Abstract
High-performance gas sensors operating at room temperature are in great demand towards monitoring the environmental hazardous pollutants such as toluene and benzene. In this work, we report synthesis of polyaniline–graphene nano-platelets (PANI–GRNPs) hybrid composites with varied content of GRNPs in PANI matrix by in situ chemical oxidative polymerization. Structural and morphological characterization of polymer nanocomposites were investigated through scanning electron microscopy (SEM) and Fourier transform infra-red spectroscopy (FTIR). SEM micrograph of polymer nanocomposite reveals uniform distribution of GRNPs in the PANI matrix. FTIR analysis of PANI–GRNPs composite revealed the presence π–π interaction between PANI and GRNPs leading to the formation of charge-transfer complex. The electrical properties of the prepared composites were tested as a function of GRNPs content in PANI matrix, the presence of GRNPs has significantly improved the conductivity and dielectric response of polymer nanocomposite in comparison to pure PANI. The sensor devices fabricated using PANI–GRNPs composite were tested for non-polar toluene and benzene vapors at ambient temperature using laboratory-made sensor setup. PANI–GRNPs composites used in the present investigation have shown enhanced sensitivity for toluene and benzene gases in comparison to pure PANI. Due to excellent sensitivity, faster response and recovery time, these PANI–GRNPs composites may find extensive technological applications as a conductometric sensor towards detection of toluene and benzene gases at lower concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
One of the main reasons for the rapid development of sensor technology is to provide safety and security to the mankind. Air pollution influences human health and can cause a number of diseases. The detection of hazardous pollutants such as benzene and toluene at low concentrations using the cost-effective means is a serious concern of today’s sensor technology.
Metal oxides such as TiO2, ZnO, SnO2, MoO3 have been utilized as materials for gas-sensing applications over many years with some drawbacks such as their operation at room temperature and long-term stability [1,2,3,4]. In recent past, conducting polymer-based gas sensors have attracted the attention of many researchers due to easy synthesis, environmental stability, and tunable electrical properties and can be easily operated at room temperatures [5,6,7,8]. Polyaniline (PANI) is one of the most interesting conducting polymers that can be used for gas-sensing applications due its sensitivity for number of gases such as NH3, CO, H2, NO2, methanol, H2S, hydrazine, volatile organic compounds, etc. [9,10,11,12,13,14,15,16]. The intrinsic redox and acid/base reaction that PANI can undergo upon doping results into range of electrical properties, which makes it a suitable material for gas-sensing applications [16,17,18,19]. The gas-sensing properties of conducting polymers can be tailored significantly by suitable formation of composites with nano-fillers. In recent years, graphene has emerged as a prominent nano-filler due to its superior electrical and mechanical properties. The pristine graphene is not suitable for gas-sensing applications due its low adsorption energy at the surface for different gases [20,21,22,23]. The incorporation of graphene into conducting polymer matrix results in a polymer nanocomposite; this combination of multiphase material forms a hybrid system with improved material properties. Recently, numerous studies have been reported on graphene-based polymer nanocomposites for their structural, optical and thermal properties [23,24,25,26]. Owing to the technological promises of polyaniline and graphene, polyaniline/graphene nanocomposites are attracting immense interest of scientific community [27,28,29,30,31,32,33]. Studies on gas-sensing behavior of these composites started very recently [32,33,34], yet the reports on detection of benzene and toluene gases at low concentrations are very limited. In this work, we present a simple and economic way of preparing PANI–GRNPs composites via in situ polymerization of aniline in the presence of GRNPs. A detailed study on transport properties of these composite were reported. We report the fabrication of chemo-sensor based on PANI–GRNPs composites that can operate at normal atmospheric conditions. The gas-sensing properties of prepared materials were investigated for detection of toluene and benzene gases at low concentrations.
2 Materials and methods
Chemicals used in the present investigation were procured from Sigma Aldrich (India). Aniline was distilled under reduced pressure prior to its use, whereas other reagents were used without further purification. Graphene nano-platelets were procured from Cheap-tubes Inc (Brattleboro, VT, USA). These GRNPs have specific surface area of 600–750 m2/g with an average platelet diameter of less than 2 µm. These GRNPs were used as received to prepare composites with polyaniline.
