30° to 80°. The four main peaks observed in the X-ray diffractograms correspond to (111), (200), (220), and (311) planes of FCC structure (JCPDS file No. 06–0480) of silver nanoparticles [38]. The XRD pattern of PANI-AgNPs reflects sharp and well-defined peaks, indicating the crystallinity of the synthesized composite films. With the addition of AgNPs, the degree of interchain stacking between the phenyl rings of PANI increased and sharpened resulting in higher regularity of PANI stacked inside AgNPs. The XRD pattern of PANI-AgNPs also reflects that it has retained the major characteristic peaks which correspond to both PANI and AgNPs, and hence supports the formation of composite structure.
3.5 Thermogravimetric Analysis (TGA)
The thermal stability of the PANI-AgNPs composites was investigated in comparison to pure PANI using TGA analysis, as shown in Fig. 5. The thermograms of both PANI and PANI-AgNPs composite (20 wt% of AgNPs in PANI) show a three-step weight loss. A small fraction of weight loss that occurs up to 150° in both PANI and PANI-AgNPs composite is due to the evaporation moisture content from the samples. The weight loss that occurs in PANI between 150° and 300° C is due to the evaporation of oxidizing agents and degradation of PANI chains [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. The poor thermal stability of the PANI can significantly be improved by the addition of AgNPs in polymer matrix. The initial degradation of the PANI chains was improved from 150° C to 225° C in the case of PANI-AgNPs composite. Pure PANI shows a residual weight of 27% when subjected to a temperature of 500 °C, whereas the PANI-AgNPs composite shows a residual weight of ~ 40% for the same temperature. Hence, the TGA studies conducted on both samples indicates a significant enhancement in the thermal stability of the PANI-AgNPs composite used in the present investigation.
3.6 Electrical Conductivity
The effect of AgNPs concentration on temperature-dependent conductivity of PANI was investigated at 100° C and depicted in Fig. 6. It is interesting to note that the conductivity of PANI depends on the filler concentration and shows a percolation threshold at 20 wt% of AgNPs in PANI. The conductivity of pure PANI increases from ~ 0.3 S/cm to 139 S/cm for PANI-AgNPs (20 wt%) composite. The increase in conductivity up to percolation threshold could be attributed to the enlarged surface area of PANI-AgNPs composite film. The relatively large surface area of PANI-AgNPs (20 wt.%) strengthened the stacking of polymer chains along a particular direction, thereby promoting the conjugation length and increased the crystalline phase of the film [39]. In such a condition, the intra-chain electron transfer becomes significant resulting in a high-quality conducting composite film. Increase in conjugation length is one of the important factors affecting the temperature dependence of conductivity and enhancing the conductivity rapidly in the composite. Another reason for improved conductivity is the formation of π-π conjugation due to the interactions between PANI and AgNPs which enhances the charge carrier density and better conducting pathways arising from the conformational changes on the polymer backbone. For the AgNPs content beyond 20 wt% in PANI, the π-π conjugation in the polymer backbone is greatly affected due to the formation of porous structures in the composite and blocks the ease of charge carrier hopping in the composite, and hence the conductivity decreases drastically. The conductivity studies clearly suggest that the composites investigated in the present study demonstrate percolative conduction with ultimate conductivity at 20 wt% of AgNPs in PANI.
3.7 Electrochemical Studies for Anti-corrosion Protection
The potentiodynamic polarization behavior of pure PANI and PANI-AgNPs composites with the different concentrations of silver nanoparticles in one molar HCl corrosive media is represented in Fig. 7. The Tafel curves are extrapolated to estimate corrosion current, corrosion potential, anodic and cathodic Tafel constants. In the plot, it is being observed that 20wt.% of PANI-AgNPs composite film exhibits the highest value of the corrosion potential (Ecorr) and the lowest value of the corrosion current (Icorr). The detailed analysis of the results has shown that PANI-AgNPs (20wt.%) thin film exhibits better performance for anti-corrosion on aluminum substrate compared to pure PANI coating. The improved properties in 20wt.% of PANI-AgNPs composite film is believed to be due to highly dispersed silver nanoparticles in PANI matrix, resulting in good anti-corrosion protection towards exposure of acidic media such as HCl. In the presence of water vapor and oxygen gas, the formation of corrosion and rust in 6061 aluminum alloy substrate could simply be illustrated in the following reactions.