2.1 Synthesis of PANI–GRNP composites
Polyaniline was synthesized by oxidative chemical polymerization of aniline using ammonium peroxydisulfate (APS) as an oxidant [35,36,37]. The chemical oxidative polymerization of monomer aniline was carried out in the presence of GRNPs in acidic medium with APS as an oxidant. 1M hydrochloric acid (HCl) and GRNPs were homogenized by vigorous stirring for 1 h, to this 0.5 M of aniline was added with vigorous stirring to form an emulsion. The oxidant APS was added drop-by-drop with continuous stirring for 6 h by maintaining the reaction mixture at 0–5 °C. The resulting precipitate was filtered and washed thoroughly with water and acetone, dried at 50 °C under a dynamic vacuum to obtain the fine powder. Different compositions of the PANI–GRNPs composites with varying content of GRNPs in PANI were synthesized (2, 4, 6, 8 and 10 wt% of GRNPs in PANI). Suspensions of PANI–GRNPs composites were prepared by dispersing GRNPs in dimethyl propylene urea (DMPU) under sonication followed by vigorous stirring to get a good dispersion. The resulting suspension was spin coated onto glass substrate at 1000 rpm, dried under dynamic vacuum at 50 °C for 12 h to obtain films of PANI–GRNPs composite. PANI–GRNPs composite films with varying content of GRNPs (2, 4, 6, 8 and 10 wt%) in PANI were prepared and aluminum electrodes were deposited by thermal vapor deposition method.
2.2 Experimental techniques
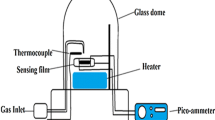
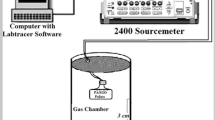
Surface morphology of PANI–GRNPs composite films were investigated using scanning electron microscopy (SEM) (Hitachi S-3000N), the structural analysis of the composites were performed using Fourier transform infra-red (FTIR) spectrometer (Thermo-Nicolet 6700) in KBr medium. The temperature-dependent electrical characterization of the nanocomposite films were studied by using four probe methods (Keithley 2410 Source meter) in the temperature range 30–200 °C. The frequency-dependent conductivity and dielectric attributes were analyzed using LCR impedance analyzer (Wayne Kerr 6500B) in the frequency range 100 Hz–1 MHz. Sensing behavior of pure PANI and PANI–GRNPs composite films were investigated for toluene and benzene vapors using a laboratory-made sensor setup (Fig. 1). The sensor setup was calibrated prior to use by flushing N2 gas over the test samples. During the process of N2 gas run, the resistance of the sample increased with N2 gas concentration and attains the base resistance when the N2 gas was completely flushed out.
The sensing response of the test gases were evaluated using the relation:
where Rg and Ra are the resistances of the film in gas and air, respectively.
3 Results and discussion
3.1 Scanning electron microscopy (SEM)
The surface morphologies of pure PANI, GRNPs and PANI–GRNPs composites (6 wt%) recorded by scanning electron microscope are shown in Fig. 2a–c. The SEM micrograph of pure PANI (Fig. 2a) shows an agglomerated morphology with granular particles of few microns. The surface morphology of pure GRNPs (Fig. 2b) reflects a flaky structure with distinct edges and flat surfaces. The micrograph of PANI–GRNPs composite (Fig. 2c) shows a homogeneous distribution of GRNPs in PANI matrix. The GRNPs in PANI matrix were completely covered by PANI layers during in situ polymerization of aniline in the presence of GRNPs and forming a glue-like network. The presence of GRNPs sheets in PANI matrix leads to the formation of macro-pores through which the gas molecules can easily diffuse leading to higher sensitivity, short response time and good reversibility [38]. The SEM micrographs of the nanocomposite films have large surface area in comparison to pure PANI and the homogeneous distribution of GRNPs in PANI matrix provides high aspect ratios for the nanocomposites [39]. These morphological modifications of pure PANI matrix due to the addition of GRNPs significantly contribute towards improved electrical and sensing characteristics of PANI–GRNPs composites.
3.2 Fourier transform infra-red spectroscopy (FTIR)
The structural aspects of pure PANI and PANI–GRNPs (6 wt%) composites were analyzed by FTIR spectroscopy and are shown in Fig. 3a, b. The FTIR spectra of pure PANI (Fig. 3a) show the important characteristic peaks at 1560 cm−1 (C=C quinoid rings), 1447 cm−1 (C=C, benzenoid rings), 1303 cm−1 (C–H in plane bending), 1276 cm−1 (C–N in plane bending) [35,36,37, 40]. The spectra of PANI–GRNP composite (Fig. 3b) show characteristic peaks at 3450 cm−1 (O–H stretching of GRNP), 3000 cm−1 (C=O of GRNP), 2348 cm−1 (C–OH of GRNP). The major characteristics peaks of pure PANI 1447 cm−1 and 1303 cm−1 appears to be shifted in the composite spectra, which suggests a weak Van der Waals interaction between PANI and GRNPs in the composite [38]. The FTIR spectra of the PANI–GRNPs composite show a slight shift towards higher wavenumbers also suggest a π–π interactions developed between PANI and GRNPs phases forming a charge-transfer complex.
3.3 Temperature-dependent conductivity
Temperature-dependent conductivities of pure PANI and PANI–GRNPs composites in the temperature range 30–200 °C are represented in Fig. 4. The conductivity shows three-step variations with temperature for pure PANI and PANI–GRNP composites. The increase in conductivity for all the samples with temperature is mainly due to thermally assisted charge carrier hopping, which is a characteristic feature of disordered materials. The presence of GRNPs in PANI matrix significantly enhances the conductivity of composite in comparison to pure PANI. The conductivities of the composites depend on the content of GRNPs in PANI matrix, this enhancement in the conductivity arises due to the synergetic interactions between PANI and GRNPs phases through charge transfer between delocalized P-orbitals. It is observed that the conductivity of the composite increases upto 6 wt% content of GRNPs in PANI and decreases thereafter. Addition of GRNPs (upto 6 wt%) in PANI matrix marginally alters the chain length of PANI backbone thereby facilitating the charge carrier hopping between favorable sites [41]. For higher content of GRNPs in PANI (beyond 6 wt%), there is a pronounced alteration of chain length in polymer backbone leading to partial blocking of charge carrier hopping between favorable sites thereby reducing the conductivity. Similar behavior has been observed in PANI based nanocomposites, where the electrical conductivity shows strong dependence on percolation threshold [35,36,37, 40]. These results on conductivity measurement suggests that, GRNPs in PANI matrix plays a significant role in enhancing the conductivity of pure PANI by several orders of magnitude.
3.4 Frequency dependent conductivity
The variation of conductivity as a function of frequency for pure PANI and PANI–GRNPs composites in the frequency range 100 Hz to 1 MHz is shown in Fig. 5. The conductivity of pure PANI and PANI–GRNPs composites increases with increasing frequencies thus obeying universal power law. The conductivity was found to exhibit low value plateau in the frequency range 105 Hz and increases thereafter with increase in frequency, which is a characteristic property of disordered materials. At higher frequencies the polymer chains with shorter conjugation length compared to interatomic separation participate significantly in polarization mechanism leads to better conductivity. The presence of GRNPs in PANI matrix has greater influence on the frequency dependent conductivity values. The conductivity was found to increase up-to 6 wt% content of GRNPs in PANI matrix and decreases thereafter. The initial increase in the conductivity is due to the chain alterations caused by GRNPs presence that facilitate the charge carrier hopping between favorable sites assisted by interfacial polarizations at grain boundaries. Higher content of GRNPs (beyond 6 wt%) will cause greater disorder in the chain alignments on polymer backbone and with broad interfaces and large granular separation affects the delocalization of conduction electrons as well as their hopping between favorable sites [35,36,37, 40]. The percolation threshold for frequency dependent conductivity was observed at 6 wt% content of GRNPs in PANI matrix.
3.5 Dielectric studies
The variation of real and imaginary parts of permittivity as well as dielectric loss for PANI and PANI–GRNPs composites as a function of frequency is shown in Figs. 6, 7 and 8, respectively. It is observed that, the real and imaginary parts of permittivity decreases at lower frequencies and becomes almost constant at higher frequency for all the samples, this behavior may be attributed due to the polarization effects induced by applied frequency. Both ε′ and ε″ shows a strong dependency on GRNPs content in PANI matrix, the values of ε′ and ε″ decreases up-to 6 wt% content of GRNPs and increases thereafter in comparison to pure PANI. The presence of GRNPs in PANI matrix increases the electrode polarization effects at the grain boundaries for lower content of GRNPs thereby reducing the ε′ and ε″ values. The variation of dielectric loss shown in Fig. 8 is almost constant at lower frequencies and gradually decreases at higher frequencies. An increase in filler concentration creates large number of favorable hopping sites in the polymer matrix which facilitates the ease of charge carrier transport, which leads to decrease in dielectric loss with increasing content of GRNPs in PANI. For the frequencies above 1 MHz, the dielectric loss values approaching to zero indicates the possibility of using these composites as loss less materials. The variation of dielectric loss for PANI–GRNPs composites have shown similar dependency on GRNPs content in PANI matrix and was found to be minimum for 6 wt% composite.