If by some means this mechanism is prevented, the corrosion inhibition becomes more effective. Hence, good anti-corrosion protection properties can be achieved by the diminution of diffusion pathways for oxygen and water vapors in the polymer matrix.
The corrosion protection performance of the synthesized composite films was studied using electrochemical impedance spectroscopy (EIS) technique by measuring parameters like coating resistance (Rc) and double-layer charge capacitance (Cct). Figures 8 and 9 represent the variation of capacitance and resistance as a function of immersion time for PANI and PANI-AgNPs composite of different weight percentages, respectively. The Rc and Cct can be attributed to the barrier ability and anti-permeability behavior of a particular sample in an electrolyte solution [40]. Based on the detailed analysis of EIS results, it has been observed that the Rc value increases whereas Cct values tend to decrease with immersion time. The higher the Rc value is, the higher the anti-corrosion ability of the film [41]. The increasing Rc values and decreasing Cct value are found to increase with an increase in the AgNPs concentration and time of immersion. They were observed to be optimized for 20 wt.% of PANI-AgNPs composite film because the highly cross-linked layered structure creates tortuous paths acting as a barrier diffusion against corrosive species, and ultimately prevents corroding agents to reach metallic substrates. The enhancement in anti-permeability and anti-corrosion ability of PANI-AgNPs composite (20 wt.%) at percolation threshold was due to the slow diffusion and lengthening of the diffusion pathway of electrolyte. Compared to pure PANI, the nanocomposite film efficiently protects the metal substrate due to its enhanced barrier properties between the metal surface and corrosive medium.
The electrical measurements under impedance spectroscopy can easily be automated and can often be associated with complex material properties such as corrosion, defects, mass transport, dielectric attributes, and the influence of the composition on the conductivity of solids. The Cole–Cole plots obtained by plotting \(Z^{\prime}\) VS \(Z^{\prime\prime}\) for pure PANI and PANI- AgNPs composite films of different weight percentages are shown in Fig. 10. The plot results as semicircular arcs in these composites representing the capacitive loops implying the resistance of corrosion. With the decrease in diameter, the resistance decreases significantly. The impedance diagrams show that by increasing the concentration of inhibitor, the size of the capacitive loop increased and the diameter of the semicircular arc was larger at 20 wt.% of PANI-AgNPs composite compared to pure PANI and any other composite sample, indicating higher charge transfer resistance and hence lower corrosion rate [42]. The analysis of the results suggests a strong barrier ability of charge transfer and thus a higher corrosion resistance efficiency offered by the PANI-AgNPs composite (20 wt.%) compared to pure PANI, thereby demonstrating more protective effects preventing the metal to corrode and the results observed are in good agreement with Tafel results. The investigated PANI-AgNPs composite in the present study not only shows the improved anti-corrosion performance in comparison to pure PANI, but also exhibits better results in comparison to plain epoxy-coated Al substrates [43, 44].
Figure 11 shows the oxygen gas permeability of pure PANI and PANI-AgNPs composites at different concentrations of silver nanoparticles. From the plot it is clear that 20wt.% of PANI-AgNPs thin film exhibits a 56% reduction in oxygen gas permeability due to the high aspect ratio of 20wt.% AgNPs thin film. The obtained results show that 20wt.% AgNPs thin film shows improved gas barrier property for anti-corrosion compared to pure PANI. These studies reveal that PANI-AgNPs composites thin films can be looked at as advanced anti-corrosion materials replacing the polymer-ceramic composite materials due to the existence of more tortuous pathways in the polymer backbone. The percolation threshold was optimized at 20wt.% of PANI-AgNPs sample, whereas by increasing the concentration of AgNPs above 20wt.%, the oxygen permeability also gradually increases due to reduction of tortuous pathways in the polymer chain [45].