3.6 Sensor studies
The sensing response of PANI and PANI–GRNPs composite films were studied for toluene and benzene vapors at different concentrations. The variation of sensitivity as a function of toluene and benzene gas concentrations is shown in Figs. 9 and 10. The experimental results show that the sensitivity of PANI and PANI–GRNPs composites for both the test gases increases linearly with increasing concentration of test gases. Both toluene and benzene being hydrogenated and non-polar, they can release free electrons and alters the work function of polymer matrix due interaction with analyte molecules, which leads to change in conductivity of the composite resulting into increased sensitivity. The observed change in sensitivity of PANI–GRNPs composites may be due to the presence of active sites on GRNPs (junctions between GRNPs flakes) that helps in chemisorption of toluene and benzene gases. These absorbed gas molecules or saturated molecules of analytes can diffuse well into the deeper layers of composite film cause the swelling effects and increased interaction between analyte and polymer. The swelling effects of polymer chains in PANI–GRNPs composites may deteriorate the degree of connectivity thereby drastically increasing the resistance [42, 43]. Addition of GRNPs in the PANI matrix results in the formation of p–n junction [44] and positively charged depletion layer being formed may increase the resistance at the junction, thereby enhancing the sensitivity in nanocomposites. Apart from swelling action, the following reasons do contribute towards the enhancement of sensitivity in PANI–GRNPs composites (1) large surface area for analyte reaction can be achieved due to presence of PANI particles on the surface of GRNPs which helps in adsorption of gas molecules and deprotonation at the hybrid interface. This adsorption/desorption assisted by deprotonation at the interface enhances the sensing performance of the composites. (2) The GRNPs sheets provide high carrier mobility at atmospheric conditions thereby facilitate rapid change in the composite resistance, which reduces the response time in the presence of analytes. (3) The rate of electron transfer that occurs due to π–π interaction between PANI and GRNPs during the sensing process increases, thereby increasing the sensitivity of the composites.
The sensitivity of the PANI–GRNPs composites was found to be greater than that of pure PANI for both the test gases, the sensitivity shows a strong dependency on the GRNPs content in PANI matrix. It is observed that, for both the test gases, the sensitivity increases upto 6 wt% content of GRNPs in PANI matrix and decreases thereafter; these results are in well agreement with the behavior observed in case of conductivity and dielectric studies. The electronic conduction in PANI mainly occurs through the delocalization of conduction electrons that hops between favorable sites; the presence of GRNPs in PANI leads to chain disorder and π-electron delocalization. For GRNPs content beyond 6 wt%, the polymer chain disorder will increase that makes electron hopping more difficult and results in lowering of sensitivity [45]. PANI–GRNPs composite (6 wt%) shows a maximum sensitivity of 90% and 80% for toluene and benzene gases, respectively, which may be due to the rate at which the gas molecules can diffuse into PANI–GRNPs composite. The rate at which the toluene gas diffuses into PANI–GRNPs composite is expected to be higher than that of benzene, hence leading to better sensitivity for toluene. The improved sensitivity for toluene in comparison to benzene can also be attributed to their different bond dissociation energy values. The PANI–GRNPs composite used in the present investigation shows better sensitivity for toluene and benzene gases as well as improved surface resistance (in KΩ) in comparison to previous works [7, 10, 16]. Sensitivity of PANI and PANI–GRNPs composites for the complete cycle of absorption and desorption of toluene and benzene gases as a function of time is shown in Figs. 11 and 12, respectively. From Figs. 11 and 12, it is observed that both PANI and PANI–GRNPs composites exhibit perfect conductometric behavior over the complete cycle of absorption and desorption of test gases. Sensitivity was found to increase with concentration of gas molecules during gas inlet due to absorption and decreases due to desorption when the gas was flushed out.
The response and recovery time are important parameters that describes sensing response of the material under investigation. The response time defines the time taken for 90% of resistance change in comparison to the baseline resistance during absorption and recovery time determines the time required to attain the base line resistance during desorption [46, 47]. Response and recovery times for PANI–GRNPs composites for both the test gases at different concentrations calculated from Figs. 11 and 12 and are shown in Table 1. The PANI–GRNPs composites exhibit better values of response and recovery times towards toluene and benzene gases (at lower gas concentration) in comparison to pure PANI. Sensitivity as well as response and recovery times of PANI–GRNPs composites show better result for toluene and benzene gases in comparison to reports available in literature [34]. Hence these PANI–GRNPs composites may find extensive technological applications as conductometric gas sensors towards sensing toluene and benzene gases at lower concentrations.
4 Conclusions
In summary, polyaniline–graphene nano-platelet (PANI–GRNPs) composites were successfully synthesized with varying content of GRNPs in PANI matrix via simple in situ polymerization of aniline in the presence of GRNPs. The SEM micrographs indicate uniform distribution of GRNPs in PANI matrix and formation of glue-like conducting network structure. FTIR spectra of the composite reveal a shift in the characteristic peaks of PANI indicating a π–π interaction between PANI and GRNPs resulting in a charge-transfer complex. The presence of GRNPs in the PANI matrix significantly enhances the conductivity and dielectric attributes of the composite. The PANI–GRNPs composites were tested as an active element in gas sensor devices to detect toluene and benzene gases at ambient temperature. The sensing material developed in the present investigation is sensitive to change of analyte gas concentration as well as chemical nature of vapor (toluene/benzene). Studies on sensing behavior of these composites show a linear increase in sensitivity for toluene and benzene as a function of gas concentration. The sensitivity variation shows a strong dependency on GRNPs content in PANI matrix. The absorption and desorption studies over a complete cycle shows perfect conductometric behavior of the composites. The PANI–GRNPs composites used in the present investigation are compatible with both the test gases. Due to higher sensitivity and small response and recovery times towards toluene, these composites may find extensive technological applications as conductometric sensors towards the detection of toluene in low concentrations. The materials developed through the present investigation may lead to better detection of BTEX gases present in the environment due their superior gas sensing characteristics.
References
F. Zhang, X. Wang, J. Dong, N. Qin, J. Xu, Selective BTEX sensor based on a SnO2/V2O5 composite. Sens. Actuator B Chem. 131, 126 (2013)
G.F. Fine, L.M. Cavanagh, A. Afonja, R. Binions, Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 10, 5469 (2010)
H.F. Lu, F. Li, G. Liu, Z.G. Chen, D.W. Wang, H.T. Fang, G.Q. Lu, Z.H. Jiang, H.M. Cheng, Amorphous TiO2 nanotube arrays for low-temperature oxygen sensors. Nanotechnology 19, 405504 (2008)
S. Kotresh, Y.T. Ravikiran, S.C. Vijaya Kumari, Ch.V.V. Ramana, K.M. Batoo, Solution based-spin cast processed LPG sensor at room temperature, Sens. Actuators A Phys. 263, 687 (2017)
H. Bai, G. Shi, Gas sensors based on conducting polymers. Sensors 7, 267 (2007)
S. Bai, Y. Zhao, J. Sun, Y. Tian, R. Luo, D. Li, A. Chen, Ultrasensitive room temperature NH3 sensor based on graphene–polyaniline hybrid loaded on PET thin film. Chem. Commun. 51, 7524 (2015)
C. Murugan, E. Subramanian, D.P. Pandiyan, Enhanced sensor functionality of in situ synthesized polyaniline-SnO2 hybrids towards benzene and toluene vapors. Sens. Actuators B 205, 74 (2014)