4 Conclusions
The conducting PANI-silver nanoparticles hybrid composites prepared by in-situ polymerization were used as anti-corrosion coatings for 6061 aluminum alloys. FTIR and XRD studies show that the crystallinity of individual components was retained in the composite formation. Electron microscopy studies (SEM and TEM) revealed that the AgNPs were uniformly covered by PANI. The TGA studies confirm that PANI-AgNPs composites were thermally more stable in comparison to pure PANI. The effect of addition of AgNPs on the improvement of conductivity and anti-corrosion properties has been clearly demonstrated. The novelty of these coatings lies in the generation of corrosion inhibition by three mechanisms operating simultaneously, viz. improvement of barrier properties, redox behavior of PANI, and formation of a nonlinear current conduction behavior preventing easy charge transport. Thus, the coatings based on PANI-AgNPs could potentially be employed as a corrosion protection films in 6061 aluminum alloys used in solar panel frames.
Change history
21 October 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s40735-022-00720-x
References
Agarwal S, Ho GW (2012) J Solid State Chem 189:101–107
Bakirci K, Ozyurt O, Comaki K, Comaki O (2011) Energy 36:3224–3232
Li H, Yan J, Campana PE (2012) Int J Greenhouse Gas Control 9:272–280
Bekele G, Palm B (2010) Appl Energy 87:487–495
Nikiforov AV, Petrushina IM, Christensen E, Tomas-Garcia AL, Bjerrum NJ (2008) Int. J. Hydro. Energy 33:111–119
Palcut M, Mikkelsen L, Neufeld K, Chen M, Knibbe R, Hendriksen PV (2010) Corros Sci 52:3309
Dutta S (2010) Int J Hydrog Energy 15(1990):379
Pourbaix M (1974) Corros Sci 14:25
Chang CH, Huang TC, Peng CW, Yeh TC, Lu HI, Hung WI, Weng CJ, Yang TI, Yeh JM (2012) Carbon 50:5044–5051
KC. Chang, WF. Ji, MC Lai, YR Hsiao, CH. Hsu, TL. Chuang, Y. Wei, JM. Yeh, WR. Liu, (2014) Poly. Chem 5: 1049–1056.
Zarras P, Anderson A, Webber C, Irvin DJ, Irvin JA, Guenthner A, Stenger-Smith JD (2003) Radiat Phy Chem 68(3–4):387–394
Spinks G, Dominis A, Wallace G, Tallman D (2002) J Solid State Electrochem 6(2):85–100
Liu S, Yan H, Fang Z, Wang H (2014) Compos Sci Technol 90:40–47
Zhang Z, Zhang W, Li D, Sun Y, Wang Z, Hou H, Chen L, Cao Y, Liu Y (2015) Int. J. Mol. Sci 16:2239–2251
Qi B, Lu SR, Xiao XE, Pan LL, Tan FZ, Yu JH (2014) Polym. Lett. 8(7):467–479
Wan YJ, Gong LX, Tang LC, Wu LB, Jiang JH (2014) Compos A 64:79–89
Jiang MY, Wu LK, Hu JM, Zhang JQ (2015) Corros Sci 92:118–126
Ramezanzadeh B, Ghasemi E, Madhavian M, Changizi E, Mohammedzadeh M (2015) Carbon 93:555–573
Wang X, Xing W, Song L, Yang H, Hu Y, Yeoh GH (2012) Surf. Coat. Technol 206:4778–4784
KC. Chang, MH. Hsu, HI Lu, MC. Lai, PJ. Liu, CH. Hsu, WF. Ji, TL. Chuang, Y. Wei, JM. Yeh, WR. Liu (2014) Carbon 66: 144–153
Yang Z, Sun W, Wang L, Li S, Zhu T, Liu G (2016) Corros Sci 103:312–318
Al-Hartomy OA, Khasim S, Roy A, Pasha A (2019) Appl Phys A 125:12