S. Kotresh; Y, T. Ravikiran, S.K. Tiwari, S.C. Vijaya Kumari, Polyaniline–cadmium ferrite nanostructured composite for room-temperature liquefied petroleum gas sensing. J. Electron. Mater. 46, 5240 (2017)
R.S. Andre, F.M. Shimizu, C.M. Miyazaki, A. Riul Jr, D. Manzani, S.J.L. Ribeiro, O.N. Oliveria Jr., L.H.C. Mattoso, D.S. Corrrea, Hybrid layer-by-layer (LbL) film of polyaniline, graphene oxide and zinc oxide to detect ammonia. Sens. Actuators B 238, 795 (2017)
E. Subramanian, P. Santhanamar, C. Murugan, Sensor functionality of conducting polyaniline-oxide (TiO2/SnO2) hybrid materials films toward benzene and toluene vapors at room temperature. J. Electron. Mater. 47, 4764 (2018)
P. Stamenov, R. Madathil, J.M.D. Coey, Dynamic response of ammonia sensors constructed from polyaniline nanofiber films with varying morphology. Sens. Actuators B Chem. 161, 989 (2012)
T. Anwer, F. Mohammad, Thermal stability of electrical properties and amine vapour sensitivity of in-situ prepared polyaniline/graphene nanocomposites assisted by sodium dodecyl sulfate micelles. Polym. Polym. Compos. 23, 261 (2015)
M. Eising, C.E. Cava, R.V. Salvatierra, A.J.G. Zabrin, L.S. Roman, Doping effect on self-assembled films of polyaniline and carbon nanotube applied as ammonia gas sensor. Sens. Actuators B, 245, 25 (2017)
Y. Zou, Q. Wang, C. Xiang, C. Tang, H. Chu, S. Qiu, E. Yan, F. Xu, L. Sun, Doping composite of polyaniline and reduced graphene oxide with palladium nanoparticles for room-temperature hydrogen-gas sensing. Int. J. Hydrog. Energy 41, 5396 (2016)
C. Dhand, M. Das, M. Datta, B.D. Malhotra, Recent advances in polyaniline based biosensors. Biosens. Bioelectron. 26, 2811 (2011)
F. Xu, S. Guo, Y.-L. Luo, Novel YHBT/MWNTs-OH polyurethane conducting composite thin films for applications in detection of volatile organic compounds. Mater. Chem. Phys. 145, 222 (2014)
Z. Wu, X. Chen, S. Zhu, Z. Zhou, Y. Yao, W. Quan, B. Liu, Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite, Sens. Actuators B 178, 485 (2013)
R.G. Bavane, M.D. Shrisat, A.M. Mahajan, Ammonia gas sensing characteristics of chemically synthesized polyaniline matrix. Sens. Transducers 113, 63 (2010)
O. Abdulrazzaq, S.E. Bourdo, V. Saini, V.G. Bairi, E. Dervishi, T. Viswanathan, Z.A. Nima, A.S. Biris, Optimization of the protonation level of polyaniline-based hole-transport layers in bulk-heterojunction organic solar cells. Energy Technol. 1, 463 (2013)
G.A. Snook, P. Kao, A.S. Best, Conducting-polymer-based super capacitor devices and electrodes. J. Power Sour. 196, 1 (2011)
E.S. Forzani, H.Q. Zhang, L.A. Nagahara, I. Amlani, R. Tsui, N.J. Tao, A conducting polymer nanojunction sensor for glucose detection. Nano Lett. 4, 1785 (2004)
S. Ameen, M.S. Akhtar, M. Husain, A review on synthesis processing, chemical and conduction properties of polyaniline and its nanocomposites. Sci. Adv. Mater. 2, 441 (2010)
F. Lux, Properties of electronically conductive polyaniline: a comparison between well-known literature data and some recent experimental findings. Polymer 35, 2915 (1994)
N. Gospodinova, L. Terlemezyan, Conducting polymers prepared by oxidative polymerization: polyaniline. Prog. Polym. Sci. 23, 1443 (1998)
W. Zheng, Y. Min, A.G. MacDiarmid, M. Angelopoulos, Y.H. Liao, A.J. Epstein, Effect of organic vapors on the molecular conformation of non-doped polyaniline. Synth. Met. 84, 63 (1997)
S. Bhadra, D. Khastgir, N.K. Singha, J.H. Lee, Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 34, 783 (2009)
M. Moussa, M.F. El-Kady, Z. Zhao, P. Majewski, J. Ma, Recent progress and performance evaluation for polyaniline/graphene nanocomposites as super capacitor electrodes. Nanotechnology 27, 442001 (2016)