Khasim S (2014) Omar A Al-Hartomy. RSC-Advances 4:39844–39852
Khasim S (2019) Results in Physics 12:1073–1081
da Silva JEP, de Torresi SIC, Torresi RM (2007) Pro Org Coat 58(1):33–39
Jafarzadeh S, Adhikari A, Sundall PE, Pan J (2011) Prog. Org. Coat 70(2–3):108–115
de Souza S (2007) Surf Coat Technol 201(16–17):7574–7581
Kulkarni MV, Vishwanath AK, Marimuthu R, Mulik UP (2004) J Mater Sci Mater Electron 15:781–785
Kulia BK, Garai A, Nandi AK (2007) Chem Mater 19:5443–5452
Haes AJ, Hall WP, Chang L, Klein WL, Van Duyne RP (2004) Nano Lett 4:1029–1034
Lakshmi M, Roy AS, Parveen A, Al-Hartomy OA, Khasim S (2015) J Mater Res 30(15):2310–2318
Sapurina I, Stejska J (2008) Polym Int 57:1295–1325
Rajyalakshmi Timmapuram, Apsar Pasha, Syed Khasim, Mohana Lakshmi, MV. Murugendrappa , Nacer Badi (2020) Journal of Electronic Materials, 49:341–352
Perrin FX, Phan TA, Nguyen DL (2015) J Polym Sci Part A: Polym Chem 53:1606
Badi N, Khasim S, Roy AS (2016) J Mater Sci: Mater Electron 27:6249–6257
Huang J, Wan M (1999) J Poly Sci Chem 37:1277
Jyothi K, Baunthiyal M, Singh A (2016) Journal of Radiation Research and Applied Sciences 9:217–227
Li Y, Li Z, Zheng F (2015) J Appl Polym Sci 132:42785
Kim M, Lee C, Jang J (2014) Adv Funct Mater 24:489–2499
Liu X, Xiong J, Lv Y, Zuo Y (2009) Prog Org Coat 64:497–503
Sun W, Wang L, Wu T, Liu G (2014) Corros Sci 82:1–6
Amirudin A, Thierry D (1995) ‘Prog. Org Coat 26(1):1–28
Zhang S, He Y, Zhang T, Wang G, Xu Du (2018) Materials 11:965
HosseiniRad R, Toorani M, Zarei HamidReza (2019) Anti-Corrosion Methods and Materials 66(1):138–147
Stankovich S, Piner RD, Nguyen ST, Ruoff SS (2006) Crabon 44:3342–3347
Acknowledgements
Authors NB & SK would like to acknowledge the financial support towards this research from Deanship of Scientific Research (DSR), University of Tabuk, Tabuk, Saudi Arabia, under research Grant No.S-1440-0185.
Author information
Authors and Affiliations
Contributions
Authors NB and SK contributed towards synthesis of polyaniline-AgNPs nanocomposites and prepared their coatings for anti-corrosion studies. They also contributed towards investigation of electrochemical characterizations and preparation of manuscript (drafting & editing). Authors AP, ASA, and ML contributed towards materials characterization such as morphological and structural analysis of the nanocomposite films using SEM, TEM, FTIR, and TGA. They have also contributed towards analysis of charge transport properties. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s40735-022-00720-x
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Badi, N., Khasim, S., Pasha, A. et al. RETRACTED ARTICLE: Silver Nanoparticles Intercalated Polyaniline Composites for High Electrochemical Anti-Corrosion Performance in 6061 Aluminum Alloy-Based Solar Energy Frameworks. J Bio Tribo Corros 6, 123 (2020). https://doi.org/10.1007/s40735-020-00417-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00417-z