S.M. Imran, Y.N. Kim, G.N. Shao, M. Hussain, Y. Choa, H.T. Kim, Enhancement of electroconductivity of polyaniline/graphene oxide nanocomposites through in situ emulsion polymerization. J. Mater. Sci. 49, 1328 (2014)
Y. Jafari, S.M. Ghoreishi, M. Shabani-Nooshabadi, Polyaniline/graphene nanocomposite coatings on copper: electropolymerization, characterization, and evaluation of corrosion protection performance. Synth. Met. 217, 220 (2016)
R. Gupta, N. Vadodariya, A. Mahto, J.P. Chaudhary, D.B. Parmar, D.N. Srivastava, S.K. Nataraj, R. Meena, Functionalized seaweed derived graphene/polyaniline nanocomposite as efficient energy storage electrode. J. Appl. Electrochem. 48, 37 (2018)
M. Dinari, M. Momeni, Mohsen, Goudarzirad, meysam, dye-sensitized solar cells based on nanocomposite of polyaniline/graphene quantum dots. J. Mater. Sci. 51, 2964 (2016)
S.-J. Lin, H.-J. Sun, T.-J. Peng, L.-H. Jiang, Synthesis of high-performance polyaniline/graphene oxide nanocomposites. High Perform. Polym. 26, 790 (2014)
X.S. Zhou, T.B. Wu, B.J. Hu, G.Y. Yang, B.X. Han, Synthesis of graphene/polyaniline composite nanosheets mediated by polymerized ionic liquid. Chem. Commun. 46, 3663 (2010)
M. Parmar, C. Balamurugan, D.-W. Lee, PANI and graphene/PANI nanocomposite films-comparative toluene gas sensing behavior. Sensors 13, 16611 (2013)
K.C. Sajjan, A.S. Roy, A. Parveen, S. Khasim, Analysis of DC and AC properties of a humidity sensor based on polyaniline–chromium oxide composites. J. Mater. Sci. Mater. Electron. 25, 1237 (2014)
M. Faisal, S. Khasim, Broadband electromagnetic shielding and dielectric properties of polyaniline-stannous oxide composites. J. Mater. Sci. Mater. Electron. 24, 2202 (2013)
M. Faisal, S. Khasim, E. Conductivity, Dielectric behavior and EMI shielding effectiveness of polyaniline-yttrium oxide composites. Bull. Korean Chem. Soc. 34, 99 (2013)
H. Tai, Y. Jiang, G. Xie, J. Yu, X. Chen, Fabrication and gas sensitivity of polyaniline–titanium dioxide nanocomposite thin film. Sens. Actuators B 125, 644 (2007)
L. Al-Mashat, K. Shin, K. Kalantar-zadeh, J.D. Plesis, S.H. Han, R.W. Kojima, R.B. Kaner, D. Li, X. Gou, S.J. Ippolito, W. Wlodarski, Graphene/polyaniline nanocomposite for hydrogen sensing. J. Phys. Chem. C 114, 16168 (2010)
S. Khasim, O. Al-hartomy, Fabrication and gas sensitivity in hetero-structures of ortho-chloropolyaniline-ZnO nanocomposites. RSC Adv. 4, 39844 (2014)
N. Badi, S. Khasim, A.S. Roy, Micro-Raman spectroscopy and effective conductivity studies of graphene nano-platelets/polyaniline composites. J. Mater. Sci. Mater. Electron. 27, 6249 (2016)
M. Matsuguchi, I. Io, G. Sugiyama, Y. Sakai, Effect of NH3 gas on the electrical conductivity of polyaniline blend films. Synth. Met. 128, 15 (2002)
L.T. Liu, X.Y. Ye, K. Wu, Z.Y. Zhou, D.J. Lee, T.H. Cui, Room temperature methane sensor based on graphene nanosheets/polyaniline nanocomposite thin film. IEEE Sens. J. 9, 1308 (2009)
D. Majumdar, M. Baskey, S.K. Saha, Epitaxial growth of crystalline polyaniline on reduced graphene oxide. Macromol. Rapid Commun. 32, 1277 (2011)
Z. Wu, C. Xiangdong, S. Zhou, Y. Yao, W. Quan, B. Liu, Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens. Actuators B 178, 485 (2013)
M. Parmar, K. Rajanna, Copper (II) oxide thin film for methanol and ethanol sensing. Int. J. Smart Sens. Intell. Syst. 4, 710 (2011)
B. Liu, H. Yang, H. Zhao, L. An, L. Zhang, R. Shi, L. Wang, L. Bao, Y. Chen, High toluene sensing properties of NiO–SnO2 composite nanofiber sensors operating at 330 °C. Sens. Actuator B Chem. 156, 251 (2011)
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under Grant no. J-88-130-38. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Hartomy, O.A., Khasim, S., Roy, A. et al. Highly conductive polyaniline/graphene nano-platelet composite sensor towards detection of toluene and benzene gases. Appl. Phys. A 125, 12 (2019). https://doi.org/10.1007/s00339-018-2317-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-018-2317-